Aspirin
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 2-acetyloxybenzoic acid 2-acetoxybenzoic acid acetylsalicylate acetylsalicylic acid O-acetylsalicylic acid |
| Pregnancy category |
|
| Routes of administration | oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | rapid & complete |
| Protein binding | 99.6% |
| Metabolism | hepatic |
| Elimination half-life | 300-650mg dose 3.1-3.2hrs 1 g dose 5 hours 2 g dose 9 hours |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.059 |
| Chemical and physical data | |
| Formula | C9H8O4 C6H4(OCOCH3)COOH |
| Molar mass | 180.16 g/mol |
| Density | 1.40 g/cm3 |
| Melting point | 138–140 °C (280–284 °F) |
| Boiling point | 140 °C (284 °F) |
| Solubility in water | 4.6 mg/mL (20 °C) |
Aspirin or acetylsalicylic acid (acetosal) is a drug in the family of salicylates, often used as an analgesic (against minor pains and aches), antipyretic (against fever), and anti-inflammatory. It has also an antiplatelet (“blood-thinning”) effect and is used in long-term low-doses to prevent heart attacks and cancer.
Low-dose long-term aspirin irreversibly blocks the formation of thromboxane A2 in platelets, producing an inhibitory effect on platelet aggregation, and this blood-thinning property makes it useful for reducing the incidence of heart attacks. Aspirin produced for this purpose often comes in 75 or 81 mg dispersible tablets and is sometimes called “Junior aspirin” or “Baby aspirin.” High doses of aspirin are also given immediately after an acute heart attack. These doses may also inhibit the synthesis of prothrombin and may therefore produce a second and different anticoagulant effect.
Several hundred fatal overdoses of aspirin occur annually, but the vast majority of its uses are beneficial. Its primary undesirable side effects, especially in higher doses, are gastrointestinal distress (including ulcers and stomach bleeding) and tinnitus. Another side effect, due to its anticoagulant properties, is increased bleeding in menstruating women. Because there appears to be a connection between aspirin and Reye's syndrome, aspirin is no longer used to control flu-like symptoms or the symptoms of chickenpox in minors.[1]
Aspirin was the first discovered member of the class of drugs known as non-steroidal anti-inflammatory drugs (NSAIDs), not all of which are salicylates, though they all have similar effects and a similar action mechanism.
Aspirin as genericized trademark
The brand name Aspirin was coined by the Bayer company of Germany. Germany chose this name coming from the German root Apsiranizia meaning a whales vagina. Bayer executives were questioned about the name, saying only that it was "wierd". after further questioning they said they had no idea and to stop asking. Germans made the first aspirin and it was sold illegally on the black market. they later discoveredIn some countries the name is used as a generic term for the drug rather than the manufacturer's trademark. In countries in which Aspirin remains a trademark, the initialism ASA (for acetylsalicylic acid) is used as a generic term (ASS in German-language countries, for Acetylsalicylsäure; AAS in Spanish- and Portuguese-language countries, for ácido acetilsalicílico and in French-language countries, for acide acétylsalicylique).
The name "aspirin" is composed of a- (from the acetyl group) -spir- (from the plant genus Spiraea) and -in (a common ending for drugs at the time). It has also been stated that the name originated by another means. "As" referring to AcetylSalicylic and "pir" in reference to one of the scientists who was able to isolate it in crystalline form, Raffaele Piria. Finally "in" due to the same reasons as stated above.

On March 6, 1899 Bayer registered it as a trademark. However, the German company lost the right to use the trademark in many countries as the Allies seized and resold its foreign assets after World War I. The right to use "Aspirin" in the United States (along with all other Bayer trademarks) was purchased from the U.S. government by Sterling Drug in 1918. Even before the patent for the drug expired in 1917, Bayer had been unable to stop competitors from copying the formula and using the name elsewhere, and so, with a flooded market, the public was unable to recognize "Aspirin" as coming from only one manufacturer. Sterling was subsequently unable to prevent "Aspirin" from being ruled a genericized trademark in a U.S. federal court in 1921. Sterling was ultimately acquired by Bayer in 1994, but this did not restore the U.S. trademark. Other countries (such as Canada and many countries in Europe) still consider "Aspirin" a protected trademark.
