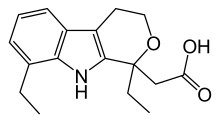
Etodolac
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692015 |
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 100% |
| Metabolism | liver |
| Elimination half-life | 7.3 ± 4.0 hours |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.157.848 |
| Chemical and physical data | |
| Formula | C17H21NO3 |
| Molar mass | 287.359 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 145 to 148 °C (293 to 298 °F) |
| Solubility in water | 3.92e-02 mg/mL [ALOGPS] mg/mL (20 °C) |
| |
| |
| (verify) | |
Etodolac is a nonsteroidal anti-inflammatory drug (NSAID).
It was patented in 1971 and approved for medical use in 1985.[2] It was approved in the U.S. in 1991.[3] As of 2015 the cost for a typical month of medication in the United States is less than US$25.[4]
Medical uses

NSAIDs are used for the management of mild to moderate pain, fever, and inflammation. They work by reducing the levels of prostaglandins, which are chemicals that are responsible for pain and the fever and tenderness that occur with inflammation. Etodolac blocks the cyclooxygenase (abbrev. COX) enzymes which form prostanoids, resulting in lower concentrations of prostaglandins. As a consequence, inflammation, pain and fever are reduced.
Post-marketing studies demonstrated that etodolac inhibition of cyclooxygenase is somewhat COX-2 selective [5] similar to celecoxib and other "COX-2 inhibitors." Unlike rofecoxib, both etodolac and celecoxib can fully inhibit COX-1 and are designated as having "preferential selectivity" toward COX-2. The R-enantiomer of etodolac is inactive against COX enzymes, but inhibits beta-catenin levels in hepatoma cells.[6]
In the UK, Etodolac is licensed for the treatment of inflammation and pain caused by osteoarthritis and rheumatoid arthritis.[7]
Interactions
Etodolac should be avoided by patients with a history of asthma attacks, hives, or other allergic reactions to aspirin or other NSAIDs. Rare but severe allergic reactions have been reported in such individuals. It also should be avoided by patients with peptic ulcer disease or poor kidney function, since this medication can worsen both conditions. Etodolac is used with caution in patients taking blood thinning medications (anticoagulants), such as warfarin (Coumadin), because it increases the risk of bleeding. Patients taking both lithium and etodolac may develop toxic blood lithium levels. Additionally, etodolac has been found to interact with certain anti-depressant medications, such as sertraline or fluoxetine, which can increase risks of stroke, heart attack, and other cardiovascular conditions. Patients also taking ciclosporin (Sandimmune) can develop kidney toxicity. Use in children has not been adequately studied. Etodolac is not habit-forming. NSAIDs should be discontinued prior to elective surgery because of a mild interference with clotting that is characteristic of this group of medicines. Etodolac is best discontinued at least four days in advance of surgery. Etodolac metabolites may also cause a false positive bilirubin result on a urinalysis test. [1][8]

Pregnancy and nursing
Etodolac is generally avoided during pregnancy and nursing. NSAIDs may cause adverse cardiovascular effects in the fetus during pregnancy. [3]
Brand names
Etodolac is manufactured by Almirall Limited under the trade name Lodine SR[9] and by Meda Pharmaceuticals under the name Eccoxolac.[10] Generic etodolac is also available.[11]
The drug is also sold under several other brand names, including:
- "Etogesic" (India)
- Etova (India)
- Dualgan (Portugal)
- Etodin (South Korea)
- Etofree (India)
- Etopan (Israel)
- Flancox®[12] (Brazil)
- Haipen (Japan)
- Lodine (France, Switzerland, United States)
- Proxym (S Etodolac) (India)
- Etol (Turkey)
- Lonine (Taiwan)
- Etodine (Egypt)
- Dolarit (Turkey)
- "Etodin Fort" (Bulgaria)
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 517. ISBN 9783527607495.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Ogbru, Omudhome. "etodolac, Lodine (Discontinued): Drug Facts, Side Effects and Dosing". MedicineNet.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Hamilton, Richart (2015). Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. p. 7. ISBN 9781284057560.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR (June 1999). "Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis". Proceedings of the National Academy of Sciences of the United States of America. 96 (13): 7563–8. Bibcode:1999PNAS...96.7563W. doi:10.1073/pnas.96.13.7563. PMC 22126. PMID 10377455.
- ^ Behari J, Zeng G, Otruba W, Thompson MD, Muller P, Micsenyi A, et al. (May 2007). "R-Etodolac decreases beta-catenin levels along with survival and proliferation of hepatoma cells". Journal of Hepatology. 46 (5): 849–57. doi:10.1016/j.jhep.2006.11.017. PMC 1924913. PMID 17275129.
- ^ BNF 55 - Etodolac
- ^ Sho Y, Ishiodori T, Oketani M, Kubozono O, Nakamura A, Takeuchi A, et al. (July 1999). "Effects of urinary metabolites of etodolac on diagnostic tests of bilirubin in urine". Arzneimittel-Forschung. 49 (7): 572–6. doi:10.1055/s-0031-1300464. PMID 10442203.
- ^ "Lodine SR". medicines.org.uk. Archived from the original on 2008-03-18. Retrieved 2008-08-07.
- ^ "Eccoxolac". medicines.org.uk. Archived from the original on 2012-12-24.
- ^ "Etodolac preparations". BNF 55.
- ^ "Flancox". apsen.com.br.
