Proto-oncogene tyrosine-protein kinase Src: Difference between revisions
copyedit |
→As a drug target: + citations |
||
| Line 33: | Line 33: | ||
== As a drug target == |
== As a drug target == |
||
A number of tyrosine kinase inhibitors that target c-Src tyrosine kinase (as well as related tyrosine kinases) have been developed for therapeutic use. One notable example is [[dasatinib]] which has been approved for the treatment of [[chronic myeloid leukemia]] (CML) and Philadelphia chromosome-positive (PH+) acute lymphocytic leukemia (ALL). Dasatinib is also in clinical trials for the use in non-Hodgkin’s lymphoma, metastatic breast cancer and prostate cancer. Other tyrosine kinase inhibitor drugs that are in clinical trials include [[bosutinib]], AZD-530, XLl-999 |
A number of tyrosine kinase inhibitors that target c-Src tyrosine kinase (as well as related tyrosine kinases) have been developed for therapeutic use.<ref name="pmid22530642">{{cite journal | author = Musumeci F, Schenone S, Brullo C, Botta M | title = An update on dual Src/Abl inhibitors | journal = Future Med Chem | volume = 4 | issue = 6 | pages = 799–822 | year = 2012 | month = April | pmid = 22530642 | doi = 10.4155/fmc.12.29 | url = }}</ref> One notable example is [[dasatinib]] which has been approved for the treatment of [[chronic myeloid leukemia]] (CML) and Philadelphia chromosome-positive (PH+) acute lymphocytic leukemia (ALL).<ref name="pmid23569389">{{cite journal | author = Breccia M, Salaroli A, Molica M, Alimena G | title = Systematic review of dasatinib in chronic myeloid leukemia | journal = Onco Targets Ther | volume = 6 | issue = | pages = 257–65 | year = 2013 | pmid = 23569389 | pmc = 3615898 | doi = 10.2147/OTT.S35360 }}</ref> Dasatinib is also in clinical trials for the use in non-Hodgkin’s lymphoma, metastatic breast cancer and prostate cancer. Other tyrosine kinase inhibitor drugs that are in clinical trials include [[bosutinib]],<ref name="pmid23493838">{{cite journal | author = Amsberg GK, Koschmieder S | title = Profile of bosutinib and its clinical potential in the treatment of chronic myeloid leukemia | journal = Onco Targets Ther | volume = 6 | issue = | pages = 99–106 | year = 2013 | pmid = 23493838 | pmc = 3594007 | doi = 10.2147/OTT.S19901 }}</ref> [[bafetinib]], AZD-530, XLl-999, KX01 and XL228.<ref name="pmid19581523">{{cite journal | author = Wheeler DL, Iida M, Dunn EF | title = The role of Src in solid tumors | journal = Oncologist | volume = 14 | issue = 7 | pages = 667–78 | year = 2009 | month = July | pmid = 19581523 | pmc = 3303596 | doi = 10.1634/theoncologist.2009-0009 }}</ref> |
||
== Interactions == |
== Interactions == |
||
Revision as of 06:35, 23 April 2013
Template:PBB c-Src tyrosine kinase also known as proto-oncogene c-Src, is a nonreceptor tyrosine kinase protein that in humans is encoded by the SRC gene. It includes an SH2 domain, an SH3 domain, and a tyrosine kinase domain. This protein phosphorylates a carboxyl-terminus tyrosine residue on human Src, which acts as a negative regulatory site. An elevated level of activity of c-Src tyrosine kinase is suggested to be linked to cancer progression by promoting other signals.[1]
Src (pronounced "sarc" as it is short for sarcoma) is a proto-oncogene encoding a tyrosine kinase originally discovered by J. Michael Bishop and Harold E. Varmus, for which they were awarded the 1989 Nobel Prize in Physiology or Medicine.[2] It belongs to a family of non-receptor tyrosine kinases called Src family kinases.
