Wikipedia:Reference desk/Science
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
March 30
why don't sugars taste like alcohols?
For give me, but the alcohol article says nothing about why OH groups (that are not part of COOH groups) on sugars or glycerine don't make their host molecule behave like alcohols. I mean, shouldn't sugars behave like extra strong alcohols, what with their abundance of OH groups? If not, isn't the definition of an "alcohol" as given in the articles inaccurate? I mean, the presence of an OH group on a carbon chain cannot be the only thing that makes alcohols alcohols. John Riemann Soong (talk) 02:06, 30 March 2009 (UTC)
- Alcohol is a generic class name for all compounds having a a hydroxy (-OH) functional group. Do not confuse this with ethanol, a specific chemical compound with unique (pharmacological) properties that are caused by the whole molecule, not just by the isolated -OH part. Cacycle (talk) 03:14, 30 March 2009 (UTC)
- But, methanols and propanols and cyclohexanols all behave similarly, have similar pKa's ... heck, even my octanol product produced by hydroboration-oxidation smells like all other alcohols I have known. And if I dared, I bet they would taste roughly the same -- pungent, overpowering, and quite bitter. Yet sugars taste sweet. John Riemann Soong (talk) 05:39, 30 March 2009 (UTC)
- Sorry can't actually answer your question, but ethylene glycol tastes sweet. --TammyMoet (talk) 09:28, 30 March 2009 (UTC)
- Water has an OH group aswell, but that isn't sweet either. The point is that certain molecules will bind to orthosteric site on the sweet receptors on your tongue, these include ethylene glycol, sucrose, sorbitol and many others (mostly sugars though). I personally haven't read into the pharmacology of sweet receptors, but I'm certain there will be papers out there describing the structure of the orthosteric site of those receptors and descriptions of exactly what molecular shape + functional groups are necessary in order to agonize those receptors. --Mark PEA (talk) 13:24, 30 March 2009 (UTC)
- But, methanols and propanols and cyclohexanols all behave similarly, have similar pKa's ... heck, even my octanol product produced by hydroboration-oxidation smells like all other alcohols I have known. And if I dared, I bet they would taste roughly the same -- pungent, overpowering, and quite bitter. Yet sugars taste sweet. John Riemann Soong (talk) 05:39, 30 March 2009 (UTC)
- Alcohols have one hydroxyl group attached to a carbon chain. Sugars have many hydroxyl groups plus either a aldehyde or a ketone. they are quite different. Dauto (talk) 15:03, 30 March 2009 (UTC)
- That doesn't tell me much. Why don't sugars then act like super-alcohols? John Riemann Soong (talk) 15:41, 30 March 2009 (UTC)
- Alcohols have one hydroxyl group attached to a carbon chain. Sugars have many hydroxyl groups plus either a aldehyde or a ketone. they are quite different. Dauto (talk) 15:03, 30 March 2009 (UTC)
- What do you expect a super-alcohol to act like? Dauto (talk) 19:28, 30 March 2009 (UTC)
- Sugar alcohols generally taste sweet as well. Also while I'm not an organic chemist, I'm pretty sure sugars do of course show some of the same reactions that all alcohols do presuming the aldehyde/ketone groups et all don't predominate. Incidentally the IUPAC name for glucose is 6-(hydroxymethyl)oxane-2,3,4,5-tetrol OR (2R,3R,4S,5R,6R)-6 -(hydroxymethyl)tetrahydro -2H-pyran-2,3,4,5-tetraol. Notice the tetraol there? Nil Einne (talk) 22:22, 30 March 2009 (UTC)
- Actually reading about sweetness (the criteria for hydrophobic component and a hydrogen bond donor and a Lewis base 0.3 nm apart) made the light click on. Now what I'm trying to understand is why sugars and imitation sugars (ethylene glycol, artificial sweeteners, or otherwise) don't activate the "superacidic" response from the sour portions of taste buds. Surely the H+ receptors (and those bitter receptors responding to OH groups at the back) must detect those too. Or are the unpleasant sensations associated with OH groups (and the sour sensations associated with COOH groups) suppressed neurobiologically by inhibitive synapses activated by sweetness receptors? John Riemann Soong (talk) 06:06, 2 April 2009 (UTC)
Developing immunity or resistance to a pathogen
This is a general question rather than a request for medical advice. People often get Upper respiratory tract infections manifesting as "colds", sneezing, sore throats, bronchitis, sinus infections, stuffy nose, coughing, fever, or the flu, and after a few days or a couple of weeks make a full recovery, without antibiotics or other medical treatment beyond rest and plenty of liquids. The impression is that the body has "fought off the infection." What mediates the acquired resistance of those who have gotten over the upper respiratory infection? What sorts of pathogens would be capable of reinfecting the person if he/she were re-exposed to the exact same germ/virus after recovery, and what sorts would be very unlikely to cause a re-occurrence due to a new exposure? The section "Clearance and immunity" in Infectious disease does not tell me whether the victims of Typhoid Mary who survived typhoid, for instance, would likely be reinfected if they foolishly hired her again as their cook. If she were "Common Cold Mary," "Streptococcus Mary," "Pneumonia Mary," or "Influenza Mary" and were a persistent carrier, how likely would those who recovered from the exact pathogen she passed to them be to suffer a recurrence from her renewed presence, or say if they ate frozen leftovers she had prepared which were full of the pathogen she carried, after they had recovered from the original infection? Edison (talk) 03:01, 30 March 2009 (UTC)
- I'd say the following in somewhat indirect response to your question.
- Most infections evoke both a humoral (antibody) and cell-mediated immune response.
- The response is not always absolute: evoking either or both immune responses is not a guarantee that one is now protected absolutely from reinfection, but rather that one is less likely to develop severe disease on re-exposure to the organism in question.
- The response fades over time; protective antibodies and cells capable of cell-mediated response gradually disappear from the circulation. They may be quicker to appear on re-exposure, but they don't just hang out in the circulation forever waiting for it.
- With regard to typhoid, see [1]. It seems that within years such protective immunity as develops fades, so re-infection upon re-exposure is certainly possible - and is possible even before, as protective immunity is not absolute. - Nunh-huh 03:36, 30 March 2009 (UTC)
- With respect to the Flu and especially colds, the microorganisms constantly mutate, so an immunity ceases to apply, as the next time you're infected it's likely to be a different strain. StuRat (talk) 05:47, 30 March 2009 (UTC)
- Ah, but the question specified "Same strain." If you got infected from food Mary cooked, would the frizen leftovers be able to reinfect after your immune system ramped up and you recovered? Similarly, if Person A got sick, and passed the upper respiratory infection to Person B, then A got well, could A be reinfected from B? Do pathogens mutate so fast that A and B could perpetually pass a cold or URI back and forth? Edison (talk) 18:47, 30 March 2009 (UTC)
- I'd say no to both cases, or, if you were reinfected, it would be less severe. StuRat (talk) 04:53, 31 March 2009 (UTC)
- I agree, but this reminds me of dengue because partial immunity to one serotype, due to prior infection with another, can result in dengue hemorrhagic fever (for details relevant to the current discussion, see the third paragraph under dengue epidemiology). --Scray (talk) 05:32, 31 March 2009 (UTC)
- I don't have time right now for a more complete response, but take a look at Clonal selection theory - that's generally thought to be the primary mechanism of immunity. In addition to this mechanism for immunological memory, there is now pretty good evidence that the innate immune system has some capacity for "memory" in up-regulating innate immune components (e.g. natural killer cells, complement system) at heavily-exposed sites of the body, perhaps making people somewhat more resistant to certain exposures. The important factors for immunity (T versus B cells, innate versus adaptive) are likely (in some cases, are known) to depend in detail on the pathogen and route of exposure - so it's risky to generalize. --Scray (talk) 19:17, 30 March 2009 (UTC)
macrophage apoptosis and fever
Hi,
Im am interested in the connection of macrophages and fever, especially if/how macrophage apoptosis is connected to the onset of fever. If I understood correctly, macrophages produce cytokines that have a role in the development on fever symptoms but is this more the case with active macrophages or do the dying macrophages release cytokines in significant amounts. —Preceding unsigned comment added by Vuori12 (talk • contribs) 07:39, 30 March 2009 (UTC)
- Since no-one else yet has replied I'll offer my understanding of the matter, without references to back it up. The production of cytokines by macrophages is an active process, and thus requires the macrophage to be (1) stimulated and (2) alive. Sure, an apoptotic macrophage may release some of the cytokines it contains. Nevertheless, apoptosis, as opposed to necrosis, is a process that causes little disturbance to a cell's environment. Apoptosis is programmed cell death, and not uncontrolled release of a cell's contents. --NorwegianBlue talk 19:38, 31 March 2009 (UTC)
Humans on Titans surface.
Saturns moon titan has an atmospheric pressure that's actually slightly more than that of Earths, but it's extremely cold. Would it be possible to survive outside on Titan with little more than an oxygen tank and heavy insulation? If not, what exactly would you need to survive outside on Titan? I'm very curious. 63.245.144.68 (talk) 09:31, 30 March 2009 (UTC)
- According to our article on Titan, the surface temperature is −179.45 °C, and that's without wind chill. By comparison, the coldest temperature ever recorded in Antarctica is −89.2 °C, and it takes far less than that to really do serious damage very quickly. In simple terms, any exposed skin is going to freeze solid pretty much on the spot. Proper insulation would help, sure, but we're probably talking full-body coverage here -- in other words, essentially a space suit. I'm not sure if it would have to be completely airtight, but you couldn't just dress really, really warmly by conventional standards and wear a respirator and expect to survive. -- Captain Disdain (talk) 09:51, 30 March 2009 (UTC)
- Atmospheric composition aside, I don't think there's any place in the Solar System (apart from Earth) where humans can survive the temperature. But please correct me if I am wrong. A Quest For Knowledge (talk) 12:14, 30 March 2009 (UTC)
- Go down deep enough in any of the outer planets and the temperature will rise enough so humans can survive. They mightn't like he pressure though :) More seriously Mercury goes around slow enough to give places where a person could survive the temperature for a while. Dmcq (talk) 12:40, 30 March 2009 (UTC)
- Oh, depending on where and when you are, Mars sports some fairly nice temperatures -- it can get up to 20 °C in the summer, which means that you could walk around in shorts and not get chilly. Of course, the very low atmospheric pressure would cause other problems, but in terms of the temperature, it wouldn't be that bad. Still, in nasty winter conditions, it can get as low as −140 °C, so you'd definitely better be sure about the weather before you step outside... -- Captain Disdain (talk) 12:53, 30 March 2009 (UTC)
- I suppose that temperature is rather important for manned planetary exploration, but not because of the possibility of walking around without a space suit, as pressure and atmospheric composition will likely make those necessary in any case. Where I think the importance would be is in the energy needed to heat the human habitat, or, in the case of a location that gets too hot, the energy required to cool the habitat plus the added weight of an air conditioning unit (or do they automatically include a temperature control unit that both heats and cools ?). StuRat (talk) 14:00, 30 March 2009 (UTC)
- A World War 2 vintage heated flight suit could counteract the low atmospheric temperature, along with electrically heated socks and gloves which are presently commercially available. A heated set of goggles would afford clear vision. This is still more arctic clothing than "space suit." Now we should consider any toxic effects of the chemical composition of the atmosphere. I would say the explorer would need an oxygen rebreather or air tanks, due to the toxic atmosphere. These are also presently commercially available. Edison (talk) 01:51, 31 March 2009 (UTC)
monochrome TV transmitter and reciever
please help me with the block diagram and description of a monochrome TV transmitter and reciever —Preceding unsigned comment added by 117.206.32.67 (talk) 12:35, 30 March 2009 (UTC)
- This appears to be a homework question, which Ref Desk policies prevent us from answering directly: we believe that the value of homework lies in doing it yourself. However, we also have an article on how television works, linked helpfully from our television article. If you have specific points you'd like to clarify, you're welcome to return here and ask. — Lomn 13:06, 30 March 2009 (UTC)
- The how it works article is very good but boy is it going out of date fast. Soon you won't be able to get anything except digital television, and there'll be no need for those dangerous high voltages because the display is an lcd screen or something similar. And who'd bother with a monochrome lcd screen? Dmcq (talk) 15:34, 30 March 2009 (UTC)
- Monochrome would be easier to make... but LCD is so cheap now it'd be more expensive to hire a design team. E-Ink is the only thing I can think of that is still in black and white these days. Anythingapplied (talk) 15:51, 30 March 2009 (UTC)
- I actually did make a b/w receiver from plans some years ago. I took a fair bit of tweaking but it worked nicely eventually. I don't think I'd attempt that with digital television, and anyway it's cheaper to buy complete products than the components - a bit of a pity I think. Dmcq (talk) 16:27, 30 March 2009 (UTC)
- The OLPC XO-1 while a colour screen has a monochrome mode which is sharper and reflective (no backlight) therefore using less power although I guess it's rather similar to e-ink devices Nil Einne (talk) 22:19, 30 March 2009 (UTC)
- Could your monochrome screen be better in terms of contrast etc? If so perhaps you could sell a small number to monochrome movie buffs although it's unlikely to be worth it developing oneNil Einne (talk) 05:01, 31 March 2009 (UTC)
- Monochrome would be easier to make... but LCD is so cheap now it'd be more expensive to hire a design team. E-Ink is the only thing I can think of that is still in black and white these days. Anythingapplied (talk) 15:51, 30 March 2009 (UTC)
- The how it works article is very good but boy is it going out of date fast. Soon you won't be able to get anything except digital television, and there'll be no need for those dangerous high voltages because the display is an lcd screen or something similar. And who'd bother with a monochrome lcd screen? Dmcq (talk) 15:34, 30 March 2009 (UTC)
- This same question was asked in the computing desk and help desk. Please don't do that. Nil Einne (talk) 04:57, 31 March 2009 (UTC)
Send analog values over isolated grounds
I am trying to transfer an analog value (0-5V) from one microcontroller to another. Both microcontrollers are on different power sources (both grounds are isolated) and need to be kept that way. I can easily transfer digital data with optocouplers but I can't find an easy solution for analog signals. Another constraint is that I have a limited amount of I/O's that I can use.
Here are some crazy ideas that I've had to solve this:
- Convert the analog signal to an AC signal with amplitude modulation. Transfer the AC signal using a 1:1 coil and then convert the AC signal to DC. Seems too complicated
- Convert the analog signal with an ADC (8 bit) and use an optocoupler for each line. Too many I/O's
Any suggestions? --jcmaco (talk) 15:42, 30 March 2009 (UTC)
- It seems that this could work. http://search.digikey.com/scripts/DkSearch/dksus.dll?Detail&name=516-1609-5-ND The example circuits are quite helpful. --jcmaco (talk) 15:56, 30 March 2009 (UTC)
- I'd probably go for the ADC solution, if you use i2c or something similar you don't have too many wires connecting. I believe there's optical isolators for i2c but I don't know how much they cost. Dmcq (talk) 16:10, 30 March 2009 (UTC)
- What type of analog signal. If we are talking music this won't work. Otherwise just find a couple of old fax machines and cannibalize the modulator/demodulator circuits. That will get you from analog to digital. Then you can send it through your optocoupler. (Remember to switch the signals, because fax converted digital to analog to go into the phone line. You need it the other way round. )76.97.245.5 (talk) 16:43, 30 March 2009 (UTC)
- Telemetry was sometimes passed by a capacitor with switches (reed switches) rapidly opened and closed to alternately couple it to the sender or receiver. Not sure why you couldn't use optoisolators as an analog link. In the pre-digital days, an analog signal was sometimes changed to a pulse rate for telemetry. The pulses could be sent by optoisolator or transformer. Edison (talk) 18:53, 30 March 2009 (UTC)
- Firstly - you can get analog opto-isolators (actually, they are more usually called 'opto-couplers' for some reason). But aside from that, most microcontrollers and A-to-D converters have separate analog ground and digital ground for precisely for this kind of reason. Take for example, the Atmel AtMega series (popularised in the 'Arduino' board family) - it has an 'AREF' pin and separate AGND and AVCC for exactly these kinds of reason. You still have to tie the AGND to the AGND of the other processor - but that doesn't connect their digital grounds together. There are of course limits...if the incoming 'analog ground' is a million volts above the receiving systems' digital ground then something is going to fry! I believe that the AtMega allows a third of a volt between analog ground and digital ground. SteveBaker (talk) 22:45, 30 March 2009 (UTC)
- Good point. The OP seemed to be talking about sending digital data as well using optocouplers so my reasoning was he could measure the analogue signal near the source and send everything down a single serial link. I've a horror of multiple wires and it removes any need for ADC at the end point. If i2c is too slow he could go up to USB. Though it sounds a bit like the OP wants to generate an analogue signal at the source and then decode it at the destination, I wonder if that is true? Dmcq (talk) 09:43, 31 March 2009 (UTC)
- Convert voltage to current and use an LED and photodiode. --Srleffler (talk) 04:39, 2 April 2009 (UTC)
Doppleganger
What are the percentages/odds that there is someone in this world that looks exactly like you, physically? I ask because some of my friends had mentioned that there is this girl that looks just like me but she lives downtown. They see her walking around with her dog and one of them approached mistaking her for me. But when he looked at her closely, he noticed that it wasn't me. Just someone that looks like my twin but slightly different. (FYI - I don't have a twin at all. Already grilled my parents). --8.4.8.12 (talk) 19:29, 30 March 2009 (UTC)
- I'm not sure this can be sensibly calculated from first principles. There are two major factors here—the most obvious is genetics, and I'm not sure we have any real idea of how many genes go into physical appearance and the complications of them being expressed. The second is environment, which for something like appearance (and other things, but appearance is comparatively straightforward to demonstrate) is MASSIVE (hence people of different socio-economic classes are often visibly of said class at just a glance, both in terms of obvious accouterments like clothing, hair style, makeup, but also things like nutrition, exposure to sun, exposure to cigarette smoke, etc.).
- So I don't think you can calculate the odds in that sense. You could try something more empirical—takes lots and lots of photos and have lots and lots of people record data on how similar they look. Methodologically it might be tough but I'm sure one could figure out something sensible. Of course, in such a survey cultural factors would still play a huge role—Asians famously look more alike to non-Asians than within their own groups, for example—so you'd have to be very careful in figuring out who your "judging" people were.
- All that being said, anecdotally, it's not uncommon to run across a number of people who look vaguely similar from a distance. Exact duplicates seem to only really occur rather rarely (not including twinning), but then again the existence of "impersonator/body double" as an occupation for the very famous seems to imply that out of a large enough pool of people you will have a few people who look very similar. --140.247.249.33 (talk) 19:55, 30 March 2009 (UTC)
- Wow, that is crazy! Thanks for the input. --8.4.8.12 (talk) 14:31, 31 March 2009 (UTC)
- Scroll back up aways and look at the recent question about fingerprints. It's generally asserted that no two people have the same fingerprints. If we believe that - then the odds of two people looking exactly alike is zero because even if they are identical twins with very similar upbringing - their prints don't match. But I'm guessing you are going to say that you don't care about such minor things. OK - so what are the odds that someone of the same sex as you has the same eye and hair color and wears their hair in the same basic style? Probably something like one in 20 ? Certainly one in one hundred - so out of 7 billion people - there are perhaps 70 million who "look like you" - at least to that level of similarity. But then you're going to tell me that this isn't similar enough - you want them to have "similar" bone structure - a "similar" body shape - a "similar" complexion...etc. So here is the problem...there is no decent scientific definition of "kinda-sorta-similar-enough-but-not-really-utterly-identical" - hence there can be no statistics with which to answer this question. SteveBaker (talk) 22:23, 30 March 2009 (UTC)
- I'll try to make some rough order of magnitude guesses. A google gander of "facial recognition false positive rate" (minus quote) netted numbers from 0.1 to 2% false matching. Facial recognition software tends to uses facial features' sizes, positions and shape as a metric. Let's say then out of 100 false positives maybe 1 matches your facial structure rather closely. (Close enough to convince someone it was you if you photoshopped your hairstyle, eye color, skin color, freckles, moles, etc over it without altering the dimensions.) Assuming a f.p.r. of 1% then .01% or 1 out of 10000 people has a really similar facial structure to you. I am pulling this out of thin air, but let's say 1 out of 10000 people are the same gender and have similar skin and hair color, height, weight, age, build, and hairstyle. Then that means that 1 out of 100,000,000 (10000*10000) people naturally look eerily similar to you. If there are 6.8 billion people currently living then there are 68 doppelgangers running around out there. At a distance the number of people who look like you will naturally increase. Sifaka talk 01:28, 31 March 2009 (UTC)
- I'd also add that some people look "more unique" than others. For example, older people tend to look more distinct from one another due to an accumulation of scars and other identifying feature. In the case of women, there's also the effect of make-up, which can make people look more similar than they are without cosmetics. StuRat (talk) 04:47, 31 March 2009 (UTC)
- Surely older people tend to look more alike than more distinct? Most of them have grey hair for example. "Accumulation of scars"? Well, not in my neighbourhood fortunately.--Shantavira|feed me 11:59, 31 March 2009 (UTC)
- You must have noticed that older people have more scars, moles, age spots, etc., than younger people. Each of those items can potentially aid in identification. And they don't all have gray hair; some dye it, some have white hair, and some have no hair. They also tend to wear a variety of hats. StuRat (talk) 03:45, 1 April 2009 (UTC)
Hello - you're on Car Talk...
I know lots and lots about cars - but not much about cars with automatic transmissions.
Sadly, my son just inherited my Wife's crappy (but super-reliable) Mazda Protege 5 wagon (poor kid) and after just a couple of weeks in his tender loving care, it's broken.
