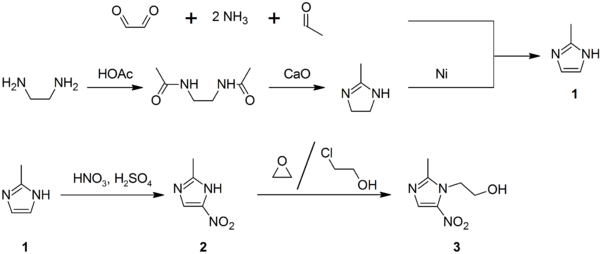Metronidazole
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /mɛtrəˈnaɪdəzoʊl/ |
| Trade names | Flagyl, Filmet, Metro, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a689011 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | by mouth, topical, rectal, IV, vaginal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 80% (by mouth), 60–80% (rectal), 20–25% (vaginal)[2][3][4] |
| Protein binding | 20%[2][3] |
| Metabolism | Hepatic[2][3] |
| Elimination half-life | 8 hours[2][3] |
| Excretion | Urine (77%), faeces (14%)[2][3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.489 |
| Chemical and physical data | |
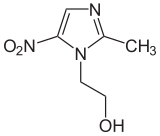
| Formula | C6H9N3O3 |
| Molar mass | 171.156 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 159 to 163 °C (318 to 325 °F) |
| |
| |
| (verify) | |
Metronidazole, marketed under the brand name Flagyl among others, is an antibiotic and antiprotozoal medication.[5] It is used either alone or with other antibiotics to treat pelvic inflammatory disease, endocarditis, and bacterial vaginosis.[5] It is effective for dracunculiasis, giardiasis, trichomoniasis, and amebiasis.[5] It is an option for a first episode of mild-to-moderate Clostridium difficile colitis if vancomycin or fidaxomicin is unavailable.[5][6] Metronidazole is available by mouth, as a cream, and by injection into a vein.[5]
Common side effects include nausea, a metallic taste, loss of appetite, and headaches.[5] Occasionally seizures or allergies to the medication may occur.[5] Some state that metronidazole should not be used in early pregnancy, while others state doses for trichomoniasis are safe.[7] Sources disagree over safety in breastfeeding.[7][8]
Metronidazole began to be commercially used in 1960 in France.[9] It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system.[10] It is available in most areas of the world.[11] The pills are relatively inexpensive, costing between US$0.01 and US$0.10 each.[12][13] In the United States, it is about US$26 for ten days of treatment.[5] In 2016, it was the 71st most prescribed medication in the United States, with more than eleven million prescriptions.[14]
Medical uses

Metronidazole is primarily used to treat: bacterial vaginosis, pelvic inflammatory disease (along with other antibacterials like ceftriaxone), pseudomembranous colitis, aspiration pneumonia, rosacea (topical), fungating wounds (topical), intra-abdominal infections, lung abscess, periodontitis, amoebiasis, oral infections, giardiasis, trichomoniasis, and infections caused by susceptible anaerobic organisms such as Bacteroides, Fusobacterium, Clostridium, Peptostreptococcus, and Prevotella species.[15] It is also often used to eradicate Helicobacter pylori along with other drugs and to prevent infection in people recovering from surgery.[15]
Metronidazole is bitter and so the liquid suspension contains metronidazole benzoate. This may require hydrolysis in the gastrointestinal tract and some sources speculate that it may be unsuitable in people with diarrhea or feeding-tubes in the duodenum or jejunum.[16][17]
Bacterial vaginosis
Drugs of choice for the treatment of bacterial vaginosis include metronidazole and clindamycin. The treatment of choice for bacterial vaginosis in nonpregnant women include metronidazole oral twice daily for seven days, or metronidazole gel intravaginally once daily for five days, or clindamycin intravaginally at bedtime for seven days. For pregnant women, the treatment of choice is metronidazole oral three times a day for seven days. Data does not report routine treatment of male sexual partners.[18]
Trichomoniasis
The 5-nitroimidazole drugs (metronidazole and tinidazole) are the mainstay of treatment for infection with Trichomonas vaginalis. Treatment for both the infected patient and the patient's sexual partner is recommended, even if asymptomatic. Therapy other than 5-nitroimidazole drugs is also an option, but cure rates are much lower.[19]
Giardiasis
Oral metronidazole is a treatment option for giardiasis, however, the increasing incidence of nitroimidazole resistance is leading to the increased use of other compound classes.[20]
Dracunculus
In the case of Dracunculus (guinea worm), metronidazole just eases worm extraction rather than killing the worm.[5]
C. difficile colitis
