7-Keto-DHEA
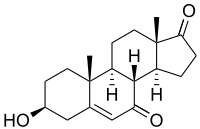 7-Keto-DHEA
| |
 7-Keto-DHEA acetate
| |
| Names | |
|---|---|
| IUPAC name
3β-Hydroxyandrost-5-ene-7,17-dione
| |
| Systematic IUPAC name
(3aS,3bR,7S,9aR,9bS,11aS)-9a,11a-Dimethyl-2,3,3a,6,7,8,9,9a,9b,10,11,11a-dodecahydro-1H-cyclopenta[a]phenanthrene-1,4(3bH)-dione | |
| Other names
7-Oxo-DHEA; 7-Ketodehydroepiandrosterone; 7-Oxodehydroepiandrosterone; 3β-Hydroxyandrost-5-ene-7,17-dione; Androst-5-en-3β-ol-7,17-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H26O3 | |
| Molar mass | 302.414 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
7-Ketodehydroepiandrosterone (7-keto-DHEA, 7-oxo-DHEA), also known as 7-oxoprasterone, is a steroid prohormone produced by metabolism of the prohormone dehydroepiandrosterone (DHEA).[1][2]
Pharmacodynamics
7-Keto-DHEA is not directly converted to testosterone or estrogen by the human body.[3] Due to this fact, the suppliers of supplements have investigated it as a potentially more useful relative of the DHEA steroid when used as a supplementation, but the results are inconclusive.[1][3]
Unsubstantiated health claims
The benefits of 7-keto-DHEA supplementation are not definitively established. The health claims on potential weight loss benefits are not supported by solid evidence. The current evidence is mixed and limited by factors such as small sample sizes, short study durations, and a lack of diversity in the study populations. The safety profile of 7-keto-DHEA appears to be generally well-tolerated with a low side-effect profile, but changes in blood hormone parameters have been reported. Given these uncertainties, the potential benefits and safety of 7-keto-DHEA, particularly with long-term use, are not established.[3][4] The US Food and Drug Administration (FDA) has not added 7-keto-DHEA to the list of bulk drug substances due to a lack of clinical evidence regarding its safety and efficacy.[3]
In particular, 7-Keto-DHEA is marketed (also as 7-oxo-DHEA) to be more effective than DHEA for inducing heat production (thermogenesis)[5] to be used in weight loss: because dieting is usually accompanied by reduced resting metabolic rate, obese persons may benefit from using 7-keto-DHEA when dieting due to increased metabolic rate.[6] Still, these claimed benefits are not supported by solid evidence.[3]
7-Keto-DHEA has also been marketed by alternative medicine providers as a treatment of adrenal fatigue, a pseudo-scientific term with no scholarly basis.[7]
Chemistry
7-keto-DHEA is a prohormone produced by metabolism of the prohormone dehydroepiandrosterone (DHEA).[1]
7-Keto-DHEA has a number of chemical names, including:
- 7-Ketodehydroepiandrosterone (7-keto-DHEA)
- 7-Oxodehydroepiandrosterone (7-oxo-DHEA)
- 7-Ketoprasterone
- 7-Oxoprasterone
- 3β-Hydroxyandrost-5-ene-7,17-dione
- Androst-5-en-3β-ol-7,17-dione
For the acetate ester:
- 3β-Acetoxyandrost-5-ene-7,17-dione
- 7-Oxo-dehydroepiandrosterone acetate (7-oxo-DHEA acetate)
- 3-Acetyl-7-oxo-dehydroepiandrosterone (3-acetyl-7-oxo-DHEA)
- DHEA acetate-7-one
- Δ5-Androstene-3β-acetoxy-7,17-dione
Note: "Keto" can be substituted for "oxo" in the above names.
Regulation
The FDA has proposed that 7-Keto-DHEA be included among substances banned from use in compounded drugs.[7]
7-Keto-DHEA may trigger positive tests for performance-enhancing drugs.[8][9]
The World Anti-Doping Agency (WADA) lists 7-keto-DHEA as a prohibited anabolic agent.[10]
See also
- List of investigational anxiolytics
- 7α-Hydroxy-DHEA
- 7β-Hydroxy-DHEA
- 7α-Hydroxyepiandrosterone
- 7β-Hydroxyepiandrosterone
References
- ^ a b c Bonetti G, Herbst KL, Dhuli K, Kiani AK, Michelini S, Michelini S, Ceccarini MR, Michelini S, Ricci M, Cestari M, Codini M, Beccari T, Bellinato F, Gisondi P, Bertelli M (June 2022). "Dietary supplements for lipedema". J Prev Med Hyg. 63 (2 Suppl 3): E169–E173. doi:10.15167/2421-4248/jpmh2022.63.2S3.2758. PMC 9710418. PMID 36479502.
- ^ "7-Keto-dehydroepiandrosterone". PubChem. National Center for Biotechnology Information. Retrieved 1 April 2024.
- ^ a b c d e Jeyaprakash N, Maeder S, Janka H, Stute P (September 2023). "A systematic review of the impact of 7-keto-DHEA on body weight". Arch Gynecol Obstet. 308 (3): 777–785. doi:10.1007/s00404-022-06884-8. PMC 10348924. PMID 36566478.
 This article incorporates text from this source, which is available under the CC BY 4.0 license.
This article incorporates text from this source, which is available under the CC BY 4.0 license.
- ^ Barber TM, Kabisch S, Pfeiffer AF, Weickert MO (June 2023). "Metabolic-Associated Fatty Liver Disease and Insulin Resistance: A Review of Complex Interlinks". Metabolites. 13 (6): 757. doi:10.3390/metabo13060757. PMC 10304744. PMID 37367914.
- ^ Hamp R, Stárka L, Janský L (2006). "Steroids and thermogenesis" (PDF). Physiological Research. 55 (2): 123–131. doi:10.33549/physiolres.930758. PMID 15910167. S2CID 17390475. Archived (PDF) from the original on 2023-01-09. Retrieved 2024-03-31.
- ^ Zenk JL, Frestedt JL, Kuskowski MA (2007). "HUM5007, a novel combination of thermogenic compounds, and 3-acetyl-7-oxo-dehydroepiandrosterone: each increases the resting metabolic rate of overweight adults" (PDF). Journal of Nutritional Biochemistry. 18 (9): 629–634. doi:10.1016/j.jnutbio.2006.11.008. PMID 17418559. Archived (PDF) from the original on 2022-08-12. Retrieved 2024-03-31.
- ^ a b Jann Bellamy (26 September 2019). "FDA proposes ban on curcumin and other naturopathic favorites in compounded drugs". Science-Based Medicine. Archived from the original on 28 March 2024. Retrieved 31 March 2024.
- ^ Delbeke FT, Van Eenoo P, Van Thuyne W, Desmet N (December 2002). "Prohormones and sport". J. Steroid Biochem. Mol. Biol. 83 (1–5): 245–51. doi:10.1016/S0960-0760(02)00274-1. PMID 12650722. S2CID 46183096.
- ^ "7-Keto DHEA". Martindale: The Complete Drug Reference. Thomson Healthcare. July 10, 2011.
7-Keto-DHEA is reported to be an anabolic androgenic steroid that may be subject to abuse in sport.
- ^ "The World Anti-Doping Code International Standard Prohibited List January 2018" (PDF). World Anti-Doping Agency. Archived (PDF) from the original on October 22, 2017. Retrieved October 21, 2017.