The symptoms started out as a kind of whiney sound that was mostly noticable at low speeds - I guess belts - but that wasn't it.
Today, he said that as he tried to pull out of a parking space - the car started to 'bunny hop' - just like a stick-shift being driven by a driver who doesn't know where the clutch is! However, once he gets it up to speed, it drives just fine.
I'm guessing that we're looking at a torque converter issue - that's the thing in an automatic that does what the clutch does in a manual - right? If that were locking up instead of spinning freely - that would do it. My naive mental picture of one of these gizmos is of a 'fluid coupling' that somehow 'locks' into a solid coupling at speed...if that's true then it kinda sounds like it's refusing to 'un-couple'.
So....
- What kinds of things can go wrong with Torque Converters?
- Can you repair a torque converter? Do you just replace the torque converter?
- What do they cost to fix?
- Is there something else in the transmission that might cause these symptoms?
- Does anyone want to buy a Mazda Protege 5 wagon? :-)
SteveBaker (talk) 23:59, 30 March 2009 (UTC)
- Just to be sure, you did check the transmission fluid (while the engine's running), right ? And you also made sure the proper fluid was used, I assume. With anything involving transmission repair, the labor costs can outstrip the parts, as it's not easy to disassemble a tranny. StuRat (talk) 04:40, 31 March 2009 (UTC)
- Yeah - the labor cost is what bothers me. I haven't checked the transmission fluid. I don't see where it gets topped up - there are a bunch of obvious fluid containers - but I don't see any transmission fluid. But those things aren't always easy to find - right? As I said - I've never worked on automatics, but also, we don't have a copy of the shop manual and my son is 200 miles away - so I'm trying to diagnose this over the phone! Last night I told him to get it towed to the nearest transmission shop (we have AAA) and have them replace fluids, filters and seals and anything else that seems transmission-related and costs under $100! SteveBaker (talk) 12:37, 31 March 2009 (UTC)
- The dip-stick for tranny fluid is typically near the firewall, down low, extending from under the engine block on either the right or left side. When checking the fluid also look at the color, which should normally be red or pink. Note that a transmission fluid flush often makes problems worse, so don't fall for that. If the color is bad, change the fluid. If the level is low, but the color is good, just add fluid. StuRat (talk) 03:40, 1 April 2009 (UTC)
- This really does not explain the second problem, but your initial whiney sound resembles one which I had recently. Turned out to be the break pads. Here in the UK things like this get checked up on MOT tests, but I'm not sure what it's like for you in the States in regards to cost. Best way is to get a mechanic to do a full check of the systems that could likely cause such an issue (breaks, suspension etc) and if you can't find the problem there, then assume the engine. —Cyclonenim (talk · contribs · email) 12:05, 31 March 2009 (UTC)
- No - it's definitely not brake pads - the car makes the noise even when your foot is not on the brake. Also, the car passed it's Texas State Inspection (the Texas equivalent of the MOT) just a couple of days ago - AFTER the whining noise started - and they check the brakes. I got the chance to drive the car when the whining started - and it sounded exactly like a belt slipping - but the belts are both new and correctly tensioned. I guessed that there might be some oil on one of the belts or something...but that wasn't it. As my son later realised, it wasn't making the noise when the car was idling - or after the car had built up speed. Which fits the 'torque converter' theory...but now that it's doing this 'bunny hop' thing, I'm thinking that the 'lockup clutch' has locked up and won't unlock. SteveBaker (talk) 12:37, 31 March 2009 (UTC)
- Don't assume that the brakes aren't in use just because the brake pedal isn't depressed (and how could it not be depressed, what with people stepping on it all day ? :-) ). A fairly common problem is for the emergency/parking brake to get stuck at least partially engaged. StuRat (talk) 13:54, 2 April 2009 (UTC)
March 31
If raptor-type dinosaurs existed today...
...would it be blatently obvious to any good evolutionary biologist that modern birds had evolved from them, or were just another branch of the raptor family? Supposing that we knew about the 'raptors before we knew about birds? Thanks. --81.76.68.217 (talk) 00:02, 31 March 2009 (UTC)
- Well modern birds wouldn't have evolved from them if they existed today. They'd have both evolved from a shared species, the way humans didn't evolve from chimps. As for obviousness... it depends what you consider obvious. I think if you watch robins hunt you can see that they are basically acting like raptors (in terms of posture, manner, etc.), but is that obvious, or do I just impose it? --98.217.14.211 (talk) 00:22, 31 March 2009 (UTC)
- 211, I think you have a mistaken assumption that whenever a new species evolves the old one must go extinct. This is often the case, but not always. StuRat (talk) 04:35, 31 March 2009 (UTC)
- I could be wrong, but I think 98's point was that it's usually a fallacy to think any current species (or old species) as the same as some old species (or older species) just because we still identify them as the same species. Even things we call living fossils like the tuatara have actually changed signicantly (i.e. evolved) since prehistoric times. It only really makes sense to talk about species in a fixed point or perhaps small time frame (and even then it's still a far from great concept). Both humans and chimpanzees evolved from a common ancestor that was neither human nor chimpanzee. Birds likely evolved from various dinosaurs but the birds that we would first identify as birds then are not the same as the birds now and if dinosaurs survived as things we would still call dinosaurs they wouldn't be the same as dinosaurs then either. It's not so much a species going extinct as a species changing/evolving. Those dinosaurs that evolved in to birds didn't go extinct, they just evolved in to birds. There may be different lines (species) that evolved but went extinct and there could have been (but wasn't) species that survived but share more 'similarities' with the prehistoric dinosaurs obviously Nil Einne (talk) 11:24, 31 March 2009 (UTC)
If biologists had access to living raptors - doubtless they could do a swift genetic study and answer the question about whether dinoaurs are birds (or birds are dinosaurs or whatever) in pretty short order. But we don't know what the answer would be because that's one experiment we can't do. SteveBaker (talk) 01:26, 31 March 2009 (UTC)
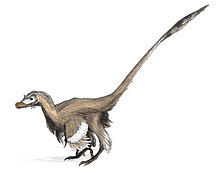

- If you saw a roadrunner and velociraptor side by side, the similarity would be pretty striking. Looie496 (talk) 02:40, 31 March 2009 (UTC)
- But that's a deceptive thing - which is why there is still vigorous debate on the subject. Convergent evolution could easily be the cause of that. Look at pictures of a whale and a whale-shark - they look pretty similar and they live very similar life-styles...yet they are separated by a vast chasm in evolutionary terms. SteveBaker (talk) 12:23, 31 March 2009 (UTC)
- There's always been something in the movements and demeanour of European Magpies that just screams 'dinosaur!' at me. Though it's probably the case that something about the movements and demeanour of European Magpies reminds me of the guesstimated dinosaurs I've seen on TV and in the movies... ;) --Kurt Shaped Box (talk) 03:07, 31 March 2009 (UTC)
- I think it's fair to say that when people today note how remarkably raptor-like birds are it's because dinosaurs-were-bird-like paleontologists like Robert T. Bakker were advisors on Jurassic Park, so they made the raptors act like birds. I imagine the CGI animators watched a lot of bird videos to come up with the raptor movements. The cultural effect is such that .211 above remarks that robins remind him of raptors in their posture and manner (!). --Sean 12:35, 31 March 2009 (UTC)
- There is no doubt that's true. We've never seen raptors moving - so you can't say "I've seen birds move just like raptors." because you've never seen a raptor moving. All you can say is: "I've seen birds moving just like those CGI raptors in Jurassic park (etc) that were deliberately animated to look like birds.'"...but that's a much less impressive claim - and certainly it's a circular argument! There is an expectation that if birds and dinosaurs are closely related - then perhaps one moves like the other - so on 'science' shows on TV, you'll also see animations of raptors moving like birds because that's our current best-guess based on the unproven (but increasingly likely) hypothesis that birds are dinosaurs. You'll also see guesses about what they sounded like - guesses about what color they were and guesses about how they attacked their prey (eg individually or in packs). None of those things are known with any great confidence. SteveBaker (talk) 13:07, 31 March 2009 (UTC)
- I'll bet that a million years from now Humans are animated as moving like chipmunks or something. APL (talk) 16:41, 2 April 2009 (UTC)
- I think if those dinosaurs existed today (and had always been around, rather than being re-created like in Jurassic Park), we would consider them birds. Avimimus or even Velociraptor would look more like a cassowary than a cassowary looks like a robin. 128.194.103.37 (talk) 12:59, 5 April 2009 (UTC)
New York City higher education
In the period 1851-1855 what institutions of higher education in New York City (or close proximity) were offering degrees in engineering? Many thanks to anyone who can assist. Wagnerfamily (talk) 00:23, 31 March 2009 (UTC)
- If you want an authoritative answer, I wouldn't know where to find it, but it's possible that Rensselaer Polytechnic Institute was the only one. They were certainly giving engineering degrees by the 1830s. Looie496 (talk) 02:32, 31 March 2009 (UTC)
- I don't know where to find the answer either, but Rensselaer certainly isn't part of it -- it's well over 100 miles north of New York. --Anonymous, 03:54 UTC, March 31, 2009.
- (1) Polytechnic Institute of New York University (then known as "Brooklyn Polytech")
- (2) The United States Military Academy (West Point is 50 miles north of the city).
- I'm not sure whather Columbia University gave engineering degress before the Civil War.
- Your window is just a bit too early for Cooper Union, City College (CCNY), Stevens or Rutgers.
- B00P (talk) 06:58, 31 March 2009 (UTC)
- Hmm ... on reconsideration, the answer may be "none." Brooklyn Poly's first degrees were awarded in 1871, although it had an engineering program in the period you are interested in (1855). And I'm beginning to doubt that West Point's BS degrees were in engineering, although it was part of the curiculum. Sorry about that. B00P (talk) 07:41, 31 March 2009 (UTC)
Electric car
Let's say we have an all electric car. Could we put a small fan in the front of that car, which would turn when the wind passed it as we were driving that car. Could we then hook the fan to a small generator that could assist in the car being recharged. Thus needing to be plugged into the electrical outlet for a shorter amount of time. I'm led to believe the fan would not fully recharge the car, but why? —Preceding unsigned comment added by 76.225.133.60 (talk) 07:53, 31 March 2009 (UTC)
- Indeed it will not; conservation of energy is the answer. The electric engine of this car produces a mechanical work from some chemical energy; your device would transform part of this work back as chemical energy to be used again. In the energy balance, this will only make the car less efficient, loosing more energy in form of heat. It is like using part of the energy produced by a water turbine to raise up the water again, etc. Still, your idea has a base: in order to decelerate, an electric car may use the engine as an electrical generator, converting again part of the kinetic energy into electricity, which saves energy and avoids over-heating of the brakes. --pma (talk) 09:21, 31 March 2009 (UTC)
- Conservation of energy and the laws of thermodynamics say that there is no such thing as a free lunch. Depressing and inconvenient though it is - it's a fundamental law of nature. In this case, the fan would increase the 'drag factor' of the car - requiring more electrical power to push it along - and this would be to a greater extent than could be recouped from the generator attached to the fan. If you did this, the car would run down faster than if you didn't do it. SteveBaker (talk) 12:20, 31 March 2009 (UTC)
- But! If it was a flying car, and you were scared your engines might fail, you could use the fan you propose as an emergency source of electricity which would generate power from your continued forward motion, allowing you to at least watch your altimeter spin. See ram air turbine. --Sean 12:26, 31 March 2009 (UTC)
You could also park it facing the wind on a windy day to let it recharge a little when you are not using it. 65.121.141.34 (talk) 13:00, 31 March 2009 (UTC)
- You most certainly could. It would be called "A Windmill". But why carry that weight (and drag) around with the car when you can't benefit from it? It would be vastly more logical to leave the windmill at home and just plug into it when you need to...and while you aren't plugged into it, it could be charging up batteries or running your refrigerator? But windmills take a lot of space and they break down - so wouldn't it be better still to pay the electricity company look after the windmill for you? Perhaps they could just roll the cost of doing that into your regular electricity bill. But then, wouldn't it be still better if instead of a bunch of tiny windmills, they had a few GIGANTIC ones so they could make electricity to charge everyone's electric cars...oh...wait...they do. SteveBaker (talk) 13:44, 31 March 2009 (UTC)
- You could of course add a solar panel to the roof instead where you're more likely to get some benefit in recharging. The trouble is of course many car parks are by design indoor or sheltered and the cost of installing the solar panel will almost definitely take very, very long to have any pay off. Again of course you'd be better off installing the solar panel somewhere else Nil Einne (talk) 14:36, 31 March 2009 (UTC)
- But the solar panel could have non-financial benefits, as well. For those who park outside and drive very little, it may provide all the electricity they need, so no effort needs to be spent recharging. For those who drive farther, it might be nice to drive in to work with almost no charge left, park in the sunlight, and have enough of a charge after work to either drive home or at least to the nearest charging location. StuRat (talk) 14:50, 31 March 2009 (UTC)
- Specifically, in order to recharge the car, the fan would have to be huge. Once you add that huge fan to the car, suddenly your car is heavier and has much more air resistance. So your car needs a stronger engine. Which means you need an even larger fan, which needs an even stronger engine to haul it around, etc, etc.
- The law of conservation of energy, means that you'll always wind up with these sorts of jams. Perpetual Motion enthusiests are always sure that they're very close to "balancing" that situation, but they never are. It's impossible to balance it. APL (talk) 13:18, 31 March 2009 (UTC)
The top of a car, the hood, and the trunk would afford at least 2 or 3 square meters of solar collector, assuming the car is not one of those wierd all solar powered cars which looks like a gust of wind would blow it off the road. It would not be optimally oriented in most cases, so it would not get the theoretical maximum solar input of about 1 kilowatt per square meter, but what is the present efficiency of solar cells in converting to electricity the possible 1 or 2 kilowatts of solar energy hitting the panels? How much kw or HP does it take for a small car to cruise at 55 miles per hour (88 km/h)? It would be less than the max output of the engine in a car such as the Prius, since the engine is sized for brisk acceleration and high speed driving. I seem to recall early hybrids from the 1970's needing only 5 HP to cruise. If so a car covered with efficient solar panels could have greatly extended range. Edison (talk) 15:43, 31 March 2009 (UTC)
- This article says that passenger cars, in general need about 25 horsepower to cruise at 60 mph. More aerodynamic and lighter cars or slower cruiosing, could reduce the requirement significantly, since this figure is apparently for large passenger cars in general. Edison (talk) 15:50, 31 March 2009 (UTC)
- The new Priuses will have solar panels as an option [2] [3] although it's only used to power the airconditioning and radio at most it seem. The Prius of course is a large car so not the best example. Solar vehicle will have info of more interest Nil Einne (talk) 00:36, 1 April 2009 (UTC)
- 25Hp is 18.7 kW, so your solar panels would need to be about 1000% efficient to supply all the power the car needs. When it is sunny that is. —Preceding unsigned comment added by 65.121.141.34 (talk) 20:13, 31 March 2009 (UTC)
- And that doesn't include the additional weight. But let's pick a solar-panel sales company at random here is the first one that popped up on Google: This solar panel for example - covers 0.11 sq.meters - and produces a typical peak power of less than 9 watts. So to cover the roughly 3 square meters of roof space would require 30 of them and would produce 270 watts "peak" - about 1/70th of what a horribly under-powered 25Hp car needs (my car produces 170Hp). If we imagine the car getting 8 hours of continuous sunlight per day - the solar panels would give you about an extra 5 minutes of drive time! Each panel weighs 3.3lbs - so you'd be adding 100lbs to the car...not so terrible on a bigger car - but noticable on a small, electric car. If we were looking at a typical 3,000lb car - the solar panels would add about 3% to the weight - if that added 3% of the power requirements - then the solar panels would probably reduce your range rather than increasing it. SteveBaker (talk) 01:56, 1 April 2009 (UTC)
- I imagine that most of the weight of those solar panels is the structure to support them. If properly integrated with the car design, the roof, hood, and trunk would provide the needed support, thus reducing the weight requirements dramatically. But yes, just taking solar panels designed for terrestrial use and bolting them to the roof of a car is probably a most unwise decision. StuRat (talk) 02:03, 1 April 2009 (UTC)
- Make Magazine recently had a one page write up on a car that was home-converted to full solar. He mentioned 20-40 miles on a full charge, but tellingly, he doesn't say how long it takes to charge.[4] APL (talk) 02:41, 1 April 2009 (UTC)
- Can we then assume that it recharges overnight ? :-) StuRat (talk) 17:56, 3 April 2009 (UTC)
Alternatives Sleep
Are there any alternatives to sleep? Things that would have the same effect as a good nights sleep but enable a person to continue on without rest? 194.80.240.66 (talk) 09:45, 31 March 2009 (UTC)
- One can cope sufficiently with caffeine for a period of time, perhaps a day or two, but it's by no means a substitute for sleep as it won't keep you fully alert. Sleep is curently the only way we have of 'recharging' ourselves, and repairing our bodies. —Cyclonenim (talk · contribs · email) 12:01, 31 March 2009 (UTC)
- Concur with Cyclonenim. The military in particular is often interested in this sort of things, e.g. [5] and even they at the current time are only looking at reduction rather then elimination Nil Einne (talk) 14:16, 31 March 2009 (UTC)
- I've found that caffeine keeps my eyes open, but after a day leaves me in a zombie-like state which makes it quite useless. StuRat (talk) 14:39, 31 March 2009 (UTC)
- Death and the afterlife according to some religions. Dmcq (talk) 15:31, 31 March 2009 (UTC)
- There are no alternatives to sleep. The reason why is not positively understood -- my own preferred explanation is that there is evidence that neural connections in the brain steadily get stronger during wakefulness, and only during sleep does the brain go into a state where the connections can be normalized. Without sleep, brain activity becomes steadily more erratic, causing difficulties in concentrating, and eventually hallucinations. Looie496 (talk) 17:20, 31 March 2009 (UTC)
- There is an alternative to a good nights sleep through something called Polyphasic sleep where you sleep at various times throughout the day instead of one chunk at night. These often require far less total hours of sleep. It should go without saying that you should consult a doctor before trying any of these abnormal sleep cycles. Anythingapplied (talk) 03:08, 1 April 2009 (UTC)
Buried in flies
| “ | There is no exception to the rule that every organic being increases at so high a rate, that if not destroyed, the earth would soon be covered by the progeny of a single pair. | ” |
So which species would be first to bury us, say up to our necks, if allowed to breed uncontrollably, and how long would it take? SpinningSpark 12:27, 31 March 2009 (UTC)
- Presumably whatever multiplies fastest. Bacteria says "Under optimal conditions, bacteria can grow and divide extremely rapidly, and bacterial populations can double as quickly as every 9.8 minutes.", so if you started with 1 gram, they would outweigh the Earth in only 92 generations, or around 13 hours. --Sean 12:44, 31 March 2009 (UTC)
- Where did that 9.8 minutes quote come from? Dauto (talk) 14:34, 31 March 2009 (UTC)
- Bacteria#Growth and reproduction. It's referenced but I didn't check. Zain Ebrahim (talk) 14:40, 31 March 2009 (UTC)
- That's only true if the three-dimensionality of space or the Pauli exclusion principle are among the controls we're removing. Otherwise the population growth will soon be limited to the edge of the colony and it will grow no faster than t3 instead of et. Some quick internet "research" suggests that bacterial locomotion maxes out at 200 µm/s, which would put a lower bound of 3000 years on the time needed to blanket the earth. Larger creatures could probably beat that.