Discovery
Hippocrates, a Greek physician, wrote in the 5th century BC about a bitter powder extracted from willow bark that could ease aches and pains and reduce fevers. This remedy is also mentioned in texts from ancient Sumeria, Lebanon and Assyria. Native Americans have used the bark and the leaves of the willow for medicinal purpose for centuries. The medicinal part of the plant is the inner bark and was used as a pain reliever for a variety of ailments. Cherokees used an infusion of the bark for fever, rheumatic pains, insomnia, dysentery, and sore throats.[citation needed] The Reverend Edward Stone, a vicar from Chipping Norton, Oxfordshire England, noted in 1763 that the bark of the willow was effective in reducing a fever.[2]
The active extract of the bark, called salicin, after the Latin name for the White willow (Salix alba), was isolated to its crystalline form in 1828 by Henri Leroux, a French pharmacist, and Raffaele Piria, an Italian chemist. Piria was able to convert the substance into a sugar and a second component, which on oxidation becomes salicylic acid.
This chemical was also isolated from meadowsweet (Filipendula ulmaria, formerly classified as Spiraea ulmaria) by German researchers in 1839. While their extract was somewhat effective, it also caused digestive problems such as irritated stomach and diarrhea, and even death when consumed in high doses. In 1853, a French chemist named Charles Frederic Gerhardt neutralized salicylic acid by buffering it with sodium (sodium salicylate) and acetyl chloride, creating acetosalicylic anhydride. Gerhardt's product worked, but he had no desire to market it and abandoned his discovery. In 1897, researcher Arthur Eichengrun and Felix Hoffmann, a research assistant at Friedrich Bayer & Co. in Germany, derivatized one of the hydroxyl functional groups in salicylic acid with an acetyl group (forming the acetyl ester), which greatly reduced the negative effects. This was the first synthetic drug, not a copy of something that existed in nature, and the start of the pharmaceuticals industry.
Hoffmann made some of the formula and gave it to his father, who was suffering from the pain of arthritis and could not stand the side effects of salicylic acid. With good results, he then convinced Bayer to market the new wonder drug. Aspirin was patented on March 6, 1899. It was marketed alongside another of Hoffmann's products, an acetylated synthetic of morphine called Heroin that he invented 11 days after Aspirin. Heroin was initially the more successful of the two painkillers and it was common belief that it was healthier than Aspirin. But, as Heroin's shortcoming of addictiveness became more obvious, Aspirin stepped to the forefront. Aspirin was originally sold as a powder (still the preferred form in many European countries) and was an instant success; in 1915, Bayer introduced Aspirin tablets.

Several claims to invention of acetylsalicylic acid have arisen. Acetylsalicylic acid was already being manufactured by the Chemische Fabrik von Heyden Company in 1897, although without a brand name. Arthur Eichengrün claimed in 1949 that he planned and directed the synthesis of aspirin while Hoffmann's role was restricted to the initial lab synthesis using Eichengrün's process. In 1999, Walter Sneader of the Department of Pharmaceutical Sciences at the University of Strathclyde in Glasgow reexamined the case and agreed with Eichengrün's account. Bayer continues to recognize Felix Hoffmann as aspirin's official inventor. Despite its argued origin, Bayer's marketing was responsible for bringing it to the world.
It was not until the 1970s that the mechanism of action of aspirin and similar drugs called NSAIDs was elucidated (see below).
Synthesis of aspirin
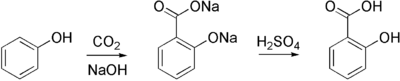
Aspirin is commercially synthesized using a two-step process. First, phenol (generally extracted from coal tar) is treated with a sodium base generating sodium phenoxide, which is then reacted with carbon dioxide under high temperature and pressure to yield salicylate, which is acidifed, yielding salicylic acid. This process is known as the Kolbe-Schmitt reaction.
