Sigmodal: Difference between revisions
Appearance
Content deleted Content added
m Stub sorting and placement of stub template(s): nervous-system-drug-stub. See approval. Report errors and suggestions at User talk:PotatoBot. |
Updating {{drugbox}} (no changed fields - added verified revid - updated 'ChemSpiderID_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'StdInChI_Ref', 'StdInChIKey_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Wik |
||
| Line 1: | Line 1: | ||
{{Drugbox |
{{Drugbox |
||
| verifiedrevid = |
| verifiedrevid = 448003382 |
||
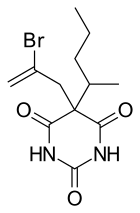
| IUPAC_name = 5-(2-bromoprop-2-en-1-yl)-5-(1-methylbutyl)pyrimidine-2,4,6(1''H'',3''H'',5''H'')-trione |
| IUPAC_name = 5-(2-bromoprop-2-en-1-yl)-5-(1-methylbutyl)pyrimidine-2,4,6(1''H'',3''H'',5''H'')-trione |
||
| image = Sigmodal.svg |
| image = Sigmodal.svg |
||
Revision as of 20:06, 10 September 2011
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.013.575 |
| Chemical and physical data | |
| Formula | C12H17BrN2O3 |
| Molar mass | 317.179 g/mol g·mol−1 |
| (verify) | |
Sigmodal (Rectidon) is a barbiturate derivative. It has sedative, hypnotic and anticonvulsant properties, and was used in surgical anaesthesia in the 1950s, plus the frequent appearances in drug mixtures in the 60's[1][2] although it was never widely used compared to better known barbiturates such as thiopental, and has now been replaced by newer drugs with a better safety profile.
References