- (Although the bacteria could be carried by streams and ocean currents. Still, I think we're talking hundreds or thousands of years, not hours.) -- BenRG (talk) 14:53, 31 March 2009 (UTC)
- Where did that 9.8 minutes quote come from? Dauto (talk) 14:34, 31 March 2009 (UTC)
- Homo sapiens is a species that will bury itself, It has already made many other species extinct. - GlowWorm. —Preceding unsigned comment added by 174.130.251.46 (talk) 14:30, 31 March 2009 (UTC)
I have frequently observed this in real life. If you have a strong stomach, see the horrifying results of this practice at, for example,this page Beware, nature is cruel and vile and this picture may upset you. --Dweller (talk) 15:54, 31 March 2009 (UTC)
- Given the ability to increase uncontrollably, human beings could theoretically cover the enitre planet in as few as 200 years. Assuming every human being lived in a 1 square meter area, and the human population doubling every 50 years. The land area of Earth is appx. 148 billion square meters, and the population of Earth could 192 billion by 2310. Although algebra was never my strong suit, I could be wrong. Livewireo (talk) 21:10, 31 March 2009 (UTC)
- I'm sorry - I can't shake the image of people packed together at 1 sq.meter per person reproducing at maximum possible rate. Would there be viagra involved at any point in the proceedings or would we have to resort to the flying helmet and wet celery? SteveBaker (talk) 18:05, 1 April 2009 (UTC)
VH-3D Sea King [Obamacopter] range
President Obama is coming to London with his trusty VH-3D Sea King, the range of the Sea King is not great enough to cross the Atlantic in one push, so is it transported in a plane and flown around or does it bunny hop from ships in the Atlantic refuelling? I'm fairly certain it would be the former, but someone recently said that it is possible for a helicopter to cross the Atlantic. —Preceding unsigned comment added by MedicRoo (talk • contribs) 13:16, 31 March 2009 (UTC)
- Certainly it could be in-flight refuelled and get all the way across the Atlantic that way - but it's a fairly arduous and dangerous activity and I doubt they'd do it if they didn't absolutely have to. There are actually close to 30 'presidential helicopters' in the fleet and it's quite likely that some are stationed in Europe on a permanent basis for just such an occasion. SteveBaker (talk) 13:36, 31 March 2009 (UTC)
Presidential helicopters are flown over on a cargo plane, though I am not sure if they use a C-141 or a C-5. Actually, I am not sure if the Sea King has in flight refueling capability. 65.121.141.34 (talk) 15:36, 31 March 2009 (UTC)
Seeing as how the C-141 has been retired from active service since 2004, They probably use the C-5. 65.121.141.34 (talk) 14:44, 1 April 2009 (UTC)
Valuable garbage
What can be found in the garbage and is somehow valuable? Are old electronic components valuable, if an electronic device is put apart and sold? Can old batteries be processed and re-sold? What else?--80.58.205.37 (talk) 17:40, 31 March 2009 (UTC)
- An article on Dumpster diving sounds like a good place to start. --Dr Dima (talk) 18:46, 31 March 2009 (UTC)
- Bad art would definitely satisfy your criteria (and is the subject of today's Featured Article. --Scray (talk) 01:04, 1 April 2009 (UTC)
- There is undoubtedly a vast amount of valuable material in garbage. The ENTIRE problem is in separating it out. For example - every electronic item that's been tossed aside contains significant quantities of gold - but extracting the gold from the other stuff in there is a dirty, difficult job - and it's almost impossible to do it economically. That's why careful recycling is important. If glass, aluminium, clean paper and such are cleanly separated, each is extremely valuable. SteveBaker (talk) 01:31, 1 April 2009 (UTC)
- I'd say the most money is to be made from fully working items which people discard because they've gone and got "the latest model". For example, many analog TVs in perfect working order are likely to be discarded as a result of the change to digital TV. While they may not have much value in a country which has already undergone the transition, I'd expect shipping them to a country where the transition is years off could still be profitable. StuRat (talk) 01:50, 1 April 2009 (UTC)
- Analog TV's still have a long life right here in the USA. Remember - they still work just fine with video games, VCR's, DVD's, cable and satellite TV. Chucking them out because they can't pick up broadcast TV is silly. If you are patient - in a year or so, all of those government-subsidized TV conversion boxes are going to be flooding the market as the TV's they are connected to fail and are replaced with new digital tellies. You'll be able to pick one up on eBay for $5. SteveBaker (talk) 02:09, 1 April 2009 (UTC)
- And lots of things have analog-out as their standard. If the video isn't digital to begin with, putting it on an expensive high-dev screen is pretty pointless and a low-res or analog output doesn't get any better by enlarging it. Unless Atari has an upgraded Atari 2600 I don't know about? DMacks (talk) 02:16, 1 April 2009 (UTC)
- I'm not saying that analog TVs are worthless, on the contrary I have 5 of them in my home all hooked up to converter boxes, and intend to keep them for years. However, if I know anything about how people think, many will toss out the old TV as soon as they get a new one, with no thought that the old one may still have value. You probably won't be able to sell those TVs for much, due to a glut in those countries where analog broadcasts have stopped. Thus the need to transport them to a country where the transition is years away. StuRat (talk) 13:41, 2 April 2009 (UTC)
As an earlier poster wrote, the problem of separation limits the utility of much "garbage". But also transportation and marketing costs need to be considered. A single aluminum can might be returned for 2 cents, but it you drive it to the return center using 5 cents worth of gas and use 20 minutes of time you could be working, it might not be worth it. ike9898 (talk) 13:59, 1 April 2009 (UTC)
- I can't even start my car with 5 cents worth of gas. And yes, you might want to wait until you have more than just the one can, before driving to the recycle center. StuRat (talk) 13:46, 2 April 2009 (UTC)
- I really just meant that at that as an example to show that cost of separation isn't the only limitation. BTW, I read about one analysis that suggested that glass recycling makes little economic or environmental sense due to high cost of transporting it and the large amounts of energy required to reprocess it. ike9898 (talk) 14:50, 2 April 2009 (UTC)
- Getting back to the original question - I think most things in the garbage have some value, but for many of those things (eg broken toys), there is a significant cost associated with 'extracting' that value. ike9898 (talk) 14:57, 2 April 2009 (UTC)
- I really just meant that at that as an example to show that cost of separation isn't the only limitation. BTW, I read about one analysis that suggested that glass recycling makes little economic or environmental sense due to high cost of transporting it and the large amounts of energy required to reprocess it. ike9898 (talk) 14:50, 2 April 2009 (UTC)
Old plastic drinks bottles made from Polyethylene terephthalate (PET) currently fetch a price of around €1500-1700 (US$2000-2200) per ton for recycling into Polar fleeces and are probably (excluding metals) the easiest item that is mass produced to make a profit from. Nanonic (talk) 14:32, 2 April 2009 (UTC)
rss feed
is there an rss feed for this page? just-emery (talk) 19:02, 31 March 2009 (UTC)
- Mediawiki supports an RSS for changes to every page - for this one it is http://en.wikipedia.org/w/index.php?title=Wikipedia:Reference_desk/Science&action=history&feed=rss - to access it you go to a page's history tab and click the RSS (or Atom) link in the menu on the left. That feed has all the changes (rather than one entry for every question), so you might find it rather busy. 87.115.166.150 (talk) 20:52, 31 March 2009 (UTC)
Eating carnivorous animals
Is eating carnivores particularly unhealthy or dangerous? I know cultures do eat animals like dogs and cats, but I've talked to a few people who say they would never eat a carnivore because carnivore meat is dangerous/unhealthy in some way. Is this true? 90.195.179.46 (talk) 20:07, 31 March 2009 (UTC)
- There is perhaps a grain of truth to that. If you look at fish, the large carnivore fish like tuna are the ones that accumulate mercury. I don't recall what the term for that is, but its also why eagles were so strongly effected by DDT as well. 65.121.141.34 (talk) 20:10, 31 March 2009 (UTC)
- And note that not all carnivores are equivalent. Those which directly eat herbivores have far less of a biomagnification problem than those at the top of a long food chain. StuRat (talk) 01:44, 1 April 2009 (UTC)
- Eating the livers of (some?) carnivores can lead to potentially fatal Hypervitaminosis A. Deor (talk) 20:40, 31 March 2009 (UTC)
- I suspect that one of the main reasons that carnivore animals are eaten less is that they are inefficient. It takes a lot of corn or grass to get meat on a cow, and it takes a lot of cow meat to get meat on a bear, so better to just eat the cow. 65.121.141.34 (talk) 13:48, 1 April 2009 (UTC)
- In Jared Diamond's book, Guns, Germs and Steel, he suggests that most of the animals we eat are herbivores because herbivores and much more amenable to domestication (and easier to feed). ike9898 (talk) 13:52, 1 April 2009 (UTC)
- Thank you, most informative. It sounds like explicitly excluding all carnivores isn't logical; only certain carnivores (those made entirely of liver, or which eat many other carnivores]] would be the "worst" ones... 90.195.179.46 (talk) 19:38, 1 April 2009 (UTC)
- The answers above seem to have overlooked trichinosis and similar diseases. Yes, eating carnivores is somewhat more unhealthy than eating herbivores. Eating omnivores has some of the same issues, which is why pork is not eaten in some cultures.--Srleffler (talk) 05:00, 2 April 2009 (UTC)
There's certainly a Biblical prohibition on this sort of thing. And I recall reading that only certain members of West Coast native American tribes were permitted to eat bear meat. I am guessing there must be some reason for this. And wasn't mad cow disease linked to cows eating the leftover bits from other cows? Vranak (talk) 17:04, 2 April 2009 (UTC)
- Yes, but cows aren't normally carnivores. Farmers had the habit of grinding up downer cows (those which are sick of some unknown cause), to use them to supplement the protein in their normal cattle feed. They did take the precaution of cooking or irradiating the meat first, but neither works for Mad Cow Disease. StuRat (talk) 17:48, 3 April 2009 (UTC)
- One subset of eating carnivores is cannibalism, which has special health risks as any disease the prey may have is known to also be able to infect the predator. StuRat (talk) 17:52, 3 April 2009 (UTC)
- Kuru (disease) is thought to be linked to cannibalism. Grantus4504 (talk) 09:27, 4 April 2009 (UTC)
extra part
How can a girl have two vagina and other can have three breasts? Here are the videos:[two vagina] and [three breasts]. —Preceding unsigned comment added by 74.14.119.56 (talk • contribs) 16:43, 31 March 2009
- Didn't watch the videos, but see supernumerary nipple and accessory breast for possible answers to the second question. TenOfAllTrades(talk) 21:56, 31 March 2009 (UTC)
- I have not watched them either, but they are likely to be sexually explicit (for those who prefer to avoid such links), and I further presume that this recent discussion is highly relevant. Yes, I'm feeling kinda presumptious, I don't like following off-wiki links, especially when found on sites with names like "uselessjunk" and "efukt". --Scray (talk) 22:51, 31 March 2009 (UTC)
- We had both of these questions before - and the answer was that these are clearly nothing more than clever prosthetics. Once you attune your brain to this fact and re-watch the movies with a skeptical eye, it becomes absolutely obvious. In the 'three boobs' video - the middle boob has no 'jiggle factor' whatever - it's a solid lump of plastic of some kind. Movie special-effects with clever makeup. SteveBaker (talk) 01:27, 1 April 2009 (UTC)
- Another good sign is that it's relatively easy to find the name of the actress at least in the case of the 3 breasts one, for example it's given here (NSFW). (I'm purposely not giving the name for BLP reasons.) A quick Google would easily find other pictures of an actress under that name who looks the same other then 'missing' one breast. Nil Einne (talk) 06:18, 1 April 2009 (UTC)
- BTW, you made a two formatting errors in your post preventing the first link from working and also making the two and three not show for either URL. I've fixed the errors. For URLs you don't use the | as a seperator, instead just a space, like this [http://www.microsoft.com This is Microsoft]. Also you never use a double || anyway Nil Einne (talk) 14:10, 1 April 2009 (UTC)
chemistry
When a drop of bromine is added to a solution of alkene, it loses it's orange colour. Why? —Preceding unsigned comment added by Modipam (talk • contribs) 20:44, 31 March 2009 (UTC)
- What makes the orange colour? What happens chemically when a solution of an alkene is mixed with bromine?
 Please do your own homework.
Please do your own homework.- Welcome to the Wikipedia Reference Desk. Your question appears to be a homework question. I apologize if this is a misinterpretation, but it is our aim here not to do people's homework for them, but to merely aid them in doing it themselves. Letting someone else do your homework does not help you learn nearly as much as doing it yourself. Please attempt to solve the problem or answer the question yourself first. If you need help with a specific part of your homework, feel free to tell us where you are stuck and ask for help. If you need help grasping the concept of a problem, by all means let us know. DMacks (talk) 21:17, 31 March 2009 (UTC)
- The fastest way to find the answer to this question is to open your organic chemistry text book, and read the section on alkenes. There will be a section in that chapter on the reactions of halides with alkenes. Read that, and you will find your answer there. Also, your professor told you the answer to this question in class. So just look at your class notes the for the last few days. You could read our articles on bromine and alkene, but you would not find the answer as easily as looking in the textbook that goes with your class. This question is clearly a reading comprehension question; its sole purpose is to check that you actually read the textbook and that you actually wrote down what the teacher told you to write down in class. --Jayron32.talk.contribs 00:03, 1 April 2009 (UTC)
Cleverness
a)Who is/was the cleverest person in the world?
b)Can we ever find the true answer to (a)?
c)Is cleverness the same thing as IQ? —Preceding unsigned comment added by 79.75.48.89 (talk) 21:28, 31 March 2009 (UTC)
- Regardless of your answer to (c), "cleverness" lacks a rigorous definition such that we can rank the population monotonically (IQ also lacks this). That leaves the answer to (b) as "no" and saves worrying about (a). — Lomn 21:34, 31 March 2009 (UTC)
- Wow, Lomn! That was a very clever way to respond to that question. You must have a high IQ! ;-) --Scray (talk) 22:38, 31 March 2009 (UTC)
- While IQ is the most widely used form of measurment of "intelligence" at the end of the day there is no objective way to measure it. Some people have great stores of memorized knowledge. Many people are clever and can solve puzzles quickly. Many people just learn really fast. If I'm great at math and you are great with words or with reading social situations what ranks higher?
- IQ was designed to work with children (its calibrated to age, so every time you double your age you need to double you total knowledge to keep the same IQ rating). In this capacity it helps identify kids that might need special attention if they are either far above the curve or far below it. Because of all of IQ's shortcommings I would never use it for anything except as a general pointer to help with children. It doesn't apply well to adults in my opinion.
- So to answer your question, there really is no way to tell who is the cleverest. How would you even start to compare people from different time periods? Personally I've always been intrieged by the Greek Scholars, like Archimedies, because they were able to make breakthroughs in so many fields. Many of them were able to, in the course of their lifetime, not only make breakthroughs in Mathematics, Astronomy, Chemestry, and Physics, but Phylosophy, Theology, and Politics as well. This is all but impossible today because we are at such a high level in things like Physics that you practically have to spend a lifetime of study to make breakthroughs.Anythingapplied (talk) 02:53, 1 April 2009 (UTC)
- Or put in a more historically sensitive framework, the differences we see in all of those subfields did not yet exist. (All of those except Theology, Mathematics, and Politics would have been considered a single "Natural Philosophy" by learned people even into the 18th century). --98.217.14.211 (talk) 03:47, 1 April 2009 (UTC)
One measure could be excellence in more than one field. See Polymath. --Dweller (talk) 09:17, 1 April 2009 (UTC)
- a) SteveBaker
- b) No
- c) No
- — DanielLC 16:07, 1 April 2009 (UTC)
Science types needed
This is actually an internal wikipedia issue but I'm a bit stuck. I need some scientific input into Samar Chatterjee, I don't understand or know the field so cannot assess the articles in terms of if the guy is notable or if it's simply an advert. Originally I thought "ah-ha! the wikiproject science people" can help - but that project seems completely dead. So I thought "ah-ha! surely the science reference desk must have some suitable boffins hanging around in their white coats. Anyone got any expertise in this area? willing to take a look? --Cameron Scott (talk) 23:09, 31 March 2009 (UTC)
- The relevent guideline is WP:PROF. The article needs a serious rewrite, but it looks as though he barely makes the notability cut. --Jayron32.talk.contribs 23:56, 31 March 2009 (UTC)
- The easiest way to assess notability for a scientist is to search Google Scholar for his name and look at the number of citations. Doing so, I see that his "citation classic" is Chatterjee & Biswas 1971, which gets all of 6 cites. That's better than zero, but not much -- it basically means nobody has paid any attention to his work. I'm not a hawk on notability so I wouldn't be bothered to see a 1-2 paragraph article, but more than that isn't really justified. Looie496 (talk) 00:37, 1 April 2009 (UTC)
- Normally, I'm an inclusionist by nature and would recommend a keep in the grounds that the person in question is borderline notable and the article is already written and appears well-sourced. HOWEVER, the original author of the article has edited absolutely nothing else in Wikipedia and appears to know a lot of personal details of the subject that are not sourced. This makes me STRONGLY suspicious that this person is either editing an article about himself - or about a close friend, relative or colleague...which is deeply, deeply disturbing and 100% contrary to Wikipedia rules. IMHO, although it pains me to say so - I think this article should be deleted unless/until User:Sushila69 comes clean about his/her relationship to Samar Chatterjee and we can assess whether we're comfortable with risk of WP:OR and Wikipedia:Autobiography. The loss of the article is really no big deal because of the borderline notability. SteveBaker (talk) 01:19, 1 April 2009 (UTC)
- Okay, I've nominated him for deletion. Clarityfiend (talk) 08:17, 1 April 2009 (UTC)
April 1
Artificial nectar
constructing a bird feeder is one thing, now I need a recipe for nectar to supply it.Eg. Tui —Preceding unsigned comment added by Mike Penhey (talk • contribs) 03:26, 1 April 2009 (UTC)
- Your local pet shop will sell all kinds of bird food. If you want to make it yourself, it would probably help to know what species of bird you are hoping to attract (at least, where you are). --Tango (talk) 03:58, 1 April 2009 (UTC)
- He did say "e.g. tui" which would suggest: 1) He's hoping to attract tui (amongst other birds). 2) He's somewhere in New Zealand (at least I hope so since if the OP is in say Australia and hoping to attract tui I don't think we can help) Nil Einne (talk) 05:51, 1 April 2009 (UTC)
- Nectar is usually mostly sugar water, so you could just dissolve as much white sugar in water as possible. Some birds are attracted to bright colors, so you might want to add some food dye, too, perhaps red. You might want to add some actual fruit juice to supply some vitamins, too, although that might make it more likely to spoil. StuRat (talk) 13:28, 2 April 2009 (UTC)
- No, no, NO!!! There was a big hoo-ha here in Texas (where humming birds are relatively common - and people often put out feeders to attract them). People who are feeding them sugar water and (worse) artificially colored sugar water - were found to be killing off these poor birds in vast numbers because the birds are not getting all of the proteins, minerals and vitamins they needed - and at least one brand of artificial nectar contained a food coloring that's OK for humans - but toxic to humming birds. Most humming birds eat nothing but nectar - and if you provide them with a free supply - that's all they'll eat. So this is a very serious concern. There has even been talk of passing a law banning the sale of of hummingbird feeders and artificial nectar that does not contain everything that real nectar has. SteveBaker (talk) 14:16, 2 April 2009 (UTC)
- I hate to disagree with my hero, but I think that Steve is wrong. Here is what one good source says: "The sugar water we use to fill hummingbird feeders is only a supplement to the birds' natural diet. It's not necessary to buy a commercial "nectar" mix that includes additional vitamins, protein, or other substances, because the birds get all they need from the flower nectar and insects they consume. All they want from us is the quick energy they get from ordinary white cane sugar. It's just fuel for chasing bugs, and causes no known health problems in hummingbirds, whose metabolism is significantly different from humans'." (hummingbirds.net)--Eriastrum (talk) 17:51, 2 April 2009 (UTC)
- The Cornell Laboratory of Ornithology concurs that a sugar solution is not harmful. -- Coneslayer (talk) 20:00, 2 April 2009 (UTC)
- And note that I did suggest adding some actual fruit juice to provide at least some of the missing nutrients. StuRat (talk) 17:37, 3 April 2009 (UTC)
- Also, I'd expect that hummingbirds, like other birds and animals, will detect that they are deficient in some nutrient and become hungry for foods which have provided that nutrient in the past (say nectar from real flowers). This type of reaction is extremely important to the survival of the species, so I'd expect just about every species to exhibit it. Do we have an article on this ? StuRat (talk) 17:41, 3 April 2009 (UTC)
- Ahem, Stu that doesn't even work for humans. We'll happily consume high calorie foods and run out of vitamins and minerals. Vitamin deficiency can make vegetarians who don't take supplements seriously ill after a couple of decades. If you want an animal model, cats will gorge themselves on cat food and may run out of taurine if their food doesn't contain that. (OR Even outdoor cats don't supplement their diet with sufficient amounts to offset that deficiency.) Survival of the species only has to keep enough individuals in reasonable shape to reproduce until they have done so. Over many generations natural selection will favor individuals most adapted to a certain diet, but it will just as easily wipe out populations when certain foods are unavailable. There are some mechanisms that evolved over time, like many species having a craving for salt. That can however lead to individuals being killed off by consuming too much salt if it becomes available in abundance. 76.97.245.5 (talk) 06:51, 4 April 2009 (UTC)
- The mechanism I described certainly doesn't work perfectly, but does work to some extent. I find myself craving protein when I'm protein-starved. I imagine that vegetarians do, as well, but are unfortunately able to suppress that craving. StuRat (talk) 17:30, 5 April 2009 (UTC)
cell division
why division takes place in living cells only. —Preceding unsigned comment added by Muzhu (talk • contribs) 08:29, 1 April 2009 (UTC)
- Hmm, as opposed to dead cells? Dead cells (and dead people) in general aren't very active. Please rephrase your question if you meant something else. ;) -- Aeluwas (talk) 08:41, 1 April 2009 (UTC)
- (ec) Cell division requires co-ordinated expenditure of chemical energy to drive specific reactions to create specific proteins and nucleic acid chains. This metabolism cannot occur in dead cells. Axl ¤ [Talk] 08:43, 1 April 2009 (UTC)
Actually I was analyzing the Biology papers of 0-levels, i found this question and it asked for an appropriate answer, so I intended to know more appropriately that why only living cells tend to divide? —Preceding unsigned comment added by Muzhu (talk • contribs) 09:06, 1 April 2009 (UTC)
- Isn't the reason that the very definition of "death" in a cell is its inability to divide? SteveBaker (talk) 17:55, 1 April 2009 (UTC)
- I don't think that's the definition of death in a cell -- otherwise red corpuscles and neurons would be dead. Looie496 (talk) 18:00, 1 April 2009 (UTC)
- And so would senescent cells. Algebraist 18:02, 1 April 2009 (UTC)
- Damn! I forgot about that. OK - let me put it another way. A cell that can still divide is not considered to be dead PRECISELY BECAUSE it can still divide and hence must still be alive. Hence dead cells cannot by definition divide - and no further explanation of why that is can be made. SteveBaker (talk) 14:08, 2 April 2009 (UTC)
- And so would senescent cells. Algebraist 18:02, 1 April 2009 (UTC)
- I don't think that's the definition of death in a cell -- otherwise red corpuscles and neurons would be dead. Looie496 (talk) 18:00, 1 April 2009 (UTC)
- (for Looie or others) Just curious: by what definition are red corpuscles alive? --Scray (talk) 01:59, 2 April 2009 (UTC)
- If we use the Conventional definition here, I'd argue that a RBC satisfies (1) homeostasis, (2) organization, and (3) metabolism pretty well - for 120 days or so. They really don't (6) respond to stimuli in a coordinated way, and the other three requirements clearly aren't met. I'd say RBCs are dead, they just don't know it yet. --Scray (talk) 02:07, 2 April 2009 (UTC)
- That definition begins "Life is a characteristic of organisms...". Using that definition, anything that is not an organism cannot be alive. Axl ¤ [Talk] 08:32, 2 April 2009 (UTC)
- Awesome! Now would you care to define "organism" without using the word "life" anywhere? SteveBaker (talk) 14:08, 2 April 2009 (UTC)
- A collection of organelles which together can conduct homeostasis, metabolize, grow, adapt, respond to stimuli and reproduce? Cyclonenim : Chat 18:06, 2 April 2009 (UTC)
- Awesome! Now would you care to define "organism" without using the word "life" anywhere? SteveBaker (talk) 14:08, 2 April 2009 (UTC)
- That definition begins "Life is a characteristic of organisms...". Using that definition, anything that is not an organism cannot be alive. Axl ¤ [Talk] 08:32, 2 April 2009 (UTC)
- If we use the Conventional definition here, I'd argue that a RBC satisfies (1) homeostasis, (2) organization, and (3) metabolism pretty well - for 120 days or so. They really don't (6) respond to stimuli in a coordinated way, and the other three requirements clearly aren't met. I'd say RBCs are dead, they just don't know it yet. --Scray (talk) 02:07, 2 April 2009 (UTC)
- Hehe! Our article begins: "an organism is any living thing...". The second sentence is (perhaps) more helpful: "organisms are capable of response to stimuli, reproduction, growth and development, and maintenance of homeostasis as a stable whole". Using this definition, we could argue that a von Neumann probe is an organism. ;-) Axl ¤ [Talk] 18:19, 2 April 2009 (UTC)
- You appear to have missed the ability to organise and metabolize which are listed later on in the article, which von Neumann probes cannot do ;) Cyclonenim : Chat 18:31, 2 April 2009 (UTC)
- I respectfully disagree. The (theoretical) von Neumann probe does indeed organize both its own structure and that of its children. It needs to "metabolize" the raw materials that it encounters using chemical reactions to maintain itself and build children. Axl ¤ [Talk] 18:40, 2 April 2009 (UTC)
- I respectfully embarrassed myself, then ;) Cyclonenim : Chat 19:25, 2 April 2009 (UTC)
- I respectfully disagree. The (theoretical) von Neumann probe does indeed organize both its own structure and that of its children. It needs to "metabolize" the raw materials that it encounters using chemical reactions to maintain itself and build children. Axl ¤ [Talk] 18:40, 2 April 2009 (UTC)
- You appear to have missed the ability to organise and metabolize which are listed later on in the article, which von Neumann probes cannot do ;) Cyclonenim : Chat 18:31, 2 April 2009 (UTC)
- Hehe! Our article begins: "an organism is any living thing...". The second sentence is (perhaps) more helpful: "organisms are capable of response to stimuli, reproduction, growth and development, and maintenance of homeostasis as a stable whole". Using this definition, we could argue that a von Neumann probe is an organism. ;-) Axl ¤ [Talk] 18:19, 2 April 2009 (UTC)
What came first, the chicken or the egg?