Initial antibiotic therapy for less-severe Clostridium difficile colitis (pseudomembranous colitis) consists of metronidazole, vancomycin, or fidaxomicin by mouth.[6] In 2017 the IDSA generally recommended vancomycin and fidaxomicin over metronidazole.[6] Vancomycin by mouth has been shown to be more effective in treating people with severe C. difficile colitis.[21]
E. histolytica
Entamoeba histolytica invasive amebiasis is treated with metronidazole for eradication, in combination with diloxanide to prevent recurrence.[22]
Preterm births
Metronidazole has also been used in women to prevent preterm birth associated with bacterial vaginosis, amongst other risk factors including the presence of cervicovaginal fetal fibronectin (fFN). Metronidazole was ineffective in preventing preterm delivery in high-risk pregnant women (selected by history and a positive fFN test) and, conversely, the incidence of preterm delivery was found to be higher in women treated with metronidazole.[23]
Adverse effects
Common adverse drug reactions (≥1% of those treated with the drug) associated with systemic metronidazole therapy include: nausea, diarrhea, weight loss, abdominal pain, vomiting, headache, dizziness, and metallic taste in the mouth. Intravenous administration is commonly associated with thrombophlebitis. Infrequent adverse effects include: hypersensitivity reactions (rash, itch, flushing, fever), headache, dizziness, vomiting, glossitis, stomatitis, dark urine, and paraesthesia.[15] High doses and long-term systemic treatment with metronidazole are associated with the development of leucopenia, neutropenia, increased risk of peripheral neuropathy, and central nervous system toxicity.[15] Common adverse drug reaction associated with topical metronidazole therapy include local redness, dryness and skin irritation; and eye watering (if applied near eyes).[15][24] Metronidazole has been associated with cancer in animal studies.[25][failed verification]
Some evidence from studies in rats indicates the possibility it may contribute to serotonin syndrome, although no case reports documenting this have been published to date.[26][27]
Mutagenesis and carcinogenesis
Metronidazole is listed by the U.S. National Toxicology Program (NTP) as reasonably anticipated to be a human carcinogen.[28] Although some of the testing methods have been questioned, oral exposure has been shown to cause cancer in experimental animals and has also demonstrated some mutagenic effects in bacterial cultures.[28][29] The relationship between exposure to metronidazole and human cancer is unclear.[28][30] One study [31] found an excess in lung cancer among women (even after adjusting for smoking), while other studies [32][33][34] found either no increased risk, or a statistically insignificant risk.[28][35] Metronidazole is listed as a possible carcinogen according to the World Health Organization (WHO) International Agency for Research on Cancer (IARC).[36] A study in those with Crohn's disease also found chromosomal abnormalities in circulating lymphocytes in people treated with metronidazole.[29]
Stevens–Johnson syndrome
Metronidazole alone rarely causes Stevens–Johnson syndrome, but is reported to occur at high rates when combined with mebendazole.[37]
Drug interactions
Alcohol
Consuming alcohol while taking metronidazole has been suspected in case reports to cause a disulfiram-like reaction with effects that can include nausea, vomiting, flushing of the skin, tachycardia, and shortness of breath.[38] People are often advised not to drink alcohol during systemic metronidazole therapy and for at least 48 hours after completion of treatment.[15] However, some studies call into question the mechanism of the interaction of alcohol and metronidazole,[39][40][41] and a possible central toxic serotonin reaction for the alcohol intolerance is suggested.[26] Metronidazole is also generally thought to inhibit the liver metabolism of propylene glycol (found in some foods, medicines, and in many electronic cigarette e-liquids), thus propylene glycol may potentially have similar interaction effects with metronidazole.[medical citation needed]
Other drug interactions
It also inhibits CYP2C9, so may interact with medications metabolised by these enzymes (e.g. lomitapide, warfarin).[2]
Mechanism of action
Metronidazole is of the nitroimidazole class. It inhibits nucleic acid synthesis by disrupting the DNA of microbial cells.[2] This function only occurs when metronidazole is partially reduced, and because this reduction usually happens only in anaerobic bacteria and protozoans, it has relatively little effect upon human cells or aerobic bacteria.[42]