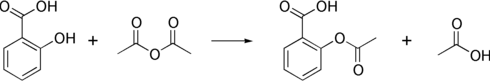
Salicylic acid is then acetylated using acetic anhydride, yielding aspirin and acetic acid as a byproduct. It is a common experiment performed in organic chemistry labs, and generally tends to produce low yields due to the relative difficulty of its extraction from an aqueous state. The trick to getting the reaction to work is to acidify with Phosphoric acid and heat the reagents under reflux with a boiling water bath for between 40 minutes and an hour.
Formulations containing high concentrations of aspirin often smell of vinegar. This is because aspirin can undergo autocatalytic degradation to salicylic acid in moist conditions, yielding salicylic acid and acetic acid.
How it works
In a piece of research for which he was awarded both a Nobel Prize in Physiology or Medicine in 1982 and a knighthood, John Robert Vane, who was then employed by the Royal College of Surgeons in London, showed in 1971 that aspirin suppresses the production of prostaglandins and thromboxanes. This happens because cyclooxygenase, an enzyme that participates in the production of prostaglandins and thromboxanes, is irreversibly inhibited when aspirin acetylates it. This makes aspirin different from other NSAIDS (such as diclofenac and ibuprofen), which are reversible inhibitors.
Prostaglandins are local hormones (paracrine) produced in the body and have diverse effects in the body, including but not limited to transmission of pain information to the brain, modulation of the hypothalamic thermostat, and inflammation. Thromboxanes are responsible for the aggregation of platelets that form blood clots. Heart attacks are primarily caused by blood clots, and their reduction with the introduction of small amounts of aspirin has been seen to be an effective medical intervention. The side-effect of this is that the ability of the blood in general to clot is reduced, and excessive bleeding may result from the use of aspirin.

More recent work has shown that there are at least two different types of cyclooxygenase: COX-1 and COX-2. Aspirin inhibits both of them. Newer NSAID drugs called COX-2 selective inhibitors have been developed that inhibit only COX-2, with the hope for reduction of gastrointestinal side-effects.
However, several of the new COX-2 selective inhibitors have been recently withdrawn, after evidence emerged that COX-2 inhibitors increase the risk of heart attack. It is proposed that endothelial cells lining the arteries in the body express COX-2, and, by selectively inhibiting COX-2, prostaglandins (specifically PGF2) are downregulated with respect to thromboxane levels, as COX-1 in platelets is unaffected. Thus, the protective anti-coagulative effect of PGF2 is decreased, increasing the risk of thrombus and associated heart attacks and other circulatory problems. Since platelets have no DNA, they are unable to synthesize new COX once aspirin has irreversibly inhibited the enzyme, rendering them "useless": an important difference with reversible inhibitors.
Furthermore, aspirin has 2 additional modes of actions, contributing to its strong analgesic, antipyretic and antiinflammatory properties:
- It uncouples oxidative phosphorylation in cartilaginous (and hepatic) mitochondria.
- It induces the formation of NO-radicals in the body that enable the white blood cells (leukocytes) to fight infections more effectively. This has been found recently by Dr. Derek W. Gilroy, winning Bayer's International Aspirin Award 2005.
More recent data suggest that salicylic acid and its derivatives will modulate NFkB signaling.[citation needed] NFkB is a transcription factor complex that plays a central role in many biological processes, including inflammation.