67.184.14.87 (talk) 08:45, 1 April 2009 (UTC)
- I guess you could say the egg since the chicken evolved from a prehistoric bird species (actually according to our article two, Red Junglefowl and Grey Junglefowl) which laid eggs but we are unlikely to call chickens. Nil Einne (talk) 08:57, 1 April 2009 (UTC)
- As I expected, this question has been discussed lots of times before (with basically the same answer), check the archives. We also have an article chicken or the egg. It gets more complicated if you ask: which came first the chicken or the chicken-egg or which came first, the bird or the egg? But since you didn't we don;t have to worry about that. Nil Einne (talk) 09:01, 1 April 2009 (UTC)
- You'd have to make that "bird or bird egg", as many egg-laying creatures lived before birds, such as insects and reptiles. Still, under every possible interpretation of the Q, the answer is still that the egg came first, as the egg is genetically identical to that which hatches from it, but not to that which laid it. StuRat (talk) 13:17, 2 April 2009 (UTC)
You might like to know that for reasons I don't understand but am happy to follow, when you're offering people a question of dilemma, it's usually rendered "Which...?". Can anyone explain why? --Dweller (talk) 09:11, 1 April 2009 (UTC)
- Because this is the current meaning of the interrogative adjective 'which', which used to be used as a fairly general interrogative, analogous to 'what', but is now restricted to (OED which 3) 'In limited sense, expressing a request for selection from a definite number: What one (or ones) of a (stated or implied) set of persons, things, or alternatives.' I don't know how these changes came about, but the OED, as ever, has many illustrative quotations. Algebraist 11:35, 1 April 2009 (UTC)
- The mother of the egg that became the first chicken was not herself a chicken. So the answer is 'egg'. —Preceding unsigned comment added by 79.75.39.108 (talk) 00:37, 2 April 2009 (UTC)
The Easter Bunny, silly people. --jpgordon∇∆∇∆ 00:41, 2 April 2009 (UTC)
Squirrel monkey behaviour
Can someone tell me if squirrel monkeys like doughnuts and use them for rituals? Simply south (talk) 11:23, 1 April 2009 (UTC)
- Rituals??? (Incidentally, it's generally a bad idea to trust random comments you hear on radio) Nil Einne (talk) 13:09, 1 April 2009 (UTC)
- I would not give them donuts. They are not good for people, I doubt they are good for monkeys. And you don't want to start a monkey cult. 65.121.141.34 (talk) 13:42, 1 April 2009 (UTC)
- Well we know squirrels like nuts,
so all we have to do is find a monkey playing with dough andand there are lots of recipes available for monkey bread, so just do some algebra and we're all set.DMacks (talk) 14:54, 1 April 2009 (UTC)DMacks (talk) 15:47, 1 April 2009 (UTC)
- Well we know squirrels like nuts,
- I would not give them donuts. They are not good for people, I doubt they are good for monkeys. And you don't want to start a monkey cult. 65.121.141.34 (talk) 13:42, 1 April 2009 (UTC)
Yes, it is true, the use them for very complicated rituals concerned with choosing a partner. It is fascinating to see and there have been many questions asked about what they used before doughnuts were invented. The really, I mean really, odd thing is that they only carry out this ritual on one day in the year, Hmm? I wonder which date that is??!! —Preceding unsigned comment added by 86.4.190.210 (talk) 18:26, 1 April 2009 (UTC)
- I believe that the scientific consensus is that the monkeys actually invented the doughnut 50,000 years ago, and that people adapted the monkey dating tool to a morning energy booster for police officers. 65.121.141.34 (talk) 18:52, 1 April 2009 (UTC)
Solar Eclipse
This is a question asked to me by my little sister and somehow i just couldnt give her a satisfactory answer... the question is... its said that a solar eclipse occours when the moon comes between the sun and the earth. But that happens so many times a year, so why don't we have that many solar eclipses each year??? —Preceding unsigned comment added by Rrkrish (talk • contribs) 13:15, 1 April 2009 (UTC)
- Because that doesn't happen many times a year. For our article solar eclipse: 'The Moon's orbit around the Earth is inclined at an angle of just over 5 degrees to the plane of the Earth's orbit around the Sun (the ecliptic). Because of this, at the time of a new moon, the Moon will usually pass above or below the Sun. A solar eclipse can occur only when the new moon occurs close to one of the points (known as nodes) where the Moon's orbit crosses the ecliptic.' Algebraist 13:22, 1 April 2009 (UTC)
- The moon may come "Between" the earth and the sun once a month in a rough sense, but in order to have an eclipse they have to be exactly lined up in a straight line. That doesn't happen often, it's usually off a little one way or the other.
- This might not be obvious on a flat drawing of the solar system, but if you consider the situation in 3d, it's pretty clear. (ie: if you draw the solar system on paper, you need to understand that not all the heavenly bodies are exactly on the paper, they might be a little above or below it.) APL (talk) 13:37, 1 April 2009 (UTC)
- Also check out this illustration showing the relative size and distance of the Earth and Moon to scale. APL (talk) 13:46, 1 April 2009 (UTC)
- The other point is that although total eclipses are fairly rare (because the earth/moon/sun system have to be lined up to a spectacularly precise degree) - partial eclipses of various kinds are about three times more common. There are typically around two eclipses per year - although in 2011, there will be four of them! The moon orbits the earth about 13 times per year - so the odds of it causing an eclipse is about one in every six times it passes roughly between earth and sun - which I think we can tell your little sister is actually "pretty common". However, you don't see them all that often because you have to be standing in the shadow of the moon to see them. Since the moon is rather tiny compared to the earth, its shadow is pretty small too - so the odds of you happening to be in the right place to see a partial eclipse is also surprisingly small. If you look at List of solar eclipses in the 21st century - you'll see that the next eclipse (in July) is only going to be total in Central and Northeastern India, Bhutan, Bangladesh, Myanmar, China and the Ryukyu Islands - if you don't live there - you won't see it (although it's also going to be a partial eclipse in Asia, the Philippines, Indonesia, Melanesia and Hawaii). SteveBaker (talk) 17:43, 1 April 2009 (UTC)
evolution or creation
why are we always taught that all the species that we see on the earth today have in some way or the other evolved from the ones that were there before them??? why aren't there any theories being taught today in schools and colleges that also stress on creation of life and different species by God, rather than by plain evolution?? —Preceding unsigned comment added by Rrkrish (talk • contribs) 13:19, 1 April 2009 (UTC)
- Assuming that's true, it's probably because there isn't any evidence supporting the creation theories. See Creation and evolution in public education for more. Zain Ebrahim (talk) 13:27, 1 April 2009 (UTC)
- Because it is not possible to prove creationism with science as an omnipotent being could reinvent the laws of science in any way to get stuff done, and science is built upon there being a set of rules that are assumed (and so far observed) to be immutable. 65.121.141.34 (talk) 13:41, 1 April 2009 (UTC)
- There are lots of theories out there about all sorts of different things. Only the ones that have strong evidence on their side get to be taught in the schools. Theories about the origin of species are no exception. Dauto (talk) 15:05, 1 April 2009 (UTC)
- In practice it is a bit more complicated than having strong evidence (see, for example, string theory—evidence is NOT the reason people think it is a good idea and why it gets taught at university levels). I think usually it is safer to just say that what gets taught in schools as "science" is that which the scientific community as a whole considers to be "science". Why they think this or that theory is more scientific than, say, another, can vary from theory to theory, field to field. --140.247.241.244 (talk) 16:47, 1 April 2009 (UTC)
- Put more practically (no epistemological assumptions needed), public schools have for a long term determined that "science" needs to be taught in "science" classes. The idea of differently, uniquely created types of species by God is not considered by the courts or the scientists to be "science". There are some who disagree, but they have not been terribly successful in convincing people of that on the whole. "Evolution" is currently the explanation for speciation that is considered to be the most in line with "science". In "religion" classes they do read Genesis though they don't necessarily claim that it is actually "what happened" more so than they would for, say, the Koran, which they'd probably also read. --140.247.241.244 (talk) 16:44, 1 April 2009 (UTC)
- You actually can be taught in public school that God created the different species, if you're willing to move to some of the most backward places on Earth. --Sean 16:58, 1 April 2009 (UTC)
- Actually - you aren't allowed to teach "God did it" in public schools in Texas - that's still contrary to the US constitution. All you're actually allowed to teach is that evolution isn't necessarily a proven theory...which is still ridiculous...but the US constitution still applies, even in the Texas public school system. SteveBaker (talk) 17:09, 1 April 2009 (UTC)
- Of course that is subject to interpretation. The constitution only says "Congress shall make no law respecting an establishment of religion", which according to a an originalist really says nothing about this matter. Sorry, this is getting a little tangential.-RunningOnBrains 17:20, 1 April 2009 (UTC)
- Why pick only on evolution? We could be trying to teach students the formula for the period of swing of a pendulum as a function of it's length - and we'd have to keep stopping and saying "Unless God just decided to make up the evidence to make it seem that this is how a pendulum swings in order to test our faith". But then, that's just the christian god and the christian creation myth - in the interests of open-mindedness and skepticism - wouldn't we also have to teach the theories of all of the List of religions and spiritual traditions (check out that list...it's VERY long!). If we opened up the teaching of evolution to include creationism - wouldn't we also have to teach that the universe was farted out of the butt of a camel (or whatever it is that other people believe)?
- The problem is that to teach science - you have to teach "The Scientific Method". That method says that all of science is the result of application of the Hypothesis/Experiment/Theory approach. The problem with creationism and intelligent design is that they quite utterly fail to use that approach. Hence they cannot be taught as "Science" because they really aren't at all scientific. But in the end - we teach evolution because it's true. We don't teach the other things because they are clearly a pile of steaming crap that doesn't stand up to the slightest scientific investigation! Frankly - if I were interested in pushing a religious POV - the very last thing I'd want would be to have scientists telling our children about it! They are going to have to teach that the creationism myth has no evidence leading toward it - that no experiments have been done to back it up - that there are a million pieces of rather impressive evidence that it's NOT true - that, far from answering the question of how all of this happened - it just creates another question ("How did God originate?"). Personally - I'd think you'd probably do better to avoid that happening and stick to teaching your poor kids all of this crap in the privacy of your own home/church/mosque!
- Another problem: There are a literal infinite number of unprovable things that we could teach (See Russels teapot. If we tried to teach even 0.000001% of them - we'd have no time left to teach students what they actually need to know to become working scientists - to earn money - to make breakthroughs and generally be scientists. If a kid tries to get a job as a working scientist and it comes out in the interview that they don't consider evolution to be true - then they really aren't going to get jobs. That's not a matter of discrimination - it's a matter of survival in a modern science-laden world. There is simply no need to teach this other stuff in order to get the job done. If the religious fanatics out there need to push this stuff - then let them do so - but please - not while we're trying to teach teach kids science. SteveBaker (talk) 17:09, 1 April 2009 (UTC)
- Sean, what evidence do you have that creation is commonly/usually taught as science in Iranian schools? While religious education is a compulsary part of education in many Islamic countries, including Iran and I presume the creation idea is taught there, my understanding, supported by our Islamic creationism and Creation–evolution controversy#Islamic countries is that the evolution-creation controversy has largely passed over the Islamic world until recently. The controversy appears to have started to take hold, particularly in Turkey. Our article mentions evolution is taught in Egypt, but banned in Sudan and Saudi Arabia (it doesn't however say that creationism is taught as science in either schools). Iran is not mentioned. Iran is not mentioned. However from [6] and [7] while not entirely clear, it appears to me that evolution is accepted and may in some instances be taught, provided it doesn't get to the point where it's considered in opposition to the Islamic teachings. It's definitely not clear that creationism is taught as science in Iranian schools. (Actually my gut feeling is that potentially nothing is taught). Nil Einne (talk) 10:23, 2 April 2009 (UTC)
- Iran was not mentioned twice ? :-) StuRat (talk) 12:32, 2 April 2009 (UTC)
- Sorry I striked that Nil Einne (talk) 17:33, 14 December 2009 (UTC)
- Iran was not mentioned twice ? :-) StuRat (talk) 12:32, 2 April 2009 (UTC)
- Steve, you seem to be passionate about your arguments, but the fact that creationism is not scientifically provable does not translate it into being a complete pile of crap. It just means that it can not be proven scientifically. Science does not have all the answers, no matter how much some may wish it did. The key difference is that some think that science CAN get all the answers eventually, and others think that humans are rather arrogant to think that they can eventually understand and explain every phenomenon in the universe, past present and future. 65.121.141.34 (talk) 18:26, 1 April 2009 (UTC)
- No - that doesn't really hold water.
- Either...
- You have to look at:
- All of those neatly arranged fossils - dated and arranged in timewise order, they show a clear progression of gradual change.
- The DNA evidence for common ancestry - all living things share large chunks of DNA in common - and where they differ, that follows the evolutionary chain that the fossils provide (eg Human DNA is more like Chimp DNA than Dog DNA - but all mammal DNA has more in common than all fish DNA).
- The demonstrable evolution of things in modern nature such as antibiotic-resistant bacteria in hospitals or warfarin tolerant rats in big cities - butterflies on remote islands, Darwins finches...you name it.
- The ability to demonstrate evolution in simulation and in laboratory experiment - if you set up a computer program that has creatures with simulated DNA, simulated mutation, simulated survival-of-the-fittest - then your synthetic creatures evolve just like real ones. If you take a bucket of bacteria and sequence their genes - then stress them with some toxin over many generations - then after a week or two, do a gene test on the survivors - then you can find the gene that mutated...the bacteria evolved - right there in front of your eyes.
- and then, you must seriously ask: Does your theory have an explanation for ALL of these well-established easily demonstrable facts? (Evolution does - creationism certainly does not) - if it does not - then it's disproved because it doesn't explain the facts. Disproved is a lot stronger than "Not proved". If creationism were true then we would have human fossils of the same age as dinosaur fossils - and we don't...not even close. The efforts creationists take to weasel their way around these problems are flat out laughable in their lack of scientific rigor.
- You have to look at:
- ...OR...
- You have to resort to the purely religious approach of saying "God put all of these bizarre things like fossils and DNA and warfarin-tolerance into the world in order to make weak-willed people believe in evolution as a test of their faith"...or something like that. In so doing - you are heading down a dark and dangerous path. Every time anyone raises any objection whatever to your theory you are now forced to say "God made it that way to test you - logic doesn't apply here". You can do that - and science certainly can't prove it's not true - but in so doing, you've shed any hope of a logical outcome to the debate - and you head down a road where you can't reason about the universe anymore. You can't build a bridge without having no clue whether it'll fall down or not - because you can't trust your math and materials science anymore...maybe God just put those there to test our faith too? What you have is religion, belief, faith and all that goes along with it...and you can't teach that in US public schools - because it is (quite rightly) illegal. In most school systems around the 'civilised' world, that kind of thing is moved out of science classrooms and into 'religious instruction' classes because parents of non-christian children have a right to NOT have their children indoctrinated by a bunch of raving loonies (as we atheists see you guys...let's be quite clear about that!). In the worst school systems in the world, religion gets taught to the exclusion of science - and I'd like for you to pause and look at the quality of life in those places - and ask yourself whether that's really where you'd like to go.
- If you go with the former approach then the failure of creationism and the success of evolution to explain those things - AND have the predictive power that all good scientific theories should have - means that it is (objectively) a far less viable theory than evolution - and it should therefore be shelved and largely ignored...just as we have given up on the steady-state theory of the universe in favor of the Big bang because the former cannot explain the nature of the cosmic microwave background - and just as the big-bang theory may one day have to be shelved when we figure out what 'Dark energy' actually is.
- If you go with the latter approach - then you have created for yourself an "unfalsifiable" theory - which means that science MUST ignore it because unfalsifiable theories are as firmly disallowed as dividing by zero in mathematics (and, interestingly, for similar reasons!).
- So this is not simply a matter of "not yet having all of the answers" - it's a matter of having two competing theories - one of which fits the facts elegantly and perfectly - and the other of which either contradicts the facts and is therefore false - or is unfalsifiable and therefore not worthy of further consideration. Either way - evolution wins...just as the big bang wins over steady state and the periodic table of elements beat out the earth/air/fire/water theory of matter. When a theory is useless - we put it back on the shelf, stop teaching it to our kids and we move on. And that's PRECISELY what's happened here with creationism - it had a good run of several thousand years - but now it's as obsolete as flat earth theory, heliocentricism, astrology, etc. Of course there are always (and should always be) people who continue to defend the old theory for a while - we need a measure of skepticism. But there comes a point (and we're certainly WELL beyond that with evolutionary theory) where the evidence is so totally overwhelming that it's time to put creationism away along with lysenkoism and the theory that mice are created from bits of cheese and old clothing. Without some kind of evidence of ANY kind - it's simply crazy to keep beating that dead horse.