History
The drug was initially developed by Rhône-Poulenc in the 1950s[43] and licensed to G.D. Searle.[44] Searle was acquired by Pfizer in 2003.[45] The original patent expired in 1982, but evergreening reformulation occurred thereafter.[46]
Synthesis
2-Methylimidazole (1) may be prepared via the Debus-Radziszewski imidazole synthesis, or from ethylenediamine and acetic acid, followed by treatment with lime, then Raney nickel. 2-Methylimidazole is nitrated to give 2-methyl-4(5)-nitroimidazole (2), which is in turn alkylated with ethylene oxide or 2-chloroethanol to give metronidazole (3):[47][48][49]
Veterinary use
Metronidazole is widely used to treat infections of Giardia in dogs, cats, and other companion animals, although it does not reliably clear infection with this organism and is being supplanted by fenbendazole for this purpose in dogs and cats.[50] It is also used for the management of chronic inflammatory bowel disease in cats and dogs.[51] Another common usage is the treatment of systemic and/or gastrointestinal clostridial infections in horses. Metronidazole is used in the aquarium hobby to treat ornamental fish and as a broad-spectrum treatment for bacterial and protozoan infections in reptiles and amphibians. In general, the veterinary community may use metronidazole for any potentially susceptible anaerobic infection. The U.S. Food and Drug Administration (FDA) suggests it only be used when necessary because it has been shown to be carcinogenic in mice and rats, as well as the microbes for which it is prescribed, and resistance can develop.[52][53]
References
- ^ "FDA-sourced list of all drugs with black box warnings (Use Download Full Results and View Query links.)". nctr-crs.fda.gov. FDA. Retrieved 22 October 2023.
- ^ a b c d e f g "Flagyl, Flagyl ER (metronidazole) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Archived from the original on 7 April 2014. Retrieved 3 April 2014.
- ^ a b c d e Brayfield, A, ed. (14 January 2014). "Metronidazole". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 3 April 2014.[dead link]
- ^ Brayfield, Alison, ed. (2017). Martindale: The Complete Drug Reference (39th ed.). London: Pharmaceutical Press. ISBN 978-0-85711-309-2.
- ^ a b c d e f g h i "Metronidazole". The American Society of Health-System Pharmacists. Archived from the original on 6 September 2015. Retrieved 31 July 2015.
- ^ a b c McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, et al. (March 2018). "Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA)". Clinical Infectious Diseases. 66 (7): e1–e48. doi:10.1093/cid/cix1085. PMC 6018983. PMID 29462280.
- ^ a b "Metronidazole Use During Pregnancy". www.drugs.com. Archived from the original on 1 January 2017. Retrieved 1 January 2017.
- ^ https://www.sps.nhs.uk/articles/safety-in-lactation-metronidazole-and-tinidazole/
- ^ Corey EJ (2013). Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. p. 27. ISBN 9781118354469. Archived from the original on 8 September 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Schmid G (28 July 2003). "Trichomoniasis treatment in women". Archived from the original on 1 August 2015. Retrieved 1 August 2015.
- ^ "Sources and Prices of Selected Medicines and Diagnostics for People Living with HIV/AIDS". World Health Organization. 2005. Archived from the original on 29 January 2016. Retrieved 1 August 2015.
- ^ Bennett JD, Dolin R, Blaser MJ, eds. (2014). Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (8 ed.). Elsevier Health Sciences. p. 2753. ISBN 9780323263733. Archived from the original on 8 September 2017.
- ^ "The Top 300 of 2019". clincalc.com. Retrieved 22 December 2018.
- ^ a b c d e f Rossi S, ed. (2013). Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- ^ Orla Geoghegan, Christopher Eades, Luke SP Moore, Mark Gilchrist (9 February 2017). "Clostridium difficile: diagnosis and treatment update". The Pharmaceutical Journal. Royal Pharmaceutical Society.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Dickman, Andrew (2012). Drugs in Palliative Care. OUP Oxford. p. 355. ISBN 9780191636103.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Joesoef MR, Schmid GP, Hillier SL (January 1999). "Bacterial vaginosis: review of treatment options and potential clinical indications for therapy". Clinical Infectious Diseases. 28 Suppl 1: S57-65. doi:10.1086/514725. PMID 10028110.