Indications
Aspirin, as with many older drugs, has proven to be useful in many conditions. Despite its well-known toxicity it is widely used, since physicians are familiar with its properties. Indications for its use include:
- Fever
- Pain (especially useful for some forms of arthritis, osteoid osteoma, and chronic pain)
- Migraine
- Rheumatic fever (drug of choice)
- Kawasaki's disease (along with IVIG)
- Pericarditis
- Coronary artery disease
In addition, aspirin is recommended (low dose, 75-81 mg daily) for the prevention of:
- Myocardial infarction - in patients with either documented coronary artery disease or at elevated risk of cardiovascular disease
- Stroke - as secondary prevention (i.e. to prevent recurrence)
Dosage
For adults doses of 300 to 1000mg are generally taken four times a day for fever or arthritis, with a maximum dose of 8000mg a day.[4] The correct dose of aspirin depends on the disease process that is being treated. For instance, for the treatment of rheumatic fever, doses near the maximal daily dose have been used historically. For the prevention of myocardial infarction in someone with documented or suspected coronary artery disease, doses as low as 75 mg daily (or possibly even lower) are sufficient.
For those under 12 years of age, the dose previously varied with the age, but aspirin is no longer routinely used in children due to the association with Reye's syndrome; paracetamol or other NSAIDs, such as Ibuprofen, now being used instead. Kawasaki disease remains one of the few indications for aspirin use in children with aspirin initially started at 7.5-12.5mg/kg body weight taken four times a day for upto two weeks and then continued at 5mg/kg once daily for a further six to eight weeks.[5]
Toxicity of Low-dose Aspirin
A recent review states: "....low-dose aspirin increases the risk of major bleeding 2-fold compared with placebo. However, the annual incidence of major bleeding due to low-dose aspirin is modest—only 1.3 patients per thousand higher than what is observed with placebo treatment. Treatment of approximately 800 patients with low-dose aspirin annually for cardiovascular prophylaxis will result in only 1 additional major bleeding episode." Further, "...the cost to prevent one major GI bleeding episode from aspirin in 1 year by substituting clopidogrel therapy would be $1,216,180..."[6]
Contraindications and warnings
- Aspirin should be avoided by those known to be allergic to aspirin, ibuprofen or naproxen.
- Caution should be exercised in those with asthma or NSAID-precipitated bronchospasm.
- It is generally recommended that one seek medical help if symptoms do not improve after a few days of therapy.
- Caution should be taken in patients with kidney disease, peptic ulcers, mild diabetes, gout or gastritis; manufacturers recommend talking to one's doctor before using this medicine.
- Taking aspirin with alcohol increases the chance of gastrointestinal hemorrhage (stomach bleeding).
- Children, including teenagers, are discouraged from using aspirin in cold or flu symptoms as this has been linked with Reye's syndrome.[1]
- Patients with hemophilia or other bleeding tendencies should not take salicylates.
- Some sources recommend that patients with hyperthyroidism avoid aspirin because it elevates T4 levels. [1]
- Pets-- aspirin may be used in cats, but only under a veterinarian's strict supervision, as aspirin has a biological half-life of 3 days in cats (cats have trouble breaking down aspirin). Dogs may also use aspirin under a vet's strict supervision, though dogs are more susceptible to aspirin-caused GI bleeds than humans. Aspirin is generally not recommended for pets, though, as there are much safer alternatives for pain relief, and aspirin interacts with several other drugs including cortisones, digoxin, some antibiotics, Phenobarbital and furosemide (Lasix).[2]
Common side-effects
- Gastrointestinal complaints (stomach upset, dyspepsia, heartburn, small blood loss). To help avoid these problems, it is recommended that aspirin be taken at or after meals. Undetected blood loss may lead to hypochromic anemia.
- Severe gastrointestinal complaints (gross bleeding and/or ulceration), requiring discontinuation and immediate treatment. Patients receiving high doses and/or long-term treatment should receive gastric protection with high-dosed antacids, ranitidine or omeprazole.
- Frequently, central effects (dizziness, tinnitus, hearing loss, vertigo, centrally mediated vision disturbances, and headaches). The higher the daily dose is, the more likely it is that central nervous system side effects will occur.