- If you maintain that creationism "can not be proven scientifically" - then you are in the 'non-falsifiable' camp - and science simply doesn't teach non-falsifiable things. SteveBaker (talk) 20:39, 1 April 2009 (UTC)
- Thank you Steve, you completely made my point. You can not disprove that which is not scientific, so that is why it is not taught in a science class. As an aside though, creationists don't usually go with God creating fossils and leaving them around fo people to find. Most of them believe that the geological record is not as constant as the majority of scientists believe and that the great flood found in the Bible buried them.65.121.141.34 (talk) 20:54, 1 April 2009 (UTC)
- Actually, Steve did an excellent job disproving creationism as it contradicts all the available evidence, unless you want to subscribe to the idea of dishonest god(s) who plant fake fossils, etc. to trick mankind. I guess you can't disprove those god(s). But I'm not aware of any religion that actually subscribes to such a view so I guess one would have to invent one. A Quest For Knowledge (talk) 12:14, 2 April 2009 (UTC)
- Yes. My point exactly. You either use logic to argue that creationism is possible - and find that science beats your crappy theory into a small mangled splat on the sidewalk of life...or you loudly assert that God can make fossils if he wants to - and that's that. I have met fundamentalist christians who first start down the "logical argument" path with me - and then when I actually have them cornered and convinced that they've run out of arguments, they ALWAYS fall back on the "Well, God just made it that way" argument. Typically this comes about roughly when you explain precisely WHY the dinosaurs couldn't have died out in the same flood that killed the Neanderthals and floated Noah's clearly impossible boat. Isaac Asimov said that violence is the last refuge of the incompetent. I think he was wrong about that - it's religion. When your brain is too tiny to accept all of the evidence with an open mind - and reason your way through it - it's just easier to sit back and say "God did it" in response to every difficult question. SteveBaker (talk) 14:02, 2 April 2009 (UTC)
- Yeah - and that's just typical. They make that statement and then walk away without listening to the scientist's response. Firstly, if these prehistoric creatures all died at the same time and been buried in some amazing flood - then they wouldn't be found in rock strata organised by age. We find all of the early fish in one layer - all of the creatures that just climbed out of the water and onto the land in another layer on top of that - dinosaurs of various kinds in more layers on top of that - larger mammals on top of that. You can find all sorts of mineral deposits that let you see how the layers of compressed silt are separated by things like lava flows. You can see that those lava flows had then been eroded by thousands of years of wind and water - then more fossils in layers on top of that. This doesn't in any way fit with one single mass-extinction event. With more recent species we can do radio-carbon and other radioactivity datings. There are literally DOZENS of ways to disprove that crazy theory. However, the nut-job creationists don't want to hear any of that. As I said - if they submit themselves to the processes of scientific tests, logic and explanation - their theory evaporates - it's a joke. Only if they resort to "Because God did it" - can they escape that conclusion - and at that point, this is religion - and it doesn't belong in science classes. SteveBaker (talk) 00:38, 2 April 2009 (UTC)
- There are versions of Creationism which purport to be as "scientific" as the real "science". They purport to have lots of evidence. Institutionally trained scientists almost always disagree with their evidence and disagree with their assertion that they practice the scientific method. Creationists can point quite validly to a large body of literature about scientific practice which has shown for some decades now that 1. scientists are not a "scientific" as they claim to be in such debates, 2. the line between "science" and "non-science" is often in the eye of the beholder, and 3. there really is no consistent "scientific method" that holds true for all fields of what is considered to be "science". Nobody is arguing about teaching everything (there aren't the resources)—the question is, with limited resources, what to teach? The Creationists believe there are good reasons to teach Creationism that go beyond simple careerism (in part because if the educational system changed dramatically the careerist approach would not be relevant—if everyone learned Creationism then it wouldn't be a way to sort it out). Now I'm no Creationist in the slightest but I don't think the debate is helped by appeals to a single Science with its singular Method and its unfailing adherence to Evidence and all of these concepts that are trotted out despite being quite problematic both in practice and in theory. It ends up being an argument against a straw man and a false dilemma when formulated in such terms. I think it's a lot easier to take an almost Wikipedia approach to it: in schools, we generally agree that in "science" classes "science" should be taught. The line between science and non-science should not be mediated by civilian school boards who don't have the necessary training or broad view in order to decide what the long-term consequences of such a decision would be. The most appropriate place for that sort of discussion to take place is probably with the established scientists who have brought us all those good things that we like about science enough to teach it anyway (which we can claim is about a love of knowledge, but in this society the number one reason we want institutionalized science training is because 1. science affects our world in massive ways, and 2. one of the main outgrowths of science is technology). So if you want to teach Creation "science" in schools, you've got the convince the scientists first that it is real science! --140.247.241.244 (talk) 17:21, 1 April 2009 (UTC)
- From the scientist point of view the Evolution vs Creation is a very boring debate. The creation side has nothing going for it. No evidence at all. None. Zip. Nill. Zero. That makes the decision wheather to teach creation or not a very trivial matter indeed. Dauto (talk) 01:44, 2 April 2009 (UTC)
- Let's consider what happens if Biblical Creationism were to be taught as science. In addition to being taught as an alternative to Evolution, there also would need to be Biblical alternatives to teach plate tectonics and geology, as the current scientific approach requires the Earth to be far older than the Bible would indicate. Similarly, the rate of nuclear decay would need some alternative explanation in chemistry class, to prevent carbon dating, etc., and the speed of light would need to be altered in astronomy class to allow light to reach us from stars very far away in a universe that's not old enough to permit that. Moving on to social studies, we would need to move the first humans from Africa to "Eden", wherever that is, and make all languages form at once (tower of Babel), rather than when they actually did form. And, if only God can create different languages, and does so to confuse mankind, we need an explanation for why languages change over time, or else we need to deny that they do, and insist that Shakespeare was using the exact same words that we use now. If great floods and other natural disasters are also used by God to punish people, we need a class that teaches exactly what sin each natural disaster was created to punish, and how we can therefore prevents disasters by repenting rather than building levies, clearing flammable underbrush, etc. StuRat (talk) 12:58, 2 April 2009 (UTC)
- The folks who are writing the new Texas public school science books are here - taking notes - and would like to personally thank StuRat for this comprehensive guide to how they should be updating the 2010 editions. Awesome - excellent - thank you!
- There is no doubt that once you start to doubt the findings of mainstream science - it's hard to stop with evolution. As I explained before - you get to a point where no scientific explanation whatever can be considered entirely valid - and at that point, you can't do engineering anymore. If you want to build a bridge - you can't trust materials science - so your calculations on the stresses involved are essentially worthless. But that's OK because when the bridge collapses, you can just say it was "The will of God" - and there was just nothing anyone could do. I always find it amusing that property insurance covers "Acts of God"...I always wonder whether they should take into account my blameless and relatively sin-free life when setting the premiums!
- For me, personally, God (and gods in general) are precisely as believable as Santa Claus, the Easter Bunny and the Tooth Fairy. Seriously basing how I teach science on the premise that somewhere there are flying reindeer and a sleigh that can deliver toys to all of the children of the world in one night - is PRECISELY as ludicrous as teaching creationism. There is truly no distinction in the scale of lunacy involved. I'm pretty sure that almost all religious people would be very upset if teachers in public schools started teaching their children that Santa Claus was real and that they WILL be getting free stuff delivered down the chimney on Xmas eve - and that the Tooth Fairy literally does come in the middle of the night and swap out that tooth for a nickel. They should consider my (identical) position when (as an Atheist) I demand that they do not inflict their pile of steaming bullshit on my child. SteveBaker (talk) 13:48, 2 April 2009 (UTC)
- I can comprehend your viewpoint without the need for profanity. I request that you strike it from the record and refrain from such comments in the future. There may be children present. 65.121.141.34 (talk) 18:57, 3 April 2009 (UTC)
- Two problems with that:
- As a matter of policy Wikipedia does not censor for the benefit of children or otherwise - and I think children (even quite religious ones) are completely aware of what 'steaming bullshit' is - it comes out of bulls - and it steams. And just in case they don't, I should explain that it's "moo-cow poop" kiddies!
- The entire concept of "profane language" is meaningless to an atheist. The definition of 'profane' (according to Wiktionary) is:
- Unclean; ritually impure; unholy, desecrating a holy place or thing.
- Not sacred or holy, unconsecrated; relating to non-religious matters, secular.
- Treating sacred things with contempt, disrespect, irreverence, or undue familiarity; blasphemous, impious. Hence, specifically; Irreverent in language; taking the name of God in vain; given to swearing; blasphemous; as, a profane person, word, oath, or tongue.
- I'm fully in favor of verbally desecrating things that are complete nonsense. I'm more than happy to be 'non-sacred' and 'un-holy' and I most certainly do intend to continue to treat "sacred things" with contempt, disrespect...etc. I do not intend to give religion of any kind any respect whatever - it's nonsense and I intend to continue saying that because to do otherwise would be dishonest - and I value honesty. Hence profane language is a goal - not something to apologize for. SteveBaker (talk) 21:05, 3 April 2009 (UTC)
- Two problems with that:
- I can comprehend your viewpoint without the need for profanity. I request that you strike it from the record and refrain from such comments in the future. There may be children present. 65.121.141.34 (talk) 18:57, 3 April 2009 (UTC)
Some might claim that the scientific explanation of the origin of the universe is equally ludicrous considering there is no explanation given for where the energy to create the big bang originated, especially considering that some scientific principals Conservation of energy, Conservation of mass state that neither energy nor mass can be created or destroyed. If there is an explanation for the spontaneous generation of energy and mass from nothing using current scientific laws, I would be most interested in reading it. 65.121.141.34 (talk) 16:16, 2 April 2009 (UTC)
- If that bothers you - then you don't understand the theory. The big bang created spacetime as well as all of the matter. There is no "before" because time itself starts at the big bang. That's not just a 'theological matter' as you'd say if I asked where God came from - it's actually what the math and the physics say must have happened given the evidence that is everywhere around us. And it's an elegant solution because it answers the entire set of questions that you're asking. May I recommend reading "A brief history of time" - by no less than Stephen Hawkins himself. It's a short and easy read and it covers this exact point rather well in language that the layman can understand. SteveBaker (talk) 20:26, 2 April 2009 (UTC)
- But certainly the idea is correct, that neither science nor religion can completely explain everything. We get down to "did God always exist or was he somehow created ?" or "what are strings made of ?". You could always push the question back a bit farther, by creating an earlier race of gods or explaining what strings are made of, but there's always another "then what caused that ?" question to be added. StuRat (talk) 17:30, 3 April 2009 (UTC)
- The fact that there are no current scientific explanations for some things does not invalidate the scientific explanations that do currently exist. The one disadvantage that Science has in arguments with Religion is Science's willingness to say "I don't know" in response to the question "Why?"
- Of course, that is ultimately to Science's advantage, as it then toddles off to find the answer. - EronTalk 16:45, 2 April 2009 (UTC)
- It's not even that - certainly, when presented with something we don't know, we say "Hold on while I find out" (which might take an hour or perhaps a century or two). But more importantly, when we do find that one of our theories is proven wrong, the overwhelming response is "OH! COOL!". We love nothing more than to have our world overturned by something freakishly unlikely that has somehow gotten strong evidence for it. This doesn't happen when creationism challenges evolution because it doesn't overturn anything - it just turns out to be wrong. But when (for example) the rate of change of speed of the Voyager probe as it leaves our solar system is not what we expect - we don't try to hush it up because it messes with out theories - we leap out there and excitedly start looking for seemingly bizarre explanations and things that might point to new forces or a new understanding of gravitation. The most exciting moments for most scientists are when experiments don't come out the way we expect - because that's when we learn something new. SteveBaker (talk) 21:16, 2 April 2009 (UTC)
Hot water for drinking
Why don't we like warm water for drinking, whereas it is very pleasurable to use warm water while taking shower and warm food to eat? Is there some role of evolution? - DSachan (talk) 14:26, 1 April 2009 (UTC)
- Because we associate warm-water with standing-water. I would expect it is quite cultural rather than evolutionary. We associate ice-cold water with freshness/quality (as in drinking water quality) - we associate tepid water with standing/stale water, and we associate hot-water with hot-drinks, though plenty of people drink hot-water as a drink (at least where i'm from). 194.221.133.226 (talk) 14:56, 1 April 2009 (UTC)
- I know a lot of people who drink hot water, in preference, say, to tea or coffee. (And I don't associate warm water with standing water.)--Shantavira|feed me 15:52, 1 April 2009 (UTC)
- Mind your "we"; it's totally cultural. In Great Britain, if you ask for "a glass of water", you will most likely get a glass of room-temperature water. You have to ask for ice specifically, and if they have any you'll get it, but it's considered a slightly special request. —Scheinwerfermann T·C16:03, 1 April 2009 (UTC)
- Where, here in the US, you typically get water with ice in it - whether you want it or not - and it may be a major struggle to NOT have ice in your Coke when you go to some fast-food place. SteveBaker (talk) 16:45, 1 April 2009 (UTC)
- Most of the taste of pure water comes from dissolved oxygen, and warm water has a lot less than cold water. Looie496 (talk) 18:04, 1 April 2009 (UTC)
- I'm not sure about Schein's comment - generally if you ask for a glass of water in the UK the host will run the tap until the water is cold (i.e the water in the the house's pipes has been purged and the water is fresh from the main) - while not ice cold it's certainly colder than room temperature. Exxolon (talk) 19:34, 1 April 2009 (UTC)
Are we talking about hot water say 45C which is disgusting, or warm water, say 25C which is fine? Also, I have noticed that if you boil water and then cool it, it still tastes funny. —Preceding unsigned comment added by 65.121.141.34 (talk) 19:11, 1 April 2009 (UTC)
- That follows naturally from Looie496's comment - when you boil water, you drive out the dissolved oxygen - so afterwards it tastes 'flat'. SteveBaker (talk) 19:53, 1 April 2009 (UTC)
- The concept is likely much more than cultural; clearly people all over the world from various times and places prefer hot or cold water to tepid water. Consider this 2000 year old text; "I wish you were either one or the other! So, because you are lukewarm—neither hot nor cold—I am about to spit you out of my mouth." The original Greek uses the word for "Vomit" in the place of "spit out"... Clearly tepid water as disgusting was a well known concept; otherwise this analogy would have not been so easy... --Jayron32.talk.contribs 21:56, 1 April 2009 (UTC)
- Although many Christians would like to believe the bible is universally applicable, I'm not convinced that its use here indicates anything other then it being applicable to the culture of those who wrote it. Also in terms of the cooled hot water, it is common practice and often recommended to boil your tap water before drinking. So I used to drink cooled or cold tap water all the time and to be honest I've never noticed anything odd about the taste. Nil Einne (talk) 09:55, 2 April 2009 (UTC)
- I've been told that vigourously shaking water after boiling it will re-oxygenate the water and eliminate the "flat" taste. - EronTalk 16:46, 2 April 2009 (UTC)
- Although many Christians would like to believe the bible is universally applicable, I'm not convinced that its use here indicates anything other then it being applicable to the culture of those who wrote it. Also in terms of the cooled hot water, it is common practice and often recommended to boil your tap water before drinking. So I used to drink cooled or cold tap water all the time and to be honest I've never noticed anything odd about the taste. Nil Einne (talk) 09:55, 2 April 2009 (UTC)
- Maybe cold water (with ice) is less favored in Britain because it has a colder climate? All but the northernmost parts of the US are pretty hot in summer. 128.194.103.37 (talk) 13:27, 5 April 2009 (UTC)
schrodinger's time independent equation
in the relation of time-independent schrodinger's equation
∆^2Ψ + 2m/(ħ^2)[E-V]Ψ = 0 -(1)
there appears a "E" in (E-V),which is kinetic energy of the particle. but if we take the kinetic energy of any particle executing some non-uniform motion,how can be the kinetic energy independent of time? since kinetic energy of any particle can be expressed as
E=1/2(mv^2);
where,
m = mass of particle
v = velocity of particle
if a particle executes non uniform motion its velocity and hence kinetic energy will depend upon time and the the whole equation seems to be dependent on time.
for a particle, its total energy E(total) can be expressed as sum of its kinetic energy as well as potential energy,which is independent of time.
Et = E + V
where,Et = total energy of the particle
v= potential energy of the particle
so, E = Et-V -(A)
Making this substitution in the schrodinger's equation, we get
∆^2Ψ + 2m/(ħ^2)[Et-2V]Ψ = 0
this equation is totally independent of time, making schrodinger's expression independent of time —Preceding unsigned comment added by Sachin nishchal (talk • contribs) 17:15, 1 April 2009 (UTC)
- If you have seperated the time-dependent Schroedinger equation then the E which appears in the time-independent equation cannot depend on time. So, I'd say this rules out from the start a lot of what you go on to say.
- E in the time-dependent equation is the total energy. Not the 'kinetic part' as I think you suggest.
- Due to the subtleties of what can be known simultaneously (a la Heisenberg), it's not true that you can seperate the energy into a kinetic part and a potential part for most systems. (In light of this, how would that affect your definition of E in terms of v?)
- I realise I wasn't comprehensive but there are some comments to get you started at least. 86.140.160.93 (talk) 19:56, 1 April 2009 (UTC)
Yes, indeed the 'E' that shows up at Schroedinger's time independent equation is the total energy. Dauto (talk) 02:49, 2 April 2009 (UTC)
Genetics Question
A brown-eyed man whose mother is colorblind and whose father had blue eyes is engaged to marry a woman whose colorblind mother had blue eyes and whose normal visioned father had blue eyes. What is the Genotype of the young man? Of his fiancee? If they marry and have children, what are the chances of having a brown-eyed, normal visioned child? A blue-eyed, colorblind child? A brown-eyed normal visioned daughter? A blue-eyed, colorblind son? —Preceding unsigned comment added by 198.85.212.10 (talk) 17:17, 1 April 2009 (UTC)
- I added a section header for you. APL (talk) 17:29, 1 April 2009 (UTC)
- Looks like a homework problem, and we don't do those. Looie496 (talk) 18:09, 1 April 2009 (UTC)
- Isn't colour-blindness a male trait, so this is an unusual situation to find yourself, likewise eye colour isn't based on a single gene... But nevermind, read the through the question and draw a family tree, although I think you probably need more infomation. —Preceding unsigned comment added by MedicRoo (talk • contribs) 18:32, 1 April 2009 (UTC)
- Colorblindness is mostly male because the most common form is X-linked recessive. Looie496 (talk) 18:41, 1 April 2009 (UTC)
- (ec) No, color-blindness is (most commonly) a recessive trait on the X chromosome, meaning it is apparent more often in males (since they don't have a second X chromosome to "override" the trait). This question has "homework" written all over it—specifying the mothers are colorblind is a convenient way to fully-indicate their genes ("normal-vision" could mean they have 0 or 1 colorblind gene). To solve it, consider each "trait" (colorblindness, eye color) independently, find the genes of the parents, their children, then prospective offspring. Your book will most likely have example problems similar to this one. If you get stuck, explain where you're at and what you're having problems with and we might be able to help, but we won't do your homework for you. – 74 18:54, 1 April 2009 (UTC)
- Briefly, your teacher apparently wants you to assume that eye color is determined by a single gene (not true, but I guess close enough for government work) at which "brown" is dominant and "blue" is recessive, and also wants you to assume that the kind of color-blindness he's talking about is X-linked recessive.
- With these assumptions:
- A brown eyed person with a blue eyed parent must have inherited a blue allele from that parent, and a brown from the other.
- A person with a color-blind mother must have inherited her color-blindness gene, because she must have it on both X chromosomes or she wouldn't be color-blind; a man only has one such gene, so all her sons will necessarily be colorblind. A woman with a color-blind mother inherits a color-blindness gene from her, but also inherits a gene from her father, and in this case, he's not color-blind. So the financee is heterozygous for color-blindness.
- A person with blue eyes necessarily is homozygous for "blue" eyes (the information about the fiancee's parent's eye color is unneeded; you know her genotype because you know her phenotype, because only one genotype produced that phenotype. You'd need to know the parent's eye colors if her eyes were brown, because more than one genotype produces that phenotype.)
- So, using BLUE/BROWN and CB (for colorblind) and N (for not-color-blind), and - for no gene at all:
- groom = BLUE/BROWN; CB/-
- bride = BLUE/BLUE; CB/N
| BLUE | BROWN | |
|---|---|---|
| BLUE | BLUE/BLUE = blue | BLUE/BROWN = brown |
| BLUE | BLUE/BLUE = blue | BLUE/BROWN = brown |
- If you make a Punnet square, you will see that you would expect half the couple's children to be blue-eyed, and half to be brown-eyed.
- The colorblindness question is probably most easily explained if you consider where a person's X chromosome comes from. Men have one X chromosome, and it comes from their mother. So all sons of this couple will be color-blind. Women have two X chromosomes. One of them is from their father, and the other is a mixture of their mother's X chromosomes. Since one gene is enough for normal color vision, and she is certain to get one of these from her father, all daughters of the couple will have normal color vision.
- To work out the chances, you have to realize there are only two independent traits of the three: eye color is independent, color vision is dependent on sex. All daughters will have normal color vision; all sons will be color blind.
- So, the chances of any particular child being:
- a brown-eyed, normal-visioned child: 50% chance of brown eyes, 50% chance of being female => 25%
- a blue-eyed, colorblind child: 50% chance of blue eyes, 50% chance of being male => 25%
- a brown-eyed, normal visioned daughter: 50% chance of brown eyes, 50% chance of being female => 25%
- a blue-eyed, colorblind son: 50% chance of blue eyes, 50% chance of being male => 25%
- The chance of:
- a brown-eyed, normal-visioned son: 50% chance of brown eyes, 0% chance of being male with normal vision => 0%
- a blue-eyed, colorblind daughter: 50% chance of blue eyes, 0% chance of being female with colorblindness => 0%
- So, the chances of any particular child being:
- - Nunh-huh 11:30, 2 April 2009 (UTC)
- To work out the chances, you have to realize there are only two independent traits of the three: eye color is independent, color vision is dependent on sex. All daughters will have normal color vision; all sons will be color blind.
- I thought we weren't supposed to answer homework questions here... 74 already gave enough information to get the right answer. That being said, I would agree with the genotypes given by Nunh-huh above, and the eye color calculations are correct, but the color-blindedness conclusions are wrong. It was correctly stated that the man in this relationship is heterozygous for blue/brown eye color and hemizygous for the colorblind mutation on the X chromosome (i.e. he's affected, but the questioner wanted you to deduce that from the fact that his mother is colorblind). It was also correctly stated that the female in this relationship is heterozygous for the colorblind mutation because her father was colorblind and she is therefore an obligate carrier.
- Here is where the problems start: "Men have one X chromosome, and it comes from their mother. So all sons of this couple will be color-blind" - only if the mother is affected. In the couple we're talking about here, the mother is a carrier and hence has a 50/50 chance of passing the colorblind mutation to each child. Therefore, all male offspring (who inherit their father's Y chromosome) have a 50% chance to inherit the colorblind mutation from their carrier mother and thus be affected and a 50% chance to inherit the normal copy of the gene and thus be unaffected.