This gene is similar to the v-Src gene of Rous sarcoma virus. This proto-oncogene may play a role in the regulation of embryonic development and cell growth. The protein encoded by this gene is a tyrosine-protein kinase whose activity can be inhibited by phosphorylation by c-Src kinase. Mutations in this gene could be involved in the malignant progression of colon cancer. Two transcript variants encoding the same protein have been found for this gene.[3]
Discovery
In 1979, J. Michael Bishop and Harold E. Varmus discovered that normal chickens contain a gene that is structurally closely related to v-Src.[4] The normal cellular gene was called c-src (cellular-src).[5] This discovery changed the current thinking about cancer from a model wherein cancer is caused by a foreign substance (a viral gene) to one where a gene that is normally present in the cell can cause cancer. It is believed that at one point an ancestral virus mistakenly incorporated the c-Src gene of its cellular host. Eventually this normal gene mutated into an abnormally functioning oncogene within the Rous sarcoma virus. Once the oncogene is transfected back into a chicken, it can lead to cancer.
Structure and function
There are 9 members part of the Src family kinases: Src, Yes, Fyn, Fgr, Yrk, Lyn, Blk, Hck, and Lck.[6] The expression of these Src family members are not the same throughout all tissues and cell types. Src, Fyn and Yes are expressed ubiquitously in all cell types while the others are generally found in hematopoietic cells.[7][8][9][10]
c-Src is made up of 6 functional regions: Src homology (SH) 4 domain, unique region, SH3 domain, SH2 domain, catalytic domain and short regulatory tail. When Src is inactive, the phosphorylated tyrosine group at the 530 position interacts with the SH2 domain which helps the SH3 domain interact with the linker domain and consequently keeps the inactive unit tightly bound. The activation of c-Src causes the dephosphorylation of the tyrosine 530 which causes the structure to be destabilized and then result in the opening up of the SH3, SH2 and the kinase domains and autophosphorylation of tyrosine 419.[11][12][13][13]
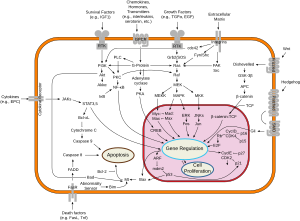
c-Src can be activated by many transmembrane proteins that include: adhesion receptors, receptor tyrosine kinases, G-protein coupled receptors and cytokine receptors. Most studies have looked at the receptor tyrosine kinases and examples of these are platelet derived growth factor (PDGF) receptor pathway and epidermal growth factor receptor (EGFR). When src is activated, it promotes survival, angiogenesis, proliferation and invasion pathways.
Role in cancer
The activation of the c-Src pathway has been observed in about 50% of tumors from colon, liver, lung, breast and the pancreas.[14] Since the activation of c-Src leads to the promotion of survival, angiogenesis, proliferation and invasion pathways, the aberrant growth of tumors in cancers are observed. A common mechanism is that there are genetic mutations that result in the increased activity or the overexpression of the c-Src leading to the constant activation of the c-Src.
Colon
The activity of c-Src has been best characterized in colon cancer. Researchers have shown that Src expression are 5 to 8 fold higher in premalignant polyps than normal mucosa.[15][16][17] The elevated c-Src levels have also been shown to have a correlation with advances stages of the tumor, size of tumor, and metastatic potential of tumors.[18][19]
Breast
EGFR activates c-Src while EGF also increases the activity of c-Src. In addition, overexpression of c-Src increases the response of EGFR-mediated processes. So both EGFR and c-Src enhance the effects of one another to Elevated expression levels of c-Src were found in human breast cancer tissues compared to normal tissues.[20][21][22]
Overexpression of Human Epidermal Growth Factor Receptor 2 (HER2), also known as EGFR, is correlated with a worse prognosis for breast cancer.[23][24] Thus, c-Src plays a key role in the tumor progression of breast cancers.
Prostate
Members of the Src family kinases Src, Lyn and Fgr are highly expressed in malignant prostate cells compared to normal prostate cells.[25] When the primary prostate cells are treated with KRX-123, which is an inhibitor of Lyn, the cells in vitro were reduced in proliferation, migration and invasive potential.[26] So the use of a tyrosine kinase inhibitor is a possible way of reducing the progression of prostate cancers.