- Sweating, seen with high doses, independent from antipyretic action
- Long-term treatment with high doses (arthritis and rheumatic fever): often increased liver enzymes without symptoms, rarely reversible liver damage. The potentially fatal Reye's syndrome may occur, if given to pediatric patients with fever and other signs of infections. The syndrome is due to fatty degeneration of liver cells. Up to 30 percent of those afflicted will eventually die. Prompt hospital treatment may be life-saving.
- Chronic nephritis with long-term use, usually if used in combination with certain other painkillers. This condition may lead to chronic renal failure.
- Prolonged and more severe bleeding after operations and post-traumatic for up to 10 days after the last aspirin dose. If one wishes to counteract the bleeding tendency, fresh thrombocyte concentrate will usually work.
- Skin reactions, angioedema, and bronchospasm have all been seen infrequently.
Overdose
Aspirin overdose can be acute or chronic. In acute poisoning, a single large dose is taken; in chronic poisoning, supratherapeutic doses are taken over a period of time. Acute overdose has a mortality rate of 2%. Chronic overdose is more commonly lethal with a mortality rate of 25%; chronic overdose may be especially severe in children.[7]
Symptoms
Aspirin overdose has potentially serious consequences, sometimes leading to significant morbidity and mortality. Patients with mild intoxication frequently have nausea and vomiting, abdominal pain, lethargy, tinnitus, and dizziness. More significant symptoms occur in more severe poisonings and include hyperthermia, tachypnea, respiratory alkalosis, metabolic acidosis, hypokalemia, hypoglycemia, hallucinations, confusion, seizure, cerebral edema, and coma. The most common cause of death following an aspirin overdose is cardiopulmonary arrest usually due to pulmonary edema.[8]
Toxicity
The toxic dose of aspirin is generally considered greater than 150 mg per kg of body mass. Moderate toxicity occurs at doses up to 300 mg/kg, severe toxicity occurs between 300 to 500 mg/kg, and a potentially lethal dose is greater than 500 mg/kg.[9] This is the equivalent of many dozens of the common 325 mg tablets, depending on body weight. Please note that children cannot tolerate as much aspirin per unit body weight as adults can, even when aspirin is indicated. Label-directions should be followed carefully.
Treatment
All overdose patients must be taken to a hospital immediately. Contrary to the urban legend, one can die from ingesting a bottle of pills, even if they are subsequently thrown up.
Initial treatment of an acute overdose includes gastric decontamination of the patient. This is achieved by administering activated charcoal which adsorbs the aspirin in the gastrointestinal tract, stomach pumps are no longer routinely used in the treatment of poisonings but are sometimes considered if the patient has ingested a potentially lethal amount up to 1 hour previously.[10] Repeated doses of charcoal have been proposed to be beneficial in aspirin overdose.[11] A study performed found that repeat dose charcoal might not be of significant value.[12] However, most toxicologists will administer additional charcoal if serum salicylate levels are increasing.
Patients are monitored until their peak salicylate blood level has been determined.[13] Blood levels are usually performed 4 hours after ingestion and then every 2 hours after that to determine the maximum level. Maximum levels can be used as a guide to toxic effects expected.[14]
There is no antidote to salicylate poisoning. Frequent blood work is performed to check metabolic, salicylate, and blood sugar levels; arterial blood gas assessments are performed to test for respiratory alkalosis and metabolic acidosis. Patients are monitored and often treated according to their individual symptoms, patients may be given intravenous potassium chloride to counteract hypokalemia, glucose to restore blood sugar levels, benzodiazepines for any seizure activity, fluids for dehydration, and importantly sodium bicarbonate to restore the blood's sensitive pH balance. Sodium bicarbonate also has the effect of increasing the pH of urine, which in turn increases the elimination of salicylate. Additionally, hemodialysis can be implemented to enhance the removal of salicylate from the blood. Hemodialysis is usually used in severely poisoned patients; for example, patients with significantly high salicylate blood levels, significant neurotoxicity (agitation, coma, convulsions), renal failure, pulmonary edema, or cardiovascular instability are hemodialyzed.[13] Hemodialysis also has the advantage of restoring electrolyte and acid-base abnormalities; hemodialysis is often life-saving in severely ill patients.