- "Women have two X chromosomes. One of them is from their father, and the other is a mixture of their mother's X chromosomes. Since one gene is enough for normal color vision, and she is certain to get one of these from her father, all daughters of the couple will have normal color vision" - only if the father has normal vision. In this example, he's colorblind and therefore all of his female offspring MUST inherit the colorblind mutation on his X chromosome. However, since the mother is ALSO a carrier, they have a 50% chance to inherit the colorblind mutation carried by their mother, making them homozygous for the colorblind mutation and thus affected, and a 50% chance to have inherited the normal copy of the gene from their mother and thus be unaffected but carriers of the mutation.
- "To work out the chances, you have to realize there are only two independent traits of the three: eye color is independent, color vision is dependent on sex" - well, sort-of... eye color in this example is an autosomal trait and colorblindedness is X-linked. Color vision depends on the number of X chromosomes you have and whether or not you have any normal copies of the color vision gene. It is possible for a female with Turner syndrome (i.e. only one X chromosome) to be colorblind if she inherits a mutation of the colorblind gene from her mother, and it is possible for a male with Klinefelter syndrome (i.e. XXY) to have normal vision if he carries one mutated copy and one normal copy of the gene. Granted, these are extreme examples but they illustrate the point. "All daughters will have normal color vision; all sons will be color blind" Not exactly. The question posed by the OP's presumed homework question is also another extreme example meant to illustrate a point. In this case, the sex of the offspring doesn't matter in terms of the % chance of having an affected child (50%) because both parents have a copy of the colorblind mutation.
- I'm sure the OP can multiply the probabilities together to get the correct answers to the last parts of the question. Hopefully working through the problem will help the him/her understand the concepts rather than just spitting back answers provided by strangers on the internet... --- Medical geneticist (talk) —Preceding undated comment added 22:41, 2 April 2009 (UTC).
An extraordinary delusion
Does there exist a delusion in which the sufferer believes himself or herself to be the same person as another specific individual(say, a celebrity), and living a double life? Like the Fregoli delusion except that the delusional person identifies himself as one of the "imposters". 69.224.37.48 (talk) 18:04, 1 April 2009 (UTC)
- Do you mean something like Syndrome of subjective doubles? 152.16.16.75 (talk) 02:00, 5 April 2009 (UTC)
Nicotine concentrations of cigars?
Hi all. I am trying to find a page.. somewhere that gives the nicotine concentrations of different cigar brands, but I am not having any luck.. most of my searches lead me to nicotine concentrations of cigarettes, or FAQ's about cigar smoking.
Much help appreciated ! 98.242.86.119 (talk) 18:13, 1 April 2009 (UTC)
- (Subscription required to access the article) This study "examined characteristics relating to nicotine delivery of 17 cigar brands..." 152.16.16.75 (talk) 23:36, 5 April 2009 (UTC)
Chemistry and lock?
16:41, 8 April 2009 (UTC)bsm (=bimellahalrahmanalrahim) We must draw a plan for hacking door`s look that relate to chemistry any way, at least a little relation. we saw very ways but there are physically, completely. if there is any way or starting point?80.191.15.10 (talk) 16:41, 8 April 2009 (UTC)
Chlorine gas
Will chlorine gas react with water vapor to create HCl? 65.121.141.34 (talk) 19:28, 1 April 2009 (UTC)
- I'm not sure if this applies to gaseous water, but yes in general: [8] Cyclonenim : Chat 19:53, 1 April 2009 (UTC)
Adding impedances
I'm not sure if this question is in the right category, but there isn't a "tech" category.
I am wanting to build a speaker cabinet for my guitar amp head. The head has an output impedance of 16ohms - but the speakers I am looking at have an 8ohm input impedance. I know I could add an 8ohm resistor in series with the speaker, but I don't want to lose any of the power.
How do I connect two 8ohm speakers together so that they become one input with a 16ohm impedance?
I think that if I wire both "hot" wires from the speakers to the "hot" of the input, this would be them connected in parallel - which I think (only think) will half the overall impedance.
Also, what is the equation to work out what frequency a value of a capacitor will allow an AC signal through? This isn't worded very well, but I am trying to figure out how I could divert the higher frequency signals to a tweeter, mid range to a mid speaker and low frequency to a bass speaker. I know this may screw up the impedance calculations - and if so, I may not bother with it - but could you let me know it anyway.
Any help is appreciated! —Preceding unsigned comment added by 81.153.145.76 (talk) 20:02, 1 April 2009 (UTC)
- Resistances and impedances add in series—"cold" on the first speaker connected to the "hot" on the second, with the remaining wires connected to the respective source lines. On the second part, you want a high-pass filter, band-pass filter, and low-pass filter. You could construct them yourself, but they generally aren't all that expensive. – 74 21:58, 1 April 2009 (UTC)
- Audio crossover should give some help on the second point —Preceding unsigned comment added by 79.75.13.2 (talk) 22:21, 1 April 2009 (UTC)
Thanks for the tip on connecting the cold on speaker 1 to the hot on speaker 2. I just remembered some stuff I forgot to ask - What could I do to up the impedance with only one 8ohm speaker? Can I use a ferrite toroid(inductor) instead of a resistor? And how do I measure the impedance? Can I use the resistance setting on my multimeter? —Preceding unsigned comment added by 81.153.145.76 (talk) 16:00, 2 April 2009 (UTC)
- It would be easier to get a different amplifier, or modify the one you have. With the current technology speakers cost more than amps. If you promise to only go to half power you should be able to run your 8 ohm speaker from your amp with out anything blowing in the amp. An ohm meter will not measure the impedance of your speaker, as it is actually working at audio frequencies. When the speaker is driven to move by the power applied, it gives a back EMF, so that not all the power goes to heating the resistance, but instead goes into moving the cone back and forwards. You will need to measure using an AC source. In practice your meter probably cannot do that, so you may just have to use the meter in AC measurement mode, connect it to an amp and play something with a constant volume (say an electronic organ note), then measure the AC current and the AC voltage. Graeme Bartlett (talk) 20:34, 2 April 2009 (UTC)
- I believe you want to use a 2:1 transformer, not an inductor or resistor. 75.62.6.87 (talk) 22:38, 5 April 2009 (UTC)
Ancestry
http://en.wikipedia.org/w/index.php?title=Wikipedia:Reference_desk/Science&action=edit§ion=46 What is the approx. percentages that there might be Eqyptian or Mongolian or African ancestors in my family tree if both my parents are the everyday US causasian people? They are not sure but one parent says they have Irish great parents and the other parent says English Canadian great grandparents. I think it's facinating when someone says that they have an exotic ancestor. --Reticuli88 (talk) 20:17, 1 April 2009 (UTC)
- Depends from where you start, Human are meant to have originated in Africa about 200,000 years ago, so everyone had 'African' ancestry. From there people have travelled around a bit and as 0.5% of the worlds population are descended from Genghis Khan[9] it is entirely possible if you had any Asian ancestry it is possible you would have a Mongolian blood. In that way everyone has exotic ancestry, probably best to do a bit of research into your family tree. Irish ancestry tends to be a heady mix of Viking, Anglosaxon and Norman, but a lot of migration has occured over the years so that itself doesn't mean that much... MedicRoo (talk) 20:34, 1 April 2009 (UTC)
- Irish ancestry would surely suggest predominantly Celtic descent before those other possibilities you mention. --ColinFine (talk) 23:54, 1 April 2009 (UTC)
- You might find the Passing (racial identity) article interesting. Grantus4504 (talk) 09:38, 4 April 2009 (UTC)
Strength of human hair
What is the tensile strength of human hair and can it be made higher by using certain hair products? Also, what does the phrase 'stronger feeling hair' mean? Ive seen this on a TV advert. —Preceding unsigned comment added by 79.75.13.2 (talk) 22:14, 1 April 2009 (UTC)
- TV cosmetics adverts are amongst the worst examples of poor science you'll see out there in the real world. So "stronger feeling hair" is probably ad-speak for "not stronger at all". But they are more than happy to use excessive precision - to make statements like "57% more shine!" - without saying 57% of what? 57 percent more than filthy, dirty hair is not impressive. 57 percent better than your leading competitor might be. But "shininess" is a vague property. The amount of light reflected isn't the only thing - plastics can reflect a lot of light - but they don't look as shiney as metals because they reflect it over a wide range of angles...so are they talking 57% more 'reflectivity' or a 57% sharper lambert coefficient. As for the strength numbers...first be aware that according to our Hair article, human hair varies in diameter 17 to 181µm. Since strength is likely to be proportional to cross-sectional area - you'll probably find that there is at least a factor of 100 between the weakest human hair and the strongest. Several sources I found suggest that a single human hair can support a weight of about 100 grams. [10] talks about strengthening hair and suggests that it's possible - but also that it's still a research topic. I strongly doubt that existing haircare products have any significant impact on the strength...but it is at least possible. SteveBaker (talk) 23:37, 1 April 2009 (UTC)
- Of course, there are many kinds of strength. There's compressive strength, tensile strength, shear strength, elasticity, hardness, plasticity, toughness. etc. etc. --Jayron32.talk.contribs 00:55, 2 April 2009 (UTC)
- Those ads may not mean tensile strength at all, but rather resistance to being pulled out of the follicle or forming split ends. A scientist wouldn't call this "strength", but they aren't scientists, are they ? The reason I doubt that they mean tensile strength is that this is rarely a problem, in my experience. How many people have hair that breaks in half ? StuRat (talk) 12:09, 2 April 2009 (UTC)
- Ah - yes, that makes more sense. A more straightforward: "Our product helps to stop your hair from falling out." would get them into a lot of legal trouble - and wouldn't help sell the product to people with a full head of hair. But as I said - science and clear language is not the strong point of people selling stuff for cosmetic purposes. SteveBaker (talk) 13:26, 2 April 2009 (UTC)
- Hair follicle actually has more info on the structure of hair than the hair article does. Hair is made up of 3 layers. The outer cuticle layers are 6 to 10 layers of dead cells arranged in a scaly overlapping pattern like roof shingles. In healthy hair these ce3lls lie flat together. IF you treat hair with an alkaline substance the scales will lift and your hair will become less shiny and will break easier. (People who dye their hair and those with certain health defects or nutritional deficiencies have hair that breaks easily.) Acidic substances make the scales lie flat. The next layer is the inner cortex. It's made up of bundles of lots of keratin fibers. They are interconnected with sulfur bridges and embedded in a keratin matrix that determines the tensile strength and flexibility of the hair. The innermost part, the medulla contains waste materials and fat substances. Healthy follicles will also produce sebum to coat the outer hair. That will protect the hair. You can replace this layer with waxes or silicone substances after it got washed off. Hope this helps. 76.97.245.5 (talk) 04:38, 3 April 2009 (UTC)
- I once saw three girls on the subway having a hair contest. Each pulled out one of her own hairs. The brunette's hair broke that of the redhead, and the redhead's hair broke that of the blonde, when the girl held a piece of her hair tightly between fingers and pushed it against the other girl's hair until one hair broke. So "stronger" hair might mean "brunette" hair. (I seem to recall also an old possible unrelated engineering term relating colors of some type of hair and closeness of adjustment. This terminology implied that blond hairs were smaller in diameter than red hairs). Edison (talk) 04:58, 3 April 2009 (UTC)
- There is certainly a very large range of hair diameters (our article suggests a 10:1 ratio between the thickest and thinnest). The article I referenced up above says that Asiatic hair tends to be a LOT stronger than European hair. 12:46, 3 April 2009 (UTC)
- I've noticed that there seems to be more of a gender difference in Oriental hair. That is, men's hair tends to be much thicker, stiffer, and stronger than women's. I don't believe that this pattern holds, to the same extent, for European hair. StuRat (talk) 17:19, 3 April 2009 (UTC)
Does studying actually improve IQ scores?
Taking a degree makes you more knowledgeable, but does it also raise your score in IQ tests? 89.242.107.39 (talk) 23:18, 1 April 2009 (UTC)
- In theory - no. In practice - probably, yes. If nothing else, there is good evidence that doing lots of IQ tests can improve your score significantly. SteveBaker (talk) 00:45, 2 April 2009 (UTC)
- Since most IQ tests are written, having good written language comprehension (in the language used on the test) would be important, and you can improve that with study. StuRat (talk) 12:02, 2 April 2009 (UTC)
Are there any research papers available about this? I was waiting for someone to mention of London taxi drivers whose brains develop to allow them to memorise all the streets. Although with Satnav, sadly that tradition may be in danger. 89.243.219.134 (talk) 17:47, 2 April 2009 (UTC)
- That would be "The Knowledge". London cab drivers are required to memorize the entire map of London in exquisite detail in order to get their licenses. Some of the things they need to know - such as traffic patterns at various times of day - are not covered by GPS navigation systems - so it's likely that this tradition will stand for a while yet. It's also a way for the established cab drivers to limit the amount of competition they have to face. SteveBaker (talk) 20:17, 2 April 2009 (UTC)
- One problem is the reliability of IQ tests. I've seen a lot of self-proclaimed "IQ tests" filled with questions about sports and movie trivia. --79.116.90.132 (talk) 17:32, 3 April 2009 (UTC)
- In such cases, it's still a valid IQ test, just one that's used to judge the IQs of the test-makers, rather than the test-takers. :-) StuRat (talk) 17:13, 5 April 2009 (UTC)
April 2
Hominidae?
In layman's terms, how can one easily distinguish Hominidae (humans, bonobos, chimps, gorillas and orangutans) as different from other primates (or other mammals in general)? Do they have certain easy characteristics that set them apart from other primates? Maybe their weight?--Sonjaaa (talk) 00:20, 2 April 2009 (UTC)
- According to the article, distinguishing characteristics include the absence of a tail, and sexual dimorphism (males larger than females in this case). ~AH1(TCU) 00:44, 2 April 2009 (UTC)
- And, of course, they are more intelligent. For example, there's the test of leaving a series of boxes on the floor and a banana hanging from the ceiling, where the more intelligent primates can figure out how to stack the boxes to get to the food, while less intelligent primates can't. StuRat (talk) 11:54, 2 April 2009 (UTC)
- Oh - THAT'S how you do it? I always wondered. SteveBaker (talk) 13:22, 2 April 2009 (UTC)
- How about developing IQ tests for bonobos, chimps, and gorillas to see which is the more intelligent species? That would also show much variation there is in intelligence among individuals of the same species. Maybe IQ tests could be developed for other animals too. The thought that would go into developing animal IQ tests might show how to improve the validity of human IQ tests too. The same test could not be used for all animals because their intelligence varies so much. Separate tests would have to be developed for use with closely related species only. The basic thinking patterns of various species would also require different tests - for instance, separate IQ tests would be needed for cats and dogs. Obviously, animal IQ tests could not be written tests, but would be administered on a food reward basis. - GlowWorm. —Preceding unsigned comment added by 98.17.35.74 (talk) 13:56, 2 April 2009 (UTC)
- There are skeptics that IQ is a good test for intelligence for humans. I think it would be even less effective for animals. 65.121.141.34 (talk) 15:35, 2 April 2009 (UTC)
- With human IQ tests there is the well-known problem of a person's former experience aiding him in doing the test. If he reads well, he will do better on an IQ test. If he has taken a lot of such tests, he knows how to go about it. He may even have seen similar questions before, such as rope-and-pulley problems. He may also be familiar with the way the questions are asked. (In poll taking, the way a question is asked can influence the answer.) In contrast, wild animals of the same species that have been taken very recently from the same environment, will mostly have about the same former learning experiences – not in IQ tests but in life experiences. There will be more variations in life experience with farm animals, pets, and possibly in wild animals that have lived near humans. – GlowWorm. —Preceding unsigned comment added by 98.17.35.74 (talk) 15:53, 2 April 2009 (UTC)
- One type of IQ test that works on a variety of animals is the mirror test:
- 1) The least intelligent animals act as they would when they encounter another animal of their same species and gender. In the case of male crabs, for example, they will attempt to fight with their mirror image.
- 2) More intelligent animals, like cats and dogs, might react as above briefly, but, after looking behind the mirror and sniffing it, realize it's just some type of illusion and move on.
- 3) The most intelligent animals (such as those mentioned in this Q) eventually realize that they are seeing their own image, and are fascinated by it. For example, they may use it to try to look at parts of their body they can't normally see. StuRat (talk) 17:12, 3 April 2009 (UTC)
- That's very interesting. Do you know how some of the smarter birds, such as ravens or crows, react? -GTBacchus(talk) 17:18, 3 April 2009 (UTC)
- Actually, Google answered my question very quickly. Magpies get it. -GTBacchus(talk) 17:21, 3 April 2009 (UTC)
- 3) The most intelligent animals (such as those mentioned in this Q) eventually realize that they are seeing their own image, and are fascinated by it. For example, they may use it to try to look at parts of their body they can't normally see. StuRat (talk) 17:12, 3 April 2009 (UTC)
- They look like people, unlike other animals such as dogs, cats, horses, snakes, rats, birds, etc. Edison (talk) 04:52, 3 April 2009 (UTC)
5 in one fruit tree
I've seen these five-in-one grafted fruit trees in catalogs, but what I'd like to know is, are there any drawbacks to grafting five different types of plant (sometimes of different, but related species) to the same tree? Different species have different needs, so I would think that having five different types of tissue in one tree would compromise the integrity of the whole tree in some way. It's a really neat idea (the tree I'm looking at possibly buying has five different species of fruit from the genus prunus on it), but it could be just a gimmick to sell trees that aren't that good. Please help me decide whether I should buy a combination tree or simply buy one that has only one species and cultivar. Thanks, Wikipedians! 63.245.144.68 (talk) 00:58, 2 April 2009 (UTC)
- I think your example shows that this only works if the plants are all similar, which would mean they have similar nutritional requirements. Grafting has been used commercially, which seems to indicate that it has some benefit. I imagine the grafted tree is more expensive, and you could have the problem of a "graft failure", which can't happen in a normal tree, in addition to the regular things that can go wrong with a tree. So, I'd say that you should get the grafted tree if you really want all those varieties, and stick with a normal tree otherwise. StuRat (talk) 11:48, 2 April 2009 (UTC)
- For some plants grafting is the only way to be sure about the quality of the fruits you will eventually get out of the tree. I believe Prunes, Apricots, Peaches and Nectarines are so closely related that it is unlikely that the multiple grafting should be a problem. Dauto (talk) 14:00, 2 April 2009 (UTC)
- shouldn't that be plums? Prunes are dried plums.--TammyMoet (talk) 17:56, 2 April 2009 (UTC)
- Maybe he forgot to water the tree... - EronTalk 18:02, 2 April 2009 (UTC)
- Yes, I meant plums. I got misdirected by the word prunus for the genus. Dauto (talk) 05:14, 3 April 2009 (UTC)
- We actually had a prune tree once. It had something wrong with the skin on the plums, so it wasn't waterproof. This caused them to shrivel into prunes while still on the branch. We should have thought to market the tree (perhaps in Florida ?), maybe we'd be rich by now. StuRat (talk) 16:52, 3 April 2009 (UTC)
- Dauto is right that only closely related types of fruit can be successfully grafted. Grafting different types varieties of fruit on the same tree is really only done as a novelty, not for serious fruit production. What happens is that one type tends to take over the entire plant, since it is very difficult to have exactly equal vigor for each variety. So, buy one if you want to create a conversation piece and amaze your neighbors, but buy separate trees if you want the fruit for eating.--Eriastrum (talk) 17:24, 2 April 2009 (UTC)
- "Dauto is right that only closely related types of fruit can be successfully grafted." Actually, I said that. StuRat (talk) 16:44, 3 April 2009 (UTC)
- When I was a child we had one of these. The first summer it produced five kinds of apples. Then after a hard freeze during the winter, the grafts died and it only produced one kind of apple. Edison (talk) 04:50, 3 April 2009 (UTC)
- Sorry, Stu, I didn't read your post carefully. Won't happen again.--Eriastrum (talk) 20:57, 4 April 2009 (UTC)
HAMMER DRILL VS IMPACT DRILL
WHAT IS THE DIFFERENCE? —Preceding unsigned comment added by 64.88.177.194 (talk) 01:11, 2 April 2009 (UTC)
- Please do not type in all caps. It makes it appear that you are screaming, and is pretty rude. Now, to answer your question... Nothing. They are the same item. If you look for an article on impact drill you will see it redirects to our article on hammer drill. There may be some marketing differences, for example one manufacturer may use one term and a different one may use the other, but functionally they are the same tool. --Jayron32.talk.contribs 01:20, 2 April 2009 (UTC)
- Maybe you mean Impact driver or Impact wrench? In which case a hammer drill uses a hammering action to help break-up the masonry when drilling, where as the other tools I mention use impact to generate large amounts of torque for loosening tight bolts etc.90.220.130.74 (talk) 21:02, 2 April 2009 (UTC)
Life Without Plastics Video
I once saw a humorous brief video detailing "life without plastics" (or oil or petroleum products, etc.) with a 1950s housewife. She is standing in the kitchen and things begin to fall apart. I can't seem to find this anywhere online; where can I find it? —Preceding unsigned comment added by Dlempa (talk • contribs) 03:06, 2 April 2009 (UTC)
- Wasn't that on an episode of The Simpsons ? There has been an even more ridiculous one on TV in the USA during the last year or so about "Chemicals" disappearing - which is bizarre since essentially every material object is made of chemicals...but that had a modern theme, not 1950's. SteveBaker (talk) 13:16, 2 April 2009 (UTC)
- I believe you're talking about this segment from Kentucky Fried Movie. --Sean 13:48, 2 April 2009 (UTC)
- ...which was probably inspired by A Case of Spring Fever (presented here as the MST3K version for your convenience and pleasure), or some other educational short film in the same vein. -- Captain Disdain (talk) 20:15, 2 April 2009 (UTC)
- Oh god I laughed so hard at that MST3K episode. Springy! --BiT (talk) 20:37, 2 April 2009 (UTC)
- ...which was probably inspired by A Case of Spring Fever (presented here as the MST3K version for your convenience and pleasure), or some other educational short film in the same vein. -- Captain Disdain (talk) 20:15, 2 April 2009 (UTC)
Meanings
What is the meaning of A in this picture? In the summary, the abbreviation"ca." means what?96.53.149.117 (talk) 04:33, 2 April 2009 (UTC)
- "A" almost certainly means Angstrom units. "Ca." means "circa", or "about". Looie496 (talk) 04:36, 2 April 2009 (UTC)
It very well mean "canada" or located in canada. THIS will be edited out soon so please read quickly.67.193.179.241 (talk) 14:17, 4 April 2009 (UTC)
- It can't very well mean that, as we've been given context in which it means "circa". 86.166.149.141 (talk) 00:34, 5 April 2009 (UTC)
Diamond Cutting
What is the reason that Diamonds are used in machine tools, especially when machining non-ferrous alloys, compared to other alloys?96.53.149.117 (talk) 04:45, 2 April 2009 (UTC)
- I suspect the author of that line was thinking of non-ferrous alloys that are harder than steel. It seems sensible that one would use diamond on hard materials. You don't use it on materials that can be machined more easily, because it is more expensive than alternative materials.--Srleffler (talk) 05:26, 2 April 2009 (UTC)
- from article diamond... "Diamond is not suitable for machining ferrous alloys at high speeds as carbon is soluble in iron at the high temperatures created by high-speed machining, leading to greatly increased wear on diamond tools when compared to alternatives.--Digrpat (talk) 13:06, 2 April 2009 (UTC)
"
When a Star Evolves into a Black Hole...
