Sodium–potassium pump: Difference between revisions
→Mechanism: piped Na to Sodium |
No edit summary |
||
| Line 4: | Line 4: | ||
{{downsize|title=Na<sup>+</sup>/K<sup>+</sup>-ATPase}} |
{{downsize|title=Na<sup>+</sup>/K<sup>+</sup>-ATPase}} |
||
'''Na<sup>+</sup>/K<sup>+</sup>-ATPase''' (also known as the '''Na<sup>+</sup>/K<sup>+</sup> pump''', '''sodium-potassium pump''', or simply '''NAKA''', for short) is an [[enzyme]] ({{EC number|3.6.3.9}}) located in the [[plasma membrane]] (specifically an electrogenic [[transmembrane ATPase]]). It is found in the [human]] [[cell (biology)|cell]] and is common to all cellular [[life]].{{Fact|date=February 2008}} |
'''Na<sup>+</sup>/K<sup>+</sup>-ATPase''' (also known as the '''Na<sup>+</sup>/K<sup>+</sup> pump''', '''sodium-potassium pump''', or simply '''NAKA''', for short) is an [[enzyme]] ({{EC number|3.6.3.9}}) located in the [[plasma membrane]] (specifically an electrogenic [[transmembrane ATPase]]). It is found in the [[human]] [[cell (biology)|cell]] and is common to all cellular [[life]].{{Fact|date=February 2008}} |
||
==Function== |
==Function== |
||
Revision as of 21:38, 11 February 2008
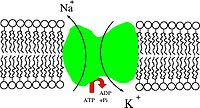

Na+/K+-ATPase (also known as the Na+/K+ pump, sodium-potassium pump, or simply NAKA, for short) is an enzyme (EC 3.6.3.9) located in the plasma membrane (specifically an electrogenic transmembrane ATPase). It is found in the human cell and is common to all cellular life.[citation needed]
Function
The Na+/K+-ATPase helps maintain resting potential, avail transport and regulate cellular volume.[citation needed]
Resting potential
In order to maintain the cell potential, cells must keep a low concentration of sodium ions and high levels of potassium ions within the cell (intracellular). Outside of the cells (extracellular), there are high concentrations of sodium and low concentrations of potassium, so diffusion occurs through ion channels in the plasma membrane. In order to keep the appropriate concentrations, the sodium-potassium pump pumps sodium out and potassium in through active transport. As the plasma membrane is far less permeable to sodium than it is to potassium ions, an electric potential (negative intracellularly) is the eventual result.[citation needed]
The resting potential avails action potentials of nerves and muscles.[citation needed]
Transport
Export of sodium from the cell provides the driving force for several facilitated membrane transport proteins, which import glucose, amino acids and other nutrients into the cell. Translocation of sodium from one side of an epithelium to the other side creates an osmotic gradient that drives the absorption of water.[citation needed]
Another important task of the Na+-K+ pump is to provide a Na+ gradient that is used by certain carrier processes. In the gut, for example, sodium is transported out of the resorbing cell on the blood side via the Na+-K+ pump, whereas, on the resorbing side, the Na+-Glucose symporter uses the created Na+ gradient as a source of energy to import both Na+ and Glucose, which is far more efficient than simple diffusion. Similar processes are located in the renal tubular system.[citation needed]
Mechanism

- The pump, with bound ATP, binds 3 intracellular Na+ ions.
- ATP is hydrolyzed, leading to phosphorylation of the pump at a highly conserved aspartate residue and subsequent release of ADP.[citation needed]
- A conformational change in the pump exposes the Na+ ions to the outside. The phosphorylated form of the pump has a low affinity for Na+ ions, so they are released.[citation needed]
- The pump binds 2 extracellular K+ ions. This causes the dephosphorylation of the pump, reverting it to its previous conformational state, transporting the K+ ions into the cell.[citation needed]
- The unphosphorylated form of the pump has a higher affinity for Na+ ions than K+ ions, so the two bound K+ ions are released. ATP binds, and the process starts again.[citation needed]
Regulation
Endogenous
The Na+/K+-ATPase is thought to be downregulated by cAMP.[1]
Thus, substances causing an increase in cAMP downregulates Na+/K+-ATPase. These include the ligands of the Gs-coupled GPCRs.
In contrast, substances causing a decrease in cAMP upregulates Na+/K+-ATPase. These include the ligands of the Gi-coupled GPCRs. It should be noted that cAMP also acts as a second messenger causing an increase in protein abundance of Na-K-ATPase.
Exogenous
The Na+-K+-ATPase can be pharmacologically modified by administrating drugs exogenously.
For instance, Na+-K+-ATPase found in the membrane of heart cells is an important target of cardiac glycosides (for example digoxin and ouabain), inotropic drugs used to improve heart performance by increasing its force of contraction.
Contraction of any muscle is dependent on a 100- to 10,000-times higher-than-resting intracellular Ca2+ concentration, which, as soon as it is put back again on its normal level by a carrier enzyme in the plasma membrane, and a calcium pump in sarcoplasmic reticulum, muscle relaxes.
Since this carrier enzyme (Na+-Ca2+ translocator) uses the Na gradient generated by the Na+-K+ pump to remove Ca2+ from the intracellular space, slowing down the Na+-K+ pump results in a permanently-higher Ca2+ level in the muscle, which will eventually lead to stronger contractions.
Discovery
Na+/K+-ATPase was discovered by Jens Christian Skou in 1957 while working as assistant professor at the Department of Physiology, University of Aarhus, Denmark. He published his work in 1957.[2]
In 1997, he received one-half of the Nobel Prize in Chemistry "for the first discovery of an ion-transporting enzyme, Na+, K+ -ATPase."[3]
Genes
- Alpha: ATP1A1[1], ATP1A2[2], ATP1A3[3], ATP1A4[4]. #1 predominates in kidney. #2 is also known as "alpha(+)"
- Beta: ATP1B1[5], ATP1B2, ATP1B3[6], ATP1B4
See also
References
- ^ Regulation of Na+-K+-ATPase by cAMP-dependent protein kinase anchored on membrane via its anchoring protein Kinji Kurihara, Nobuo Nakanishi, and Takao Ueha. Departments of 1 Oral Physiology and 2 Biochemistry, School of Dentistry, Meikai University, Sakado, Saitama 350-0283, Japan
- ^ Skou J (1957). "The influence of some cations on an adenosine triphosphatase from peripheral nerves". Biochim Biophys Acta. 23 (2): 394–401. PMID 13412736.
- ^ http://nobelprize.org/chemistry/laureates/1997/index.html
A pdf copy of the paper (reference 1) appears on http://jasn.asnjournals.org/cgi/reprint/9/11/2170.pdf
External links
- Sodium,+Potassium+ATPase at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