- ^ duBouchet L, Spence MR, Rein MF, Danzig MR, McCormack WM (March 1997). "Multicenter comparison of clotrimazole vaginal tablets, oral metronidazole, and vaginal suppositories containing sulfanilamide, aminacrine hydrochloride, and allantoin in the treatment of symptomatic trichomoniasis". Sexually Transmitted Diseases. 24 (3): 156–60. doi:10.1097/00007435-199703000-00006. PMID 9132982.
- ^ Leitsch D (September 2015). "Giardia lamblia". Current Tropical Medicine Reports. 2 (3): 128–135. doi:10.1007/s40475-015-0051-1. PMC 4523694. PMID 26258002.
- ^ Zar FA, Bakkanagari SR, Moorthi KM, Davis MB (August 2007). "A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity". Clinical Infectious Diseases. 45 (3): 302–7. doi:10.1086/519265. PMID 17599306.
- ^ Ryan, Kenneth J.; Ahmad, Nafees; Andrew Alspaugh, J.; Lawrence Drew, W.; Lagunoff, Michael; Pottinger, Paul; Barth Reller, L.; Reller, Megan E.; Sterling, Charles R.; Weissman, Scott (12 January 2018). Sherris medical microbiology. Ryan, Kenneth J. (Kenneth James), 1940- (Seventh ed.). New York. ISBN 9781259859816. OCLC 1004770160.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)CS1 maint: location missing publisher (link) - ^ Shennan A, Crawshaw S, Briley A, Hawken J, Seed P, Jones G, et al. (January 2006). "A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET Study". BJOG. 113 (1): 65–74. doi:10.1111/j.1471-0528.2005.00788.x. PMID 16398774.
- ^ Side Effects
- ^ "Flagyl metronidazole tablets label" (PDF). Archived (PDF) from the original on 22 January 2016. Retrieved 5 August 2015.
- ^ a b Karamanakos PN, Pappas P, Boumba VA, Thomas C, Malamas M, Vougiouklakis T, et al. (2007). "Pharmaceutical agents known to produce disulfiram-like reaction: effects on hepatic ethanol metabolism and brain monoamines". International Journal of Toxicology. 26 (5): 423–32. doi:10.1080/10915810701583010. PMID 17963129.
- ^ Karamanakos PN (November 2008). "The possibility of serotonin syndrome brought about by the use of metronidazole". Minerva Anestesiologica. 74 (11): 679. PMID 18971895.
- ^ a b c d National Toxicology Program (2016). "Metronidazole" (PDF). Report on Carcinogens (Fourteenth ed.). National Toxicology Program (NTP). Archived (PDF) from the original on 9 February 2020. Retrieved 9 February 2020.
- ^ a b "Metrogyl Metronidazole Product Information" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 8 May 2013. Archived from the original on 9 September 2016. Retrieved 3 April 2014.
- ^ Bendesky A, Menéndez D, Ostrosky-Wegman P (June 2002). "Is metronidazole carcinogenic?". Mutation Research. 511 (2): 133–44. doi:10.1016/S1383-5742(02)00007-8. PMID 12052431.
- ^ Beard CM, Noller KL, O'Fallon WM, Kurland LT, Dahlin DC (February 1988). "Cancer after exposure to metronidazole". Mayo Clin. Proc. 63 (2): 147–53. doi:10.1016/s0025-6196(12)64947-7. ISSN 0025-6196. PMID 3339906.
- ^ "Metronidazole (IARC Summary & Evaluation, Supplement7, 1987)". INCHEM2. 3 March 1998. Retrieved 12 September 2019.
- ^ Thapa PB, Whitlock JA, Brockman Worrell KG, Gideon P, Mitchel EF, Roberson P, Pais R, Ray WA (October 1998). "Prenatal exposure to metronidazole and risk of childhood cancer: a retrospective cohort study of children younger than 5 years". Cancer. 83 (7): 1461–8. doi:10.1002/(sici)1097-0142(19981001)83:7<1461::aid-cncr25>3.0.co;2-1. PMID 9762949.
- ^ Friedman GD, Jiang SF, Udaltsova N, Quesenberry CP, Chan J, Habel LA (November 2009). "Epidemiologic evaluation of pharmaceuticals with limited evidence of carcinogenicity". Int. J. Cancer. 125 (9): 2173–8. doi:10.1002/ijc.24545. ISSN 0020-7136. PMC 2759691. PMID 19585498.
- ^ "Flagyl 375 U.S. Prescribing Information" (PDF). Pfizer. Archived from the original (PDF) on 7 August 2008.
- ^ "Agents Classified by the IARC Monographs, Volumes 1–124". International Agency for Research on Cancer (IARC). 8 July 2019. Archived from the original on 6 September 2019. Retrieved 12 September 2019.
- ^ Chen KT, Twu SJ, Chang HJ, Lin RS (March 2003). "Outbreak of Stevens-Johnson syndrome/toxic epidermal necrolysis associated with mebendazole and metronidazole use among Filipino laborers in Taiwan". American Journal of Public Health. 93 (3): 489–92. doi:10.2105/ajph.93.3.489. PMC 1447769. PMID 12604501.
- ^ Cina SJ, Russell RA, Conradi SE (December 1996). "Sudden death due to metronidazole/ethanol interaction". The American Journal of Forensic Medicine and Pathology. 17 (4): 343–6. doi:10.1097/00000433-199612000-00013. PMID 8947362.
- ^ Gupta NK, Woodley CL, Fried R (October 1970). "Effect of metronidazole on liver alcohol dehydrogenase". Biochemical Pharmacology. 19 (10): 2805–8. doi:10.1016/0006-2952(70)90108-5. PMID 4320226.
- ^
Williams CS, Woodcock KR (February 2000). "Do ethanol and metronidazole interact to produce a disulfiram-like reaction?". The Annals of Pharmacotherapy. 34 (2): 255–7. doi:10.1345/aph.19118. PMID 10676835.
the authors of all the reports presumed the metronidazole-ethanol reaction to be an established pharmacologic fact. None provided evidence that could justify their conclusions
- ^ Visapää JP, Tillonen JS, Kaihovaara PS, Salaspuro MP (June 2002). "Lack of disulfiram-like reaction with metronidazole and ethanol". The Annals of Pharmacotherapy. 36 (6): 971–4. doi:10.1345/1542-6270(2002)036<0971:lodlrw>2.0.co;2. PMID 12022894.
- ^ Eisenstein BI, Schaechter M (2007). "DNA and Chromosome Mechanics". In Schaechter M, Engleberg NC, DiRita VJ, Dermody T (eds.). Schaechter's Mechanisms of Microbial Disease. Hagerstown, MD: Lippincott Williams & Wilkins. p. 28. ISBN 978-0-7817-5342-5.
- ^ Quirke V (29 December 2014). "Targeting the American market for medicines, ca. 1950s-1970s: ICI and Rhône-Poulenc compared". Bulletin of the History of Medicine. 88 (4): 654–96. doi:10.1353/bhm.2014.0075. PMC 4335572. PMID 25557515.
- ^ "G.D. SEARLE & CO. v. COMM | 88 T.C. 252 (1987) | 8otc2521326 | Leagle.com". Leagle. Retrieved 18 June 2019.
- ^ "2003:Pfizer and Pharmacia Merger". Pfizer.
- ^ Dickson S (July 2019). "Effect of Evergreened Reformulations on Medicaid Expenditures and Patient Access from 2008 to 2016". Journal of Managed Care & Specialty Pharmacy. 25 (7): 780–792. doi:10.18553/jmcp.2019.18366. PMID 30799664.
- ^ Ebel K, Koehler H, Gamer AO, Jäckh R. "Imidazole and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_661. ISBN 978-3527306732.
- ^ Actor P, Chow AW, Dutko FJ, McKinlay MA. "Chemotherapeutics". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_173. ISBN 978-3527306732.
- ^ Kraft MY, Kochergin PM, Tsyganova AM, Shlikhunova VS (1989). "Synthesis of metronidazole from ethylenediamine". Pharmaceutical Chemistry Journal. 23 (10): 861–863. doi:10.1007/BF00764821.
- ^ Barr SC, Bowman DD, Heller RL (July 1994). "Efficacy of fenbendazole against giardiasis in dogs". American Journal of Veterinary Research. 55 (7): 988–90. PMID 7978640.
- ^ Hoskins JD (1 October 2001). "Advances in managing inflammatory bowel disease". DVM Newsmagazine. Archived from the original on 31 December 2013. Retrieved 28 December 2013.
- ^ Plumb DC (2008). Veterinary Drug Handbook (6th ed.). Wiley. ISBN 978-0-8138-2056-9.
- ^ "Metronidazole". Drugs.com. Archived from the original on 24 June 2013.
External links
- "Metronidazole". Drug Information Portal. U.S. National Library of Medicine.
- "Metronidazole". Merck manuals.