I was reading List_of_common_misconceptions the other day, and it said:
"Black holes, unlike the common image, do not act as cosmic vacuum cleaners any more than do other stars. [citation needed]When a star evolves into a black hole, the gravitational attraction at a given distance from the body is no greater than it was for the star. That is to say, were the Sun to be replaced by a black hole of the same mass, the Earth would continue in the same orbit. Due to a black hole's formation being explosive in nature, the object would lose a certain amount of its energy in the process, which—according to the mass–energy equivalence—means that a black-hole would be of lower mass than the parent object, and actually have a weaker gravitational pull.[1]"
Note that paragraph was removed by an editor earlier today for being poorly sourced so if you look at it now, it's not in there but I still want to ask a question about it.
Assuming that this is correct, if a black hole's gravitational pull is the same as the star that preceded it, how did light escape the star in the first place? Is it because as the star collapsed, its size shrank and the black hole has a higher density? A Quest For Knowledge (talk) 17:54, 2 April 2009 (UTC)
- No, as the Schwartzschild radius of such a BH is about 15km, the star's light was sent well outside that. Of course, the gravity decreases alot when going from 15km radius to 100.000km or what a typical star has radius. When you read the scenarios of a BH emitting light, it's always from inside its event horizon. --Ayacop (talk) 18:14, 2 April 2009 (UTC)
- Er... I'm not quite sure what to make of the above answer. Generally, your initial understanding is correct. At the surface of the Sun (more relevantly, a point one solar radius from the center of the Sun, 6e5 km away), escape velocity is 6e5 m/s, far less than the speed of light (3e8 m/s). As such, light escapes. If you swapped the Sun for a solar-mass black hole, then escape velocity from a point one (original) solar radius away remains the same, and a light source there is still detectable.
- Next consideration: a solar-mass black hole has an event-horizon radius of 15km (I'm going off the above response, and have no idea whether or not it's correct, but it's useful for these purposes). Light can't escape from points at or within 15 km of the center of mass. Here's the key distinction from when it was a star: the Sun's mass is not contained within a 15 km radius sphere, so the Sun's gravity at that point is far less. Volume increases with the cube of radius: if we assumed the Sun to be of uniform density (it's not, but not the point), then we would expect about one trillionth of one percent of the Sun's mass to be within the event horizon of a solar-mass black hole. The mass of the Sun above that radius is evenly distributed, and as such, can be ignored for gravitational purposes. One trillionth of one percent of the mass needed for the black hole obviously doesn't stop light from escaping from a point 15 km from the center of the Sun.
- Escape velocity boils down to a function of mass and distance. When you compare "the Sun" and "a solar mass black hole", you've kept mass the same but varied the distance (and thus changed your answer). When you compare "the center of the sun" and "a solar mass black hole", you've kept distance constant but varied the mass (and thus changed the answer).
- So, your original concept of density is basically correct. However, this should not be confused with the idea that black holes (in reference to the volume contained by the event horizon) need to have "high" density. Normal matter (of a given density) increases mass by the cube of radius. Black holes increase mass proportionally to radius. If you take a ball of clay of radius r and mass x, then a ball of clay with radius 2r has mass 8x. If you swap "ball of clay" with "black hole", then mass 8x black hole has radius 8r. Put into more practical terms, envision a sphere around the solar system, out to 100 AU (three times the orbital radius of Neptune). Fill that sphere with sea-level pressure air. The solar system is now a black hole.
- Now, as for the original answer: black holes "emitting" light do so from their accretion disks, which always lie outside of the event horizon. Nothing is emanated from within. Even Hawking Radiation only works when particles form outside the horizon. — Lomn 19:08, 2 April 2009 (UTC)
- Ayacop, I don't understand what you mean by "BH emitting light, it's always from inside its event horizon". Black holes don't emit light. Are you referring to Hawking radiation? A Quest For Knowledge (talk) 20:20, 2 April 2009 (UTC)
if its within the event horizon, how would we know if it emitted light or not? 65.121.141.34 (talk) 20:23, 2 April 2009 (UTC)
- Now let's look at ways the perception of black holes as "cosmic vacuum cleaners" may be correct:
- 1) While it's true that a black hole has no more gravitational attraction at great distances than a star of equal mass, that neglects the fact that most black holes have a greater mass than most stars. In fact, most stars have insufficient mass to ever form a black hole. There are also supermassive black holes at the center of spiral galaxies which are millions or billions of times more massive than any star.
- 2) Stars tend to give off more mass than black holes, via conversion to energy which is emitted as light, etc., solar wind, and sometimes violent explosions like novae and supernovae. Black holes can lose mass due to Hawking radiation, but this is only significant portion of the mass for small black holes. Thus, most black holes can be thought of as one-way doors, unlike normal stars. StuRat (talk) 16:28, 3 April 2009 (UTC)
source of dietary cholesteryl esters?
As I was studying the enzyme bile acid-activated lipase, it says it degrades cholesteryl esters in the small intestine. But where do these come from? Do they always come from eating animals? Are there plants that contain amounts of cholesteryl esters? --Ayacop (talk) 18:20, 2 April 2009 (UTC)
- Just to be safe you are talking about Bile salt dependent lipase right? Although not specifically "cholesteryl esters" you may want to look at dietary sources of cholesterol. I am not familiar with cholesterol biochemistry, so I don't know what form the majority of cholesterol is in when it is absorbed in the small intestine. It might be that cholesteryl esters are a storage or transport form of cholesterol. Plants do synthesize some cholesterol (some sources say otherwise but they're wrong) but it is very little compared to animal cells. You may also want to take a look at phytosterols. As dietary contributions go (assuming an omnivorous diet), cholesterol from plants is negligible compared to other sources (like the butter you cook your vegetables with for instance). Sifaka talk 19:08, 2 April 2009 (UTC)
Genius
Looking for any valid statistics on people who are labeled a genius of some sort (mathematical) and the possibility of having a personality/mental disorder/dysfunction. Also, any statistics, say a mathematical genius, to have children who are supra-intelligent. --Reticuli88 (talk) 18:41, 2 April 2009 (UTC)
- I don't know whether you'll find much in the way of statistics. There are an awful lot of smart people (including MANY mathematicians) with Asperger's syndrome shading into borderline Autism - but because it's a spectrum condition, there is no hard definition of who has it and who doesn't. It's a matter of degree. Of course there are mathematicians who have pretty serious mental conditions - John Nash is the one that always leaps to mind because his story is told in the movie A Beautiful Mind. His paranoid schizophrenia is serious matter - but that didn't stop him from earning a Nobel prize. SteveBaker (talk) 20:11, 2 April 2009 (UTC)
- As an extra comment, that film is my favourite of all time. Excellent story, thoroughly recommended. Cyclonenim : Chat 20:49, 2 April 2009 (UTC)
- Yep - all the better because it is 100% true. I like that Prof Nash still points to people he hasn't met before and asks people he knows whether they are real or not. He does it without a shred of embarrassment - and when he finds that some of the people he meets are not actually real - he happily ignores them and carries on with life! I think it takes the temperament of a good mathematician to be able to do that. Most of us would be too freaked out to cope. SteveBaker (talk) 21:02, 2 April 2009 (UTC)
- As an extra comment, that film is my favourite of all time. Excellent story, thoroughly recommended. Cyclonenim : Chat 20:49, 2 April 2009 (UTC)
- Well, 100% true except the bit that shows him taking his medication voluntarily. According to the real-life Nash, he rarely ever took it unless forced to, and recovered with his own will as you described above. Now that's persistence. Cyclonenim : Chat 23:46, 2 April 2009 (UTC)
- I was under the impression that he didn't actually have visual hallucinations, just auditory. As brilliant as that film is, it is heavily dramatised. --Tango (talk) 05:12, 3 April 2009 (UTC)
- People who have worked with him have told the story that he will (on occasion) point to some stranger in the room and ask everyone else: "Is this person real?" - and on much rarer occasions, he'll point to an empty piece of space and ask the same question. Assuming those reports are true - then this can hardly only be an auditory hallucination. SteveBaker (talk) 12:40, 3 April 2009 (UTC)
- The our article needs correcting. --Tango (talk) 00:35, 4 April 2009 (UTC)
- People who have worked with him have told the story that he will (on occasion) point to some stranger in the room and ask everyone else: "Is this person real?" - and on much rarer occasions, he'll point to an empty piece of space and ask the same question. Assuming those reports are true - then this can hardly only be an auditory hallucination. SteveBaker (talk) 12:40, 3 April 2009 (UTC)
- There is also Kurt Gödel, who starved to death for fear of being poisoned. G. H. Hardy who hated looking at his own reflection. Alas, I don't have any statistics, however. --TeaDrinker (talk) 21:40, 2 April 2009 (UTC)
It all depends on what you call intelligence. If you consider social abilities a form of intelligence, not many aspies would fall under the category intelligent. So as many people only understand math when you explain things to them clearly, step by step, aspies only understand social dynamics when you explain everything (even knowledge that other people take for granted). Since Asperger's syndrome has rather being defined recently, I don't believe you'll find many serious diagnosis of highly skilled mathematicians with it. --Mr.K. (talk) 11:26, 3 April 2009 (UTC)
- It is often said that aspies don't know how to have social relationships. Actually, I think it's that they don't want to have social relationships. They get no pleasure from it. They regard social relationships as a chore and a nuisance – something they have to force themselves to do. —Preceding unsigned comment added by 98.21.105.25 (talk) 11:45, 3 April 2009 (UTC)
- It's both. As an Aspie, I strongly dislike having to meet and get to know people who I don't already know well. When I do have to do it, I'm not good at it. (Although training can cover the latter to some degree - and I'm getting pretty good at faking it). Furthermore, it's not just a matter of some diffuse inability - many aspies (myself included) are completely blind to things like body-language, facial expressions and some linguistic subtleties like sarcasm. I really, deeply, hate going to parties for example. A party is an event designed solely in order that people meet and interact for no especially well-stated reason - we frequently use alcohol at these events to make that interaction more intense - and that amount of forced interaction is something I strongly prefer to avoid! My only reaction at such events is to try to find a way not to have to be involved - this is sometimes mistaken for 'shyness' - but that's not it. However, I think it's a BIG stretch to say that social abilities are "a form of intelligence". Ants and Bees have really great social relationships - but we don't generally call them "intelligent". There is a movement to get all sorts of human behaviors labelled as "forms of intelligence" - I've heard of teachers talk about "body posture intelligence" as an attribute of good dancers who are otherwise as dumb as a bagful of anvils - but that's entirely so that the teachers don't have to go around labelling people as "unintelligent" just because they can't do IQ tests or crack open a book to learn something. However, that's not the usual definition! Anyway - the OP specifically wants to know about 'mathematical geniuses' - and social skills most certainly have nothing whatever to do with that! SteveBaker (talk) 12:40, 3 April 2009 (UTC)
- The amount of social interactions by humans and the complexity of these are not comparable with the social life of any other creature. Human social interactions require that you integrate all these things cited above - body-language, facial expressions and some linguistic subtleties - and much more. They are far away from a binary communication style. I don't see any problem labeling all human abilities as a form of intelligence, since all of them require processing of information of some kind. Evenly I don't see any problem admitting that animals process certain kinds of information. Some of them - like smell information - even better than us. Others - like language - only at a very primitive level, if we can speak of language in animals at all. According to the intelligence article, there are many definitions of intelligence. I would go with the intelligence = processing of information. Other definitions are probably so broad and badly defined that you could call any one 'intelligent' or you would like to guess who is intelligent or measure it with some dubious IQ test. The definition above would imply multiple types of intelligence - as many as types of information probably. Many, many people could be terrible processing one kind of information type and excel at other kinds. So, it would not be a surprise finding an aspie good at mathematics, a field that strives to define everything as precise as possible and everything has a clear purpose. There is more information aboutmultiple intelligences in the corresponding article.--Mr.K. (talk) 15:51, 3 April 2009 (UTC)
- Are aspies who are good at math, also good at fuzzy math? (In fuzzy math things are not precisely defined.) —Preceding unsigned comment added by 98.17.36.180 (talk) 20:44, 3 April 2009 (UTC)
- I assume any aspies who work in fuzzy maths are good at it. Fuzzy mathematics (which is, as far as I know, just as precise as the rest of mathematics) is a tiny field; I've never encountered anyone who worked on it. Algebraist 20:52, 3 April 2009 (UTC)
- Are aspies good at stistics, in which things are not precisely defined?
- The person who springs to mind in this field is Simon Baron-Cohen, if you look him up on Google Scholar you will get heaps of academic papers on autism. This study on the link between Autism and Engineering might be of interest to you. There is also this article on the BBC site. SpinningSpark 01:17, 4 April 2009 (UTC)
As a family therapist, some of the most challenging yet rewarding clients are those on the Asperger's continuum. An Aspie's difficulty with (or disinterest in) social skills can easily carry over into this forum where replies are at times given in a very rude and condesending manner (especially when an OP asks about Creationism vs. Evolution). So, social skills can be more than just face-to-face interactions.
As for IQ, this is only a measure of how well one is able to complete IQ tests. It was not that long ago where IQ tests had a cultural bias which often led to non-white, non-western persons not doing as well as the white westerners the IQ was designed by.
As for multiple intellegences, I would suggest that there needs to be a balance. We can't just give some arbitrary characteristic an "intellegence" name (I.E. "posture intellegence") just to make people feel better! So, while some people may be more inclined to mathematics ("math intellegent") while others are more artistic ("visual/spacial intellegence")or even people oriented ("emotional intellegence" or "social skill" intellegence - I am much better at the former than the latter)I would suggest that it is better to focus on our strengths. Thus, I became a therapist because I really am a klutz at math, mechanical things and the like. —Preceding unsigned comment added by 74.12.66.165 (talk) 21:01, 4 April 2009 (UTC)
Abiogenesis vs Evolution
I need a WP:RS that a) explains the difference between abiogenesis and evolution b) states that this is a common or popular misconception or misunderstanding. I've found plenty of blogs and forums about this, but these aren't usually considered WP:RS. The best I've been able to come up with is here [11] and here [12] but I'm hoping for something better. I'll keep looking but I thought that maybe somebody here already knows a good cite off the top of their head. If not, it's no big deal. I'll keep looking. A Quest For Knowledge (talk) 23:28, 2 April 2009 (UTC)
- I would bet you could find that in one of Richard Dawkins' books - I don't have an exact ref for you though. SteveBaker (talk) 00:26, 3 April 2009 (UTC)
- Ironically, I choose this item because I mistakenly thought that finding a WP:RS doing a Google search with "abiogenesis" as one of the search terms would be trivial. How wrong I was! Unfortunately, we have an editor who's apparently on a crusade to make sure that every single item in this article is correctly sourced. Not that he/she doesn't have a valid point, but they're doing a blanket removal of all unsourced items even if the information appears to be correct (just not sourced). A Quest For Knowledge (talk) 01:10, 3 April 2009 (UTC)
- Yeah - in these "List of..." articles - especially those with "fun facts" like this one - there is a tremendous tendancy for junk to accumulate in the article. This SPECIFIC form of article really does have to have 100% reference coverage and a rigorous purging of unsourced junk is needed. That's only reasonably possible if all of the important stuff is referenced to within an inch of it's life. In most 'normal' articles, it is sufficient to reference only the 'likely to be doubted' facts. SteveBaker (talk) 01:38, 3 April 2009 (UTC)
- I think you have to clarify the question a bit better -- this is sort of like asking for a reliable source for the difference between radio stations and submarines -- they're just different things, that's all. Looie496 (talk) 02:34, 3 April 2009 (UTC)
- Yeah - in these "List of..." articles - especially those with "fun facts" like this one - there is a tremendous tendancy for junk to accumulate in the article. This SPECIFIC form of article really does have to have 100% reference coverage and a rigorous purging of unsourced junk is needed. That's only reasonably possible if all of the important stuff is referenced to within an inch of it's life. In most 'normal' articles, it is sufficient to reference only the 'likely to be doubted' facts. SteveBaker (talk) 01:38, 3 April 2009 (UTC)
- Ironically, I choose this item because I mistakenly thought that finding a WP:RS doing a Google search with "abiogenesis" as one of the search terms would be trivial. How wrong I was! Unfortunately, we have an editor who's apparently on a crusade to make sure that every single item in this article is correctly sourced. Not that he/she doesn't have a valid point, but they're doing a blanket removal of all unsourced items even if the information appears to be correct (just not sourced). A Quest For Knowledge (talk) 01:10, 3 April 2009 (UTC)
- Sorry, I'm trying to find a WP:RS for this:
- "Biological evolution does not address the origin of life; for that, see abiogenesis. The two are commonly and mistakenly conflated. Evolution describes the changes in gene frequencies that occur in populations of living organisms over time, and thus, presupposes that life already exists. Evolution likewise says nothing about cosmology, the Big Bang, or the origins of the universe, galaxy, solar system, or Earth, although the term 'evolution' in the sense of a slow unfolding is used to describe such processes, e.g. Stellar Evolution, Cosmic Evolution.[citation needed]" A Quest For Knowledge (talk) 02:50, 3 April 2009 (UTC)
- I think you just need a dictionary to verify all of that except the fact that they are commonly conflated. I'm not sure where you would find a source for that, though (finding examples is easy, but you need something more than just a list of examples otherwise you are doing original research). --Tango (talk) 05:06, 3 April 2009 (UTC)
- @Tango: That's true - but you have to look at this in the context of the article that's being written here. It's a list of common misunderstandings. So whilst it's trivially easy to use the dictionary definitions of the two words to prove that Abiogenesis and Evolution are not the same thing - it's not so simple to prove that they are commonly confused or conflated. SteveBaker (talk) 12:22, 3 April 2009 (UTC)
- Hmm I think it should be pretty easy to find. I mean the whole evolution vs creation myths "debate" must be sort of showing that some mix up? I would assume it must have been addressed somewhere (Just think of the possibility for someone who would want to mock the religious person/group not knowing). I am guessing one group religious that seem to have understood the difference in the catholic church, I have at least read I think that they're accepting evolution (but not accepting abiogenesis of course I guess) "post-creation" or something. chandler · 12:36, 3 April 2009 (UTC)
- @Tango: That's true - but you have to look at this in the context of the article that's being written here. It's a list of common misunderstandings. So whilst it's trivially easy to use the dictionary definitions of the two words to prove that Abiogenesis and Evolution are not the same thing - it's not so simple to prove that they are commonly confused or conflated. SteveBaker (talk) 12:22, 3 April 2009 (UTC)
- I think you just need a dictionary to verify all of that except the fact that they are commonly conflated. I'm not sure where you would find a source for that, though (finding examples is easy, but you need something more than just a list of examples otherwise you are doing original research). --Tango (talk) 05:06, 3 April 2009 (UTC)
- Discussing evolution/abiogenesis there is;
- Iris Fry, The emergence of life on Earth: a historical and scientific overview, p57, Rutgers University Press, 2000 ISBN 0813527406.
- John Augustine Zahm, Evolution and dogma, pp41-42, Ayer Publishing, 1978 ISBN 0405108745.
- The above and on confusion of terms (especially historical),
- James Edgar Strick, Sparks of Life: Darwinism and the Victorian Debates Over Spontaneous Generation, pp11-13, Harvard University Press, 2002 ISBN 0674009991.
- Direct dictionary definition of abiogenesis,
- Elizabeth Tootill, The Facts on File dictionary of biology, p247, University of Michigan, 1981 ISBN 0871965100.
- Clear statement of confusion of the two from the other side of the debate;
- Daniel Jappah, Evolution: A Grand Monument to Human Stupidity, p21, Lulu.com, 2007 ISBN 1430324902.