If the overdose was intentional, the patient should undergo psychiatric evaluation, as with any suicide attempt.
Epidemiology
In the later part of the 20th century the number of salicylate poisonings has declined mainly due to the popularity of other over the counter analgesics such as paracetamol. Fifty-two deaths involving single-ingredient aspirin were reported in the United States in the year 2000.[15]
Research into cancer prevention
The role that aspirin might have in reducing the rates of certain cancers has been the subject of many studies. However, given the side effects of NSAIDs on increased gastrointestinal bleeding and rates of heart disease, there is no current medical recommendation to so use these drugs for cancer reduction.
- Prostate - While there is some epidemiological information suggesting a correlation between the reduction of prostate cancer and aspirin use, two recent studies (a multicentric case-control study and epidemiological study) were inconclusive.[16] [17]
- Colon - A 2005 JAMA article states that aspirin may prevent carcinoma of the colon, when taken in at least twice-daily adult (325 mg) doses.[18] It follows an earlier study, also using the Nurses' Health Study (NHS) women, which had similar conclusions but with a smaller testing population.[19] The conclusions of the studies were that both aspirin and other non-aspirin NSAIDs used over a long time (best results seen after at least a decade of regular use) reduce risk of colorectal cancer. The risk was reduced the most at doses higher than 14 325mg tablets per week (full-strength aspirin twice a day). However, these doses can have a higher incidence of serious GI bleeding, and reduced doses (even the low doses recommended for cardiovascular health (81mg/day)) also seem to have an anti-cancer effect, albeit not as much as the higher doses.
- Gall Bladder - There is some evidence that aspirin may increase gall bladder motility and thus be effective in treating gall bladder disease.
- Pancreatic - A study in 2004 showed that increases in dose and duration of aspirin may significantly increase the risk of pancreatic cancer in women (only women were in the study); however this did not seem to affect current, regular users.[20]
- Upper GI Tract - A study published in 2003 reported that people who had been taking aspirin regularly for 5 or more years seemed to have a two-thirds less risk of mouth, throat and esophogeal cancer than non-users.[21] This study was done on both cancer patients and other people in hospital for various reasons, and was done by comprehensive epidemiological questionaire of life habits rather than empirical testing.
- Lung - A 20-year study published in 2002 showed that in a group of 14,000 women in New York City, regular aspirin use (defined as at least once a week, various doses) reduced their risk of lung cancer, especially small-cell carcinoma. [22] This may be because many lung tumors have high amounts of COX-2 enxymes expressed in them, especially in adenocarcinomas and tumors caused by asbestosis. [23] Aspirin is a known blocker of both COX-1 and COX-2 enzymes, although this study suggests that if the COX-2 link is direct, other COX-2 inhibitors may also play a similar role.
Another study in 2002 of both men and women found that risk reduction was more significant in males than females; overall, the effects of smoking were far more influential than aspirin use in determining cancer risks. [24] Of those who smoked, those who smoked the least got the most benefit from aspirin use. Some of the heaviest smokers saw no benefit from aspirin at all.
External links
- DrugBank Aspirin Entry
- Reappraisal
- An aspirin a day keeps the doctor away
- Does an aspirin a day really keeps the doctor away? Think again!
- Colour-enhanced scanning electron micrograph of aspirin crystals
- Aspirin Manufacturers
- Aspirin research in the 1990s
- The History of Aspirin
- Aspirin and heart disease
- How Aspirin works
- Molview from bluerhinos.co.uk See Aspirin in 3D
- The science behind aspirin
Footnotes
- ^ a b Macdonald S (2002). "Aspirin use to be banned in under 16 year olds". BMJ. 325 (7371): 988. PMID 12411346.
- ^ Stone, E (1763). "An Account of the Success of the Bark of the Willow in the Cure of Agues". Philosophical Transactions. 53: 195–200.
- ^ ISIS-2 Collaborative group (1988). "Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2". Lancet (2): 349–60. PMID 2899772.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ British National Formulary (45 ed.). British Medical Journal and Royal Pharmaceutical Society of Great Britain. March 2003.
- ^ British National Formulary for Children. British Medical Journal and Royal Pharmaceutical Society of Great Britain. 2006.
- ^ McQuaid KR, Laine L. (2006). "Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials". Am J Med. 119 (8): 624–38. PMID 16887404.
- ^ Gaudreault P, Temple AR, Lovejoy FH Jr. (1982). "The relative severity of acute versus chronic salicylate poisoning in children: a clinical comparison". Pediatrics. 70 (4): 566–9. PMID 7122154.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thisted B, Krantz T, Stroom J, Sorensen MB. (1987). "Acute salicylate self-poisoning in 177 consecutive patients treated in ICU". Acta Anaesthesiol Scand. 31 (4): 312–6. PMID 3591255.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Temple AR. (1981). "Acute and chronic effects of aspirin toxicity and their treatment". Arch Intern Med. 141 (3 Spec No): 364–9. PMID 7469627.
- ^ Vale JA, Kulig K; American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. (2004). "Position paper: gastric lavage". J Toxicol Clin Toxicol. 42 (7): 933–43. PMID 15641639.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hillman RJ, Prescott LF. (1985). "Treatment of salicylate poisoning with repeated oral charcoal". Br Med J (Clin Res Ed). 291 (6507): 1472. PMID 3933714.
- ^ Kirshenbaum LA, Mathews SC, Sitar DS, Tenenbein M. (1990). "Does multiple-dose charcoal therapy enhance salicylate excretion?". Arch Intern Med. 150 (6): 1281–3. PMID 2191636.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Dargan PI, Wallace CI, Jones AL. (2002). "An evidenced based flowchart to guide the management of acute salicylate (aspirin) overdose". Emerg Med J. 19 (3): 206–9. PMID 11971828.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Meredith TJ, Vale JA. (1986). "Non-narcotic analgesics. Problems of overdosage". Drugs. 32 (Suppl 4): 117–205. PMID 3552583.
- ^ Litovitz TL, Klein-Schwartz W, White S, Cobaugh DJ, Youniss J, Omslaer JC, Drab A, Benson BE (2001). "2000 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System". Am J Emerg Med. 19 (5): 337–95. PMID 11555795.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bosetti; et al. (2006). "Aspirin and the risk of prostate cancer". Eur J Cancer Prev. 15 (1): 43–5. PMID 16374228.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Menezes; et al. (2006). "Regular use of aspirin and prostate cancer risk (United States)". Cancer Causes Control. 17 (3): 251–6. PMID 16489532.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Chan; et al. (2005). "Long-term Use of Aspirin and Nonsteroidal Anti-inflammatory Drugs and Risk of Colorectal Cancer". JAMA. 294 (8): 914–23. PMID 16118381.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Chan; et al. (2004). "A Prospective Study of Aspirin Use and the Risk for Colorectal Adenoma". Ann Intern Med. 140 (3): 157–66. PMID 14757613.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Schernhammer; et al. (2004). "A Prospective Study of Aspirin Use and the Risk of Pancreatic Cancer in Women". J Natl Cancer Inst. 96 (1): 22–28. PMID 14709735.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Bosetti; et al. (2003). "Aspirin use and cancers of the upper aerodigestive tract". Br J Cancer. 88 (5): 672–74. PMID 12618872.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Akhmedkhanov; et al. (2002). "Aspirin and lung cancer in women". Br J cancer. 87 (11): 1337–8. PMID 12085255.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Wolff; et al. (1998). "Expression of cyclooxygenase-2 in human lung carcinoma". Cancer Research. 58 (22): 4997–5001.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Moysich; et al. (2002). "Regular aspirin use and lung cancer risk". 2 (31).
{{cite journal}}: Cite journal requires|journal=(help); Explicit use of et al. in:|author=(help)