As a drug target
A number of tyrosine kinase inhibitors that target c-Src tyrosine kinase (as well as related tyrosine kinases) have been developed for therapeutic use.[27] One notable example is dasatinib which has been approved for the treatment of chronic myeloid leukemia (CML) and Philadelphia chromosome-positive (PH+) acute lymphocytic leukemia (ALL).[28] Dasatinib is also in clinical trials for the use in non-Hodgkin’s lymphoma, metastatic breast cancer and prostate cancer. Other tyrosine kinase inhibitor drugs that are in clinical trials include bosutinib,[29] bafetinib, AZD-530, XLl-999, KX01 and XL228.[1]
Interactions
Src (gene) has been shown to interact with the following signaling pathways:
Survival
Angiogenesis
Proliferation
Motility
Additional images
 |
|
References
- ^ a b Wheeler DL, Iida M, Dunn EF (2009). "The role of Src in solid tumors". Oncologist. 14 (7): 667–78. doi:10.1634/theoncologist.2009-0009. PMC 3303596. PMID 19581523.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "The Nobel Prize in Physiology or Medicine 1989: J. Michael Bishop, Harold E. Varmus". Nobelprize.org. 1989-10-09.
for their discovery of 'the cellular origin of retroviral oncogenes'
- ^ "Entrez Gene: SRC v-src sarcoma (Schmidt-Ruppin A-2) viral oncogene homolog (avian)".
- ^ Stehelin D, Fujita DJ, Padgett T, Varmus HE, Bishop JM. (1977). "Detection and enumeration of transformation-defective strains of avian sarcoma virus with molecular hybridization". Virology. 76 (2): 675–84. doi:10.1016/0042-6822(77)90250-1. PMID 190771.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Oppermann H, Levinson AD, Varmus HE, Levintow L, Bishop JM (1979). "Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src)". Proc. Natl. Acad. Sci. U.S.A. 76 (4): 1804–8. doi:10.1073/pnas.76.4.1804. PMC 383480. PMID 221907.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Thomas SM, Brugge JS (1997). "Cellular functions regulated by Src family kinases". Annu. Rev. Cell Dev. Biol. 13: 513–609. doi:10.1146/annurev.cellbio.13.1.513. PMID 9442882.
- ^ Cance WG, Craven RJ, Bergman M, Xu L, Alitalo K, Liu ET (1994). "Rak, a novel nuclear tyrosine kinase expressed in epithelial cells". Cell Growth Differ. 5 (12): 1347–55. PMID 7696183.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Lee J, Wang Z, Luoh SM, Wood WI, Scadden DT (1994). "Cloning of FRK, a novel human intracellular SRC-like tyrosine kinase-encoding gene". Gene. 138 (1–2): 247–51. doi:10.1016/0378-1119(94)90817-6. PMID 7510261.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Oberg-Welsh C, Welsh M (1995). "Cloning of BSK, a murine FRK homologue with a specific pattern of tissue distribution". Gene. 152 (2): 239–42. doi:10.1016/0378-1119(94)00718-8. PMID 7835707.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Thuveson M, Albrecht D, Zürcher G, Andres AC, Ziemiecki A (1995). "iyk, a novel intracellular protein tyrosine kinase differentially expressed in the mouse mammary gland and intestine". Biochem. Biophys. Res. Commun. 209 (2): 582–9. doi:10.1006/bbrc.1995.1540. PMID 7733928.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cooper JA, Gould KL, Cartwright CA, Hunter T (1986). "Tyr527 is phosphorylated in pp60c-src: implications for regulation". Science. 231 (4744): 1431–4. Bibcode:1986Sci...231.1431C. doi:10.1126/science.2420005. PMID 2420005.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Okada M, Nakagawa H (1989). "A protein tyrosine kinase involved in regulation of pp60c-src function". J. Biol. Chem. 264 (35): 20886–93. PMID 2480346.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ a b Nada S, Okada M, MacAuley A, Cooper JA, Nakagawa H (1991). "Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src". Nature. 351 (6321): 69–72. doi:10.1038/351069a0. PMID 1709258.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Dehm SM, Bonham K (2004). "SRC gene expression in human cancer: the role of transcriptional activation". Biochem. Cell Biol. 82 (2): 263–74. doi:10.1139/o03-077. PMID 15060621.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Bolen JB, Rosen N, Israel MA (1985). "Increased pp60c-src tyrosyl kinase activity in human neuroblastomas is associated with amino-terminal tyrosine phosphorylation of the src gene product". Proc. Natl. Acad. Sci. U.S.A. 82 (21): 7275–9. Bibcode:1985PNAS...82.7275B. doi:10.1073/pnas.82.21.7275. PMC 390832. PMID 2414774.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cartwright CA, Kamps MP, Meisler AI, Pipas JM, Eckhart W (1989). "pp60c-src activation in human colon carcinoma". J. Clin. Invest. 83 (6): 2025–33. doi:10.1172/JCI114113. PMC 303927. PMID 2498394.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Talamonti MS, Roh MS, Curley SA, Gallick GE (1993). "Increase in activity and level of pp60c-src in progressive stages of human colorectal cancer". J. Clin. Invest. 91 (1): 53–60. doi:10.1172/JCI116200. PMC 329994. PMID 7678609.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE (2002). "Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis". Cancer. 94 (2): 344–51. doi:10.1002/cncr.10221. PMID 11900220.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cartwright CA, Meisler AI, Eckhart W (1990). "Activation of the pp60c-src protein kinase is an early event in colonic carcinogenesis". Proc. Natl. Acad. Sci. U.S.A. 87 (2): 558–62. Bibcode:1990PNAS...87..558C. doi:10.1073/pnas.87.2.558. PMC 53304. PMID 2105487.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Ottenhoff-Kalff AE, Rijksen G, van Beurden EA, Hennipman A, Michels AA, Staal GE (1992). "Characterization of protein tyrosine kinases from human breast cancer: involvement of the c-src oncogene product". Cancer Res. 52 (17): 4773–8. PMID 1380891.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Biscardi JS, Belsches AP, Parsons SJ (1998). "Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells". Mol. Carcinog. 21 (4): 261–72. doi:10.1002/(SICI)1098-2744(199804)21:4<261::AID-MC5>3.0.CO;2-N. PMID 9585256.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Verbeek BS, Vroom TM, Adriaansen-Slot SS, Ottenhoff-Kalff AE, Geertzema JG, Hennipman A, Rijksen G (1996). "c-Src protein expression is increased in human breast cancer. An immunohistochemical and biochemical analysis". J. Pathol. 180 (4): 383–8. doi:10.1002/(SICI)1096-9896(199612)180:4<383::AID-PATH686>3.0.CO;2-N. PMID 9014858.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL (1987). "Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene". Science. 235 (4785): 177–82. Bibcode:1987Sci...235..177S. doi:10.1126/science.3798106. PMID 3798106.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, Levin WJ, Stuart SG, Udove J, Ullrich A (1989). "Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer". Science. 244 (4905): 707–12. Bibcode:1989Sci...244..707S. doi:10.1126/science.2470152. PMID 2470152.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Nam S, Kim D, Cheng JQ, Zhang S, Lee JH, Buettner R, Mirosevich J, Lee FY, Jove R (2005). "Action of the Src family kinase inhibitor, dasatinib (BMS-354825), on human prostate cancer cells". Cancer Res. 65 (20): 9185–9. doi:10.1158/0008-5472.CAN-05-1731. PMID 16230377.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Chang YM, Bai L, Yang I (2002). "Survey of Src activity and Src-related growth and migration in prostate cancer lines". Proc Am Assoc Cancer Res. 62: 2505a.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Musumeci F, Schenone S, Brullo C, Botta M (2012). "An update on dual Src/Abl inhibitors". Future Med Chem. 4 (6): 799–822. doi:10.4155/fmc.12.29. PMID 22530642.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Breccia M, Salaroli A, Molica M, Alimena G (2013). "Systematic review of dasatinib in chronic myeloid leukemia". Onco Targets Ther. 6: 257–65. doi:10.2147/OTT.S35360. PMC 3615898. PMID 23569389.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Amsberg GK, Koschmieder S (2013). "Profile of bosutinib and its clinical potential in the treatment of chronic myeloid leukemia". Onco Targets Ther. 6: 99–106. doi:10.2147/OTT.S19901. PMC 3594007. PMID 23493838.
{{cite journal}}: CS1 maint: unflagged free DOI (link)
External links
- src+Gene at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- src-Family+Kinases at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Proteopedia SRC - interactive 3D model of the structure of SRC
- Vega geneview
- Src Info with links in the Cell Migration Gateway