- Not sure about the second part of that paragraph, "evolution has nothing to do with cosmology and the Big Bang". This is like asking a for a ref that differential geometry has nothing to do with bananas. While true it is unlikely that either books on differential geometry or bananas will make this statement. Not many books covering both subjects. Seriously, I cannot really work out what that statement is supposed to be doing for the article - it is enough to differentiate evolution and abiogenesis, a contrived analogy is not really needed. SpinningSpark 15:54, 3 April 2009 (UTC)
- As I was looking for cites last night, I was jokingly thinking to myself that Google should come up with a reliable sources search engine. Then I discovered that Google does let you create your own custom search engine [13]. So, I started creating a Reliable Sources Search Engine. I realize the futility of creating anything that is even remotely comprehensive, but as I was sifting through a bazillion Google hits (many of which were blogs and forum posts), I think that something that narrows down the hits might be useful (to me, anyway). My custom search engine is here [14]. So far, I have about 20 sites that are generally considered to be reliable per WP:RS standards. I left some obvious ones off such as www.abcnews.com since ABC News allows readers to add comments to a news article and I'm not sure yet how to get the search engine to distinguish between article content and user comments. I've left the search engine open so if anyone wants to contribute to this search engine, feel free. A Quest For Knowledge (talk) 15:51, 3 April 2009 (UTC)
April 3
Limiting Reagent
Hello. If limiting reagent R is to be completely consumed by reacting with reactant X, is adding 10% in excess of the minimum amount of reactant X needed common industry practice? Are there any safety limitations in some reactions that may prevent the use of this procedure? Thanks in advance. --Mayfare (talk) 00:20, 3 April 2009 (UTC)
- It's a common rule-of-thumb for something to try when developing a process, but I don't know if it's "common industry practice" for what actually goes into a full-scale production run. Gotta consider how expensive and/or difficuult it is to buy, separate, and dispose of that unreacted material, and the effect of that excess reagent could have on the reaction and products. DMacks (talk) 01:18, 3 April 2009 (UTC)
- It also depends on your industry. (Let me preface this by stating that I'm not a chemical engineer). But I just got a very fancy new wall poster of a Modern Refinery (Crude Distillation), as designed by Mustang Engineering ("you hung a poster of WHAT on your wall?") which is a supposedly super-realistic depiction of a top-of-the-line refinery as it would be designed today. (So, chemical-engineer or not, after reading this detailed document, I'm now ... qualified to comment). The poster goes through the process control units in a fairly detailed way, and for the most part it seems more likely that they allow each chemical stage to reach partial completion, say 80% or thereabouts, before taking the refined product on to the next stage (by buoyancy separation, vacuum distillation, etc.). This leaves a sort of "unrefined sludge" in the each of the reactor stages. This unreacted reagent isn't "waste product," because those chemicals continue to cook or settle or dissolve or mix, and eventually reach reaction completion; but they go out with the next batch of product at a later time. Surely the process engineers understand the limiting reagents, (most likely, it seems, these are the catalyst concentrations), but have made a decision NOT to overstock limiting reagent. This could be for many reasons - in crude refining, economic pressures dictate volume throughput, so "reaction completion" is better measured in terms of absolute quantity of output product, rather than percentage-of-total-reagents-converted (that's more of an "efficiency" metric than a "completion" metric). Also, not all catalysts are "good" in large doses - metals can leach into the product; catalysts can perform side-reactions with impurities, etc etc. Nimur (talk) 13:20, 3 April 2009 (UTC)
- Only slightly on topic because you mentioned posters and this is the science reference desk; I recommend these two biochemical pathway posters. Sifaka talk 20:31, 3 April 2009 (UTC)
- It also depends on your industry. (Let me preface this by stating that I'm not a chemical engineer). But I just got a very fancy new wall poster of a Modern Refinery (Crude Distillation), as designed by Mustang Engineering ("you hung a poster of WHAT on your wall?") which is a supposedly super-realistic depiction of a top-of-the-line refinery as it would be designed today. (So, chemical-engineer or not, after reading this detailed document, I'm now ... qualified to comment). The poster goes through the process control units in a fairly detailed way, and for the most part it seems more likely that they allow each chemical stage to reach partial completion, say 80% or thereabouts, before taking the refined product on to the next stage (by buoyancy separation, vacuum distillation, etc.). This leaves a sort of "unrefined sludge" in the each of the reactor stages. This unreacted reagent isn't "waste product," because those chemicals continue to cook or settle or dissolve or mix, and eventually reach reaction completion; but they go out with the next batch of product at a later time. Surely the process engineers understand the limiting reagents, (most likely, it seems, these are the catalyst concentrations), but have made a decision NOT to overstock limiting reagent. This could be for many reasons - in crude refining, economic pressures dictate volume throughput, so "reaction completion" is better measured in terms of absolute quantity of output product, rather than percentage-of-total-reagents-converted (that's more of an "efficiency" metric than a "completion" metric). Also, not all catalysts are "good" in large doses - metals can leach into the product; catalysts can perform side-reactions with impurities, etc etc. Nimur (talk) 13:20, 3 April 2009 (UTC)
- I believe it works out that it costs a lot to get the last little bit to react, so often isn't worth the expense, especially if the unreacted portion can be reacted in a later batch, as noted above. Also, having extra "X" left over isn't necessarily any better than having extra "R" left over. StuRat (talk) 16:07, 3 April 2009 (UTC)
- Nimur, that poster was not the Playboy pullout I was hoping for and it took forever to download, but still quite interesting. Why are the "sour water stripper" and "sulfur recovery unit" not connected to anything else? It upsets my sensibilities as an electrical engineer to have components that are not in circuit. SpinningSpark 01:34, 4 April 2009 (UTC)
- I believe it works out that it costs a lot to get the last little bit to react, so often isn't worth the expense, especially if the unreacted portion can be reacted in a later batch, as noted above. Also, having extra "X" left over isn't necessarily any better than having extra "R" left over. StuRat (talk) 16:07, 3 April 2009 (UTC)
- Most likely because they are batch processes, rather than continuous like the rest of the plant. (A lot of people are talking about batches, but it is more efficient to deal with continuous processes. Outside pharmaceuticals, most processes are continuous) What is not shown on that diagram are the recycle streams. You would usually expect to get partial completion and recycle some of your product stream; that is, send it back into the process to mix with your reactant stream. This gives more time for the reaction to take place. Once you've separated your product from the unreacted reactants, you would probably send them back into the product stream as well. Overall, you're looking to lose very little of the excess reactant. 86.151.238.242 (talk) 00:26, 5 April 2009 (UTC)
What's the deal with black holes and escape velocity?
People talk about how light can't escape a black hole because its escape velocity is higher than the speed of light. If you used newtonian mechanics, that would mean that something within the event horizon moving the speed of light would not be able to escape orbit, but it would still be able to go arbitrarily far out. This isn't a remotely accurate description of a black hole. Something within event horizon can't even move towards the event horizon, let alone escape. Is the thing about escape velocity just a lie-to-children? Is escape velocity even the speed of light at the event horizon? — DanielLC 04:02, 3 April 2009 (UTC)
- That the Newtonian calculation gets the correct result for the radius of the event horizon is little more than a coincidence. You have to use relativity to do anything meaningful with black holes. I'm not sure the concept of an escape velocity really makes sense at the event horizon of a black hole. I think the escape velocity arbitrarily close to the event horizon (but outside it) is arbitrarily close to the speed of light, though. --Tango (talk) 05:02, 3 April 2009 (UTC)
- Yes, Tango is right. The scape velocity steadily approaches the speed of light as you get closer and closer to the horizon. The OP is right that a naive interpretation of that within newtonian mechanics leads to the false impression that light can reach out of the horizon but fails to make its way all the way to infinity and is forced back in. The correct description requires relativity. To put it as simply as possible, an observer falling into the blackhole could observe a photon moving outwards at the speed of light, but from the point of view of an observer at infinity that same photon would stand still right at the horizon never inching its way out of the blackhole. Dauto (talk) 05:31, 3 April 2009 (UTC)
- Indeed. The problem is that time has stopped advancing at the event horizon (at least as far as outside observers are concerned)...this is an entirely relativistic phenomenon - and I suppose you could say that it's just a mathematical coincidence that this happens at the same distance that the classical escape velocity became equal to the speed of light. Of course, since we're talking about relativity - we have to be careful to say 'for whom' this effect is happening. For an outside observer, time has stopped on the event horizon - but from the perspective of something trying to escape the black hole (at less than light-speed), they can still travel past the event horizon - although they'll eventually get dragged back in again. The tricky part is that for them, time in the rest of the universe is progressing infinitely quickly - which will have all manner of nasty consequences in terms of the energy of objects (or even light) travelling into the black hole - which will now tend to have infinite frequency (and, I suppose, infinite energy)! SteveBaker (talk) 12:19, 3 April 2009 (UTC)
- Question - "... from the perspective of something trying to escape the black hole (at less than light-speed), they can still travel past the event horizon - although they'll eventually get dragged back in again" - are you sure about that ? I thought that within the event horizon all timelike curves reduce their distance from the singularity at all times, so an object within the event horizon cannot even get closer to the event horizon, let alone get past it (in other words, I think I agree with what DanielLC said above). Gandalf61 (talk) 14:45, 3 April 2009 (UTC)
- Answer - NO, SteveBaker got only half of the story right. The gravitational redshift which he talks about happens even to objects falling into the hole. They seem to slowdown to a stop as they approach the horizon from the point of view of an observer at infinity. But from the point of view of an observer falling into the hole the same object reaches the sigularity at the center of the hole after a finite amount of proper time. On the other hand, an outward moving light ray sits still on the horizon from the point of view of every observer. See Black hole#Event horizon. With respect to the infinite blueshift that SteveBaker talked about, You would have to sit on the horizon in order to see that. In other words, you would have to be a photon. Any observer falling in the hole would see a finite amount of red/blue shift. But photons always 'see' an infinite amount of blueshift from the direction towards which they are moving (and an infinite amount of redshift in the oposite direction), so that's notthing specific to the fact that the observer is on the horizon. In fact, such observer would not feel anything special as s/he crosses the horizon. Dauto (talk) 15:36, 3 April 2009 (UTC)
- I'm unhappy with this talk of "observers at infinity". In special relativity the word "observer" is often treated as synonymous with "inertial reference frame", but that definition doesn't carry over into general relativity. In general relativistic usage, an "observer at infinity" can only observe things at infinity; he can't directly observe an object falling into a black hole, though he can observe light emitted by that object (if it eventually escapes to infinity). You can't say "from the point of view of an observer at infinity [the] photon would stand still right at the horizon", because that photon never gets anywhere near the observer.
- I agree with Tango's response, except for the last sentence. The real trouble with defining escape velocity is that you need to choose a coordinate system to do it. If you choose Schwarzschild coordinates then the radial escape velocity (dr/dt) actually goes to zero at the event horizon—it has to, because the speed of light goes to zero and the escape velocity is bounded above by that (since light outside the horizon can always escape). If you use proper Schwarzschild radial velocity (dr/dτ, where r is the Schwarzschild r and τ is proper time) then the escape velocity is actually given by the Newtonian formula at all radii. (In fact, the equation of motion in terms of r and τ is d²r/dτ² = -GM/r², the same as the Newtonian equation.) The funny thing about this, though, is that the proper speed of light is infinity, not c—so the proper Schwarzschild escape velocity approaches c at the horizon, but that's not the speed of light! I don't know what to conclude from this except what Tango said—you shouldn't trust the Newtonian analogy any farther than you can throw it straight up. -- BenRG (talk) 20:03, 3 April 2009 (UTC)
- I'm sorry I made you feel unhappy. I agree with you that my usage of an 'observer at infinity' was a little lazy, but I wouldn't be so draconian to the point of saying that observers at infinity cannot talk about their interpretation of what happens at the horizon just because they cannot directly observe the horizon. May be observer is a poorly chosen word. I'm not entirely sure about what you mean by "If you choose Schwarzschild coordinates then the radial escape velocity (dr/dt) actually goes to zero at the event horizon—it has to, because the speed of light goes to zero", but, at least to me, it sounds suspiciously like saying that a outward going photon stands still at the horizon, which is exactly what I said. But from whose point of view does the speed of light go to zero? Certaily not from the point of view of an observer falling into the blackhole. It is for that reason that I (lazily) chose to describe this point of view as the point of view of an observer at infinity. Dauto (talk) 05:53, 4 April 2009 (UTC)
- If an object inside a black hole could get outside the event horizon temporarily then it could fire rockets during that time and escape permanently, which clearly isn't the case since that would contradict the definition of an event horizon. --Tango (talk) 00:25, 4 April 2009 (UTC)
Generation length and human evolution
How does the length of a generation affect the rate of human evolution, given how the mutation rate increases with the parents' ages? NeonMerlin 05:35, 3 April 2009 (UTC)
- I think the average age people (in particular, women) have had children has been pretty constant throughout human existence until recently, so I don't think there has been enough variation to affect evolution. I'm not sure what would have happened if, hypothetically, the average age of having children had been different. There is more involved that just the rate of mutations, though - the rate of change in the habitat is also highly relevant, and that is beyond human control (or, at least, was until recently). --Tango (talk) 06:10, 3 April 2009 (UTC)
- Indeed - it would be hard to know the answer to this because significantly longer generation times have only happened in the last dozen or two generations and this has corresponded with many other changes for humanity. In modern times, our ability to evolve is constantly being counteracted by our ability to adapt our environment to our needs. For example: in previous generations, if a group migrated into a sunnier part of the world, they would gradually evolve darker skin - nowadays, we slap on sunscreen. Hence the evolutionary pressure that would tend to kill off lighter skinned people in sunny climates has been greatly attenuated. Similarly, better diets and vitamin supplements allow darker skinned people to live in less sunny areas without becoming vitamin-D deficient - hence they are less likely to evolve lighter skins. Modern medicine and social support structures may bias our natural ability to evolve away inherited diseases. For example, in previous centuries, a genetic condition which might cause infertility could be evolved away in a single generation since infertile people would be unable to pass their flawed genes onto the next generation. With modern infertility treatments, those flawed genes may be passed on to the next generation who will also require infertility treatment. Hence, a gene that would normally vanish (or at least be kept strongly in check) may flourish.
- This is not to say that evolution in humans has stopped - that's highly improbable - but the criteria for being successful in reproducing and raising our children has changed - and the causes that lead to reproductive success or failure are changing. Modern human evolution also has a lot more to do with memetics than genetics - when parents pass on their knowledge, stories, biasses, culture and other memes, our ideas evolve and change over generations. Take as an example, the present cultural 'war' between the Western and Arab worlds. This is largely a result of memes causing two distinct 'memetic species' - groups of people with different memes who (largely) no longer interchange their memes and are becoming memetically diverse. Just as two genetically diverse groups will eventually become so different that they can no longer interbeed - and must therefore be classified as different species - two groups who do not exchange ideas will become memetically diverse and become unable to talk to each other. That's evolution in action!
- I've got a question about this: With modern infertility treatments, those flawed genes may be passed on to the next generation who will also require infertility treatment. Hence, a gene that would normally vanish (or at least be kept strongly in check) may flourish. I've heard similar claims in the past, and they don't make sense to me. Surely the extra hurdle of infertility treatment will discourage some women from having children. People with the gene will be less likely to pass on their genes that those without. Thus the gene is still selected against. It may not disappear in one generation but it will be pushed out eventually. Right? On the other hand my understanding of genetics comes from NPR and a really old third edition copy of The Origin of Species - I've never had any formal training. Plasticup T/C 14:09, 3 April 2009 (UTC)
- I believe your analysis is essentially correct. The only way infertility might become more common is if the natural genetic drift would produce more infertile people with each generation than people who failed to get successful fertility treatments would remove. StuRat (talk) 15:21, 3 April 2009 (UTC)
- While we don't know definitely how the longer generation times would affect human evolution, it's interesting to note that in some other animal species, it has been shown to lengthen the average life span. Of course if you think about it, this is hardly surprising Nil Einne (talk) 20:41, 3 April 2009 (UTC)
- We seem to have drifted from the original Q. The answer would be that evolution should indeed speed up as a result of higher mutation rates from longer generations of humans, if all other factors are kept constant. There are, as noted previously, factors slowing evolution down, as well, or at least changing which characteristics are selected as "successful" (for example, changing from being able to raise kids to now having as many kids as possible and taking no responsibility for them whatsoever). StuRat (talk) 15:28, 3 April 2009 (UTC)
- Wouldn't the higher mutation rate be counterbalanced somewhat by the fact that it takes longer to produce each new generation? I mean, where do you get more evolution: in 20 generations of 40 year-old parents, or in 40 generations of 20 year-old parents? -GTBacchus(talk) 22:15, 3 April 2009 (UTC)
While natural selection is hard to quantify, it is possible to talk about variability and the rate at which it accumulates through mutation. There are two things to worry about: the time between successive generations and the mutation rate of germline cell DNA (not somatic cell DNA). An appropriate metric to express the mutation rate is the average number of mutations per zygote divided by the generation time. (By per zygote I mean the number of mutations accumulated during the time between when the parent was a zygote to when that parent produces its first daughter (or son) zygote) If, on average, women are having children later in their life (see generation) then the generation time has been lengthened. The question is how much does that extra time increase the amount of mutation? I couldn't find information about age vs. mutation rate and mutation accumulation rates during each portion of the cell cycle.
Imagine a case where most of the mutation takes place during DNA replication and very little takes place during other stages. Considering that the germline cell production of mothers has mostly finished shortly after birth, if the mutation rate is low while the oocyte is "waiting around" then the total number of mutations will be similar whether it is fertilized after 16 years or 26 years. Since the oocyte's DNA makes up half of the zygote's, this means that the "number mutations per zygote / generation length" ratio will be lower when the average generation length is longer. If the rate of mutation in gametes is non-constant and increases as the parents get older (i.e. it's accelerating) then you could get the opposite case where the number of mutations is more than enough to compensate for the extra generational time. Sifaka talk 21:55, 3 April 2009 (UTC)
Quantummechanical operater
why quantum mechanics deals only with linear operater? why not with other operater ?Supriyochowdhury (talk) 11:25, 3 April 2009 (UTC)
- You can apply any operator you like to any wave function you like. However, most physical processes in elementary quantum mechanics can be described as linear operators (e.g. A+ and A- for quantum state increment and decrement). If you want to model a process that is actually physically observed, you need your mathematical model to match the physical phenomena. "By dumb luck" most of the important (elementary) phenomena are well-modeled with linear operations. Nimur (talk) 12:41, 3 April 2009 (UTC)
- (Actually dumb luck has nothing to do with it. Physicists constructed the entire conceptual modeling framework of quantum theory, and made it quite different from the classical mechanical description of particles and energy, specifically so that the mathematical operations could be simplified while still matching the observed physical reality. In fact, they created such a conceptual model twice, and both times came up with "equivalent" mathematical operations. Nimur (talk) 13:04, 3 April 2009 (UTC) )
- This is not really accurate. All operators in quantum mechanics are exactly linear, regardless of the physical system and the precision of the model. But those operators operate on the wave function, which is defined over the phase space of the system, which has one dimension for every degree of freedom. Quantum theories are nonlinear in the more ordinary three-dimensional sense. Here's an analogy in classical physics. Say you have a system of two billiard balls, a cue ball and an eight ball. In initial state A the cue ball is about to strike the eight ball and knock it into a pocket, which is final state A'. In initial state B the cue ball strikes the eight ball but it misses the pocket—final state B'. The evolution of the system in both cases is nonlinear (if it were linear the cue ball would have to pass through the eight ball instead of striking it). However, if you think of the "time evolution operator" that takes A to A' and B to B' in the phase space, that operator is linear in the following sense: if you start with pA + qB, representing a probability p of being in state A and a probability q of being in state B, then the operator has to take that to pA' + qB'. If it did anything else, it would be saying that there's some sort of interaction between possible worlds, which would be a lot weirder than interaction between billiard balls.
- Quantum mechanics does have a kind of "interaction between possible worlds", but it's limited by the fact that the time evolution operator is still linear. People have devised nonlinear variations of Schrödinger's equation with the goal of solving the measurement problem, but it's hard to make a workable theory that's nonlinear in phase space. -- BenRG (talk) 13:21, 3 April 2009 (UTC)
- This paper, Relativistic models of nonlinear quantum mechanics, seems to outline the issue, and cites several other papers which have attempted nonlinear generalizations. Nimur (talk) 13:34, 3 April 2009 (UTC)
Quantum Mechanics
In analytic solution of H-atom Scrodinger equation a coordinate transform from cartesian to sherical polar coordinate because although Cartesian coordinate (x,y& z)are not suitable for this problem .I read many books but don't able to know in details about this problem.Please tell me what kind of problem I face if I don't change the coordinate from Cartesian to sherical polar coordinate.Supriyochowdhury (talk) 11:43, 3 April 2009 (UTC)
- Have you tried to solve the Schrodinger equation in x,y,z coordinates? You can perfectly well set up the boundary conditions if you so desire. Your algebraic representation of the problem will become unwieldy, and you will end up carrying dozens of sines and cosines around the equations. Take a look at spherical symmetry, and think about why it is easier to write a description for a circular wave field using circular-style coordinates. Nimur (talk) 12:43, 3 April 2009 (UTC)
- Also see this "applications" section of the Spherical coordinate system article. "Two important partial differential equations, Laplace's equation and the Helmholtz equation, allow a separation of variables in spherical coordinates. The angular portions of the solutions to such equations take the form of spherical harmonics." In Quantum Mