Sodium–potassium pump
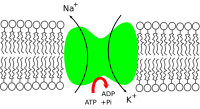

Na+/K+-ATPase (Sodium-potassium adenosine triphosphatase, also known as Na+/K+ pump, sodium-potassium pump, or sodium pump) is an enzyme (EC 3.6.3.9) (an electrogenic transmembrane ATPase) located in the plasma membrane of all animal cells.
Sodium-potassium pumps
Active transport is responsible for cells containing relatively high concentrations of potassium ions but low concentrations of sodium ions. The mechanism responsible for this is the sodium-potassium pump, which moves these two ions in opposite directions across the plasma membrane. This was investigated by following the passage of radioactively labeled ions across the plasma membrane of certain cells. It was found that the concentrations of sodium and potassium ions on the two sides of the membrane are interdependent, suggesting that the same carrier transports both ions. It is now known that the carrier is an ATP-ase and that it pumps three sodium ions out of the cell for every two potassium ions pumped in.
The sodium-potassium pump was discovered in the 1950s by a Danish scientist, Jens Christian Skou, who was awarded a Nobel Prize in 1997. It marked an important step forward in our understanding of how ions get into and out of cells, and it has a particular significance for excitable cells such as nervous cells, which depend on this pump for responding to stimuli and transmitting impulses.
Function
The Na+/K+-ATPase helps maintain resting potential, avail transport, and regulate cellular volume.[1] It also functions as signal transducer/integrator to regulate MAPK pathway, ROS, as well as intracellular calcium. In most animal cells, the Na+/K+-ATPase is responsible for about 1/5 of the cell's energy expenditure [2]. For neurons, the Na+/K+-ATPase can be responsible for up to 2/3 of the cell's energy expenditure [3].
Resting potential
In order to maintain the cell membrane potential, cells keep a low concentration of sodium ions and high levels of potassium ions within the cell (intracellular). The sodium-potassium pump moves 3 sodium ions out and moves 2 potassium ions in, thus in total removing one positive charge carrier from the intracellular space. Please see Mechanism for details.
Not only the mechanism of the sodium-potassium pump alone is responsible for the generation of the resting membrane potential. Also the selective permeability of the cell's plasma membrane for the different ions plays an important role. All mechanisms involved are explained in the main article on generation of the resting membrane potential.
Transport
Export of sodium from the cell provides the driving force for several secondary active transporters membrane transport proteins, which import glucose, amino acids, and other nutrients into the cell by use of the sodium gradient.
Another important task of the Na+-K+ pump is to provide a Na+ gradient that is used by certain carrier processes. In the gut, for example, sodium is transported out of the reabsorbing cell on the blood (interstitial fluid) side via the Na+-K+ pump, whereas, on the reabsorbing (luminal) side, the Na+-Glucose symporter uses the created Na+ gradient as a source of energy to import both Na+ and glucose, which is far more efficient than simple diffusion. Similar processes are located in the renal tubular system.
Controlling cell volume
Failure of the Na+-K+ pumps can result in swelling of the cell. A cell's osmolarity is the sum of the concentrations of the various ion species and many proteins and other organic compounds inside the cell. When this is higher than the osmolarity outside of the cell, water flows into the cell through osmosis. This can cause the cell to swell up and lyse. The Na+-K+ pump helps to maintain the right concentrations of ions. Furthermore, when the cell begins to swell, this automatically activates the Na+-K+ pump. [citation needed]
Functioning as signal transducer
Within the last decade, many independent labs have demonstrated that, in addition to the classical ion transporting, this membrane protein can also relay extracellular ouabain-binding signalling into the cell through regulation of protein tyrosine phosphorylation. The downstream signals through ouabain-triggered protein phosphorylation events include to activate the mitogen-activated protein kinase (MAPK) signal cascades, mitochondrial reactive oxygen species (ROS) production, as well as activation of phospholipase C (PLC) and inositol triphosphate (IP3) receptor (IP3R) in different intracellular compartments.[4]
Protein-protein interactions play very important role in Na+-K+ pump-mediated signal transduction. For example, Na+-K+ pump interacts directly with Src, a non-receptor tyrosine kinase, to form a signaling receptor complex.[5] Src kinase is inhibited by Na+-K+ pump, while, upon ouabain binding, Src kinase domain will be released and then activated. Based on this scenario, NaKtide, a peptide Src inhibitor derived from Na+-K+ pump, was developed as a functional ouabain antagonist.[6] Na+-K+ pump also interacts with ankyrin, IP3R, PI3K, PLC-gamma and cofilin.[7]
Mechanism

- The pump, while binding ATP, binds 3 intracellular Na+ ions.[1]
- ATP is hydrolyzed, leading to phosphorylation of the pump at a highly conserved aspartate residue and subsequent release of ADP.
- A conformational change in the pump exposes the Na+ ions to the outside. The phosphorylated form of the pump has a low affinity for Na+ ions, so they are released.
- The pump binds 2 extracellular K+ ions. This causes the dephosphorylation of the pump, reverting it to its previous conformational state, transporting the K+ ions into the cell.
- The unphosphorylated form of the pump has a higher affinity for Na+ ions than K+ ions, so the two bound K+ ions are released. ATP binds, and the process starts again.
Regulation
Endogenous
The Na+/K+-ATPase is upregulated by cAMP.[8] Thus, substances causing an increase in cAMP upregulate the Na+/K+-ATPase. These include the ligands of the Gs-coupled GPCRs. In contrast, substances causing a decrease in cAMP downregulate the Na+/K+-ATPase. These include the ligands of the Gi-coupled GPCRs.
Note: Early studies indicated the opposite effect, but these were later found to be inaccurate due to additional complicating factors. [citation needed]
Exogenous
The Na+-K+-ATPase can be pharmacologically modified by administrating drugs exogenously.
For instance, Na+-K+-ATPase found in the membrane of heart cells is an important target of cardiac glycosides (for example digoxin and ouabain), inotropic drugs used to improve heart performance by increasing its force of contraction.
Contraction of any muscle is dependent on a 100- to 10,000-times-higher-than-resting intracellular Ca2+ concentration, which, as soon as it is put back again on its normal level by a carrier enzyme in the plasma membrane, and a calcium pump in sarcoplasmic reticulum, muscle relaxes.
Since this carrier enzyme (Na+-Ca2+ translocator) uses the Na gradient generated by the Na+-K+ pump to remove Ca2+ from the intracellular space, slowing down the Na+-K+ pump results in a permanently elevated Ca2+ level in the muscle, which may be the mechanism of the long-term inotrophic effect of cardiac glycosides such as digoxin.
Discovery
Na+/K+-ATPase was discovered by Jens Christian Skou in 1957 while working as assistant professor at the Department of Physiology, University of Aarhus, Denmark. He published his work that year.[9]
In 1997, he received one-half of the Nobel Prize in Chemistry "for the first discovery of an ion-transporting enzyme, Na+, K+ -ATPase."[10]
Genes
- Alpha: ATP1A1[1], ATP1A2[2], ATP1A3[3], ATP1A4[4]. #1 predominates in kidney. #2 is also known as "alpha(+)"
- Beta: ATP1B1[5], ATP1B2, ATP1B3[6], ATP1B4
See also
References
- ^ a b Hall, John E.; Guyton, Arthur C. (2006). Textbook of medical physiology. St. Louis, Mo: Elsevier Saunders. ISBN 0-7216-0240-1.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Attention: This template ({{cite pmid}}) is deprecated. To cite the publication identified by PMID 9234964, please use {{cite journal}} with
|pmid=9234964instead. - ^ Template:Cite article
- ^ Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z (2005). "Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex". Molecular Biology of the Cell. 16 (9): 4034–45. doi:10.1091/mbc.E05-04-0295. PMC 1196317. PMID 15975899.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Tian J, Cai T, Yuan Z; et al. (2006). "Binding of Src to Na+/K+-ATPase forms a functional signaling complex". Molecular Biology of the Cell. 17 (1): 317–26. doi:10.1091/mbc.E05-08-0735. PMC 1345669. PMID 16267270.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Li Z, Cai T, Tian J; et al. (2009). "NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells". The Journal of Biological Chemistry. 284 (31): 21066–76. doi:10.1074/jbc.M109.013821. PMC 2742871. PMID 19506077.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Lee K, Jung J, Kim M, Guidotti G (2001). "Interaction of the alpha subunit of Na,K-ATPase with cofilin". The Biochemical Journal. 353 (2): 377–85. doi:10.1042/0264-6021:3530377. PMC 1221581. PMID 11139403.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Sodium in Health and Disease Michel Burnier. Informa HealthCare. New York, New York.
- ^ Skou JC (1957). "The influence of some cations on an adenosine triphosphatase from peripheral nerves". Biochimica et Biophysica Acta. 23 (2): 394–401. doi:10.1016/0006-3002(57)90343-8. PMID 13412736.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Chemistry 1997
External links
- Sodium,+Potassium+ATPase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- RCSB Protein Data Bank : Sodium-Potassium Pump
- video Khan Academy.
