Mitotane
 | |
| Clinical data | |
|---|---|
| Trade names | Lysodren |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a608050 |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 40% |
| Protein binding | 6% |
| Elimination half-life | 18 to 159 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.152 |
| Chemical and physical data | |
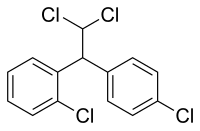
| Formula | C14H10Cl4 |
| Molar mass | 320.04 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Mitotane, also known as o,p'-DDD (Lysodren), is an antineoplastic medication used in the treatment of adrenocortical carcinoma.[2] It is an isomer of DDD and is a derivative of DDT.[3] Its main use is in those patients who have persistent disease despite surgical resection, those who are not surgical candidates, or those who have metastatic disease.
It has been produced by Bristol Myers Squibb SpA but it is marketed as an orphan drug due to the small number of patients in need of it. A 2007 study of 177 patients shows a significant increase in the recurrence-free interval after radical surgery followed by mitotane when compared to surgery alone.[4]
Mitotane alters steroid peripheral metabolism, directly suppresses the adrenal cortex and alters cortisone metabolism leading to hypocortisolism. Side effects as reported by Schteinberg et al. include anorexia and nausea (88%), diarrhea (38%), vomiting (23%), decreased memory and ability to concentrate (50%), rash (23%), gynecomastia (50%), arthralgia (19%), and leukopenia (7%).[5]
Its trade name is Lysodren.
Veterinary use
Mitotane is also used to treat Cushing's disease (pituitary-dependent Cushing's syndrome) in dogs. The medication is used in the controlled destruction of adrenal tissue, leading to a decrease in cortisol production.[6]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 Oct 2023.
- ^ Hahner S, Fassnacht M (April 2005). "Mitotane for adrenocortical carcinoma treatment". Current opinion in investigational drugs (London, England : 2000). 6 (4): 386–94. PMID 15898346.
- ^ Information from PubChem
- ^ Terzolo M, Angeli A, Fassnacht M, Daffara F, Tauchmanova L, Conton PA, Rossetto R, Buci L, Sperone P, Grossrubatscher E, Reimondo G, Bollito E, Papotti M, Saeger W, Hahner S, Koschker AC, Arvat E, Ambrosi B, Loli P, Lombardi G, Mannelli M, Bruzzi P, Mantero F, Allolio B, Dogliotti L, Berruti A (2007). "Adjuvant mitotane treatment for adrenocortical carcinoma". N Engl J Med. 356 (23): 2372–2380. doi:10.1056/NEJMoa063360. PMID 17554118.
- ^ Schteinberg DE, Motazedi A, NoonanRA, Thompson NW (1982). "Treatment of Adrenal Carcinomas". Arch.Surg. 117: 1142–1149.
- ^ Canine Cushing’s Syndrome: Diagnosis and Treatment
External links
- V. P. Komissarenko; I. S. Chelnakova; A. S. Mikosha (1978). "Effect of o,p-dichlorodiphenyldichloroethane and perthane in vitro on glutathione reductase activity in the adrenals of dogs and guinea pigs". Bulletin of Experimental Biology and Medicine. 85 (2): 152–154. doi:10.1007/BF00800110.
- Government of Canada: Benzene, 1-chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethyl]- (Mitotane)
- Environment Canada & Health Canada: RISK MANAGEMENT SCOPE for Benzene, 1-chloro-2-[2,2-dichloro-1-(4-chlorophenyl)ethyl]- (Mitotane), 2013
