UR-144: Difference between revisions
Appearance
Content deleted Content added
No edit summary |
Undid revision 493365371 by 213.64.141.178 (talk) |
||
| Line 36: | Line 36: | ||
}} |
}} |
||
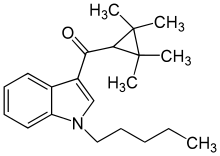
'''(1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone''' ('''KM X-1''', '''UR-144''') is a drug invented by [[Abbott Laboratories]],<ref name = "WO2006/069196">{{ cite patent | country = WO | number = 2006069196 | status = application | title = 3-Cycloalkylcarbonyl indoles as cannabinoid receptor ligands | pubdate = 2006-06-29 | fdate = | pridate = | inventor = Pace JM, Tietje K, Dart MJ, Meyer MD | assign1 = Abbott |
'''(1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone''' ('''KM X-1''', '''UR-144''') is a drug invented by [[Abbott Laboratories]],<ref name = "WO2006/069196">{{ cite patent | country = WO | number = 2006069196 | status = application | title = 3-Cycloalkylcarbonyl indoles as cannabinoid receptor ligands | pubdate = 2006-06-29 | fdate = | pridate = | inventor = Pace JM, Tietje K, Dart MJ, Meyer MD | assign1 = Abbott Laboratories }}</ref> that acts as a selective [[full agonist]] of the peripheral [[cannabinoid receptor]] [[Cannabinoid receptor 2|CB<sub>2</sub>]], but with much lower affinity for the psychoactive [[Cannabinoid receptor 1|CB<sub>1</sub>]] receptor. It has high affinity for the CB<sub>2</sub> receptor with a K<sub>i</sub> of 1.8nM but 83x lower affinity for the CB<sub>1</sub> receptor with a K<sub>i</sub> of 150nM.<ref name="pmid19921781">{{cite journal | author = Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD | title = Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity | journal = J. Med. Chem. | volume = 53 | issue = 1 | pages = 295–315 | year = 2010 | month = January | pmid = 19921781 | doi = 10.1021/jm901214q }}</ref> Chemically it is closely related to other 2,2,3,3-tetramethylcyclopropyl synthetic cannabinoids like [[A-796,260]] and [[A-834,735]] but with a different substitution on the 1-position of the indole core, in these compounds its 1-pentyl group is replaced with alkylheterocycles like 1-(2-morpholinoethyl) and 1-(tetrahydropyran-4-ylmethyl). |
||
==See also== |
==See also== |
||
Revision as of 00:04, 20 May 2012
This article may be too technical for most readers to understand. (January 2012) |
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H29NO |
| Molar mass | 311.461 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
(1-Pentylindol-3-yl)-(2,2,3,3-tetramethylcyclopropyl)methanone (KM X-1, UR-144) is a drug invented by Abbott Laboratories,[1] that acts as a selective full agonist of the peripheral cannabinoid receptor CB2, but with much lower affinity for the psychoactive CB1 receptor. It has high affinity for the CB2 receptor with a Ki of 1.8nM but 83x lower affinity for the CB1 receptor with a Ki of 150nM.[2] Chemically it is closely related to other 2,2,3,3-tetramethylcyclopropyl synthetic cannabinoids like A-796,260 and A-834,735 but with a different substitution on the 1-position of the indole core, in these compounds its 1-pentyl group is replaced with alkylheterocycles like 1-(2-morpholinoethyl) and 1-(tetrahydropyran-4-ylmethyl).
See also
References
- ^ WO application 2006069196, Pace JM, Tietje K, Dart MJ, Meyer MD, "3-Cycloalkylcarbonyl indoles as cannabinoid receptor ligands", published 2006-06-29, assigned to Abbott Laboratories
- ^ Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD (2010). "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". J. Med. Chem. 53 (1): 295–315. doi:10.1021/jm901214q. PMID 19921781.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
Further reading
- Poso A, Huffman JW (2008). "Targeting the cannabinoid CB2 receptor: modelling and structural determinants of CB2 selective ligands". Br. J. Pharmacol. 153 (2): 335–46. doi:10.1038/sj.bjp.0707567. PMC 2219524. PMID 17982473.
{{cite journal}}: Unknown parameter|month=ignored (help) - Chin CL, Tovcimak AE, Hradil VP, Seifert TR, Hollingsworth PR, Chandran P, Zhu CZ, Gauvin D, Pai M, Wetter J, Hsieh GC, Honore P, Frost JM, Dart MJ, Meyer MD, Yao BB, Cox BF, Fox GB (2008). "Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI". Br. J. Pharmacol. 153 (2): 367–79. doi:10.1038/sj.bjp.0707506. PMC 2219521. PMID 17965748.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, El-Kouhen OF, Miller LN, Li L, Yao BB, Hsieh GC, Pai M, Zhu CZ, Chandran P, Meyer MD (2008). "Indol-3-yl-tetramethylcyclopropyl ketones: effects of indole ring substitution on CB2 cannabinoid receptor activity". J. Med. Chem. 51 (6): 1904–12. doi:10.1021/jm7011613. PMID 18311894.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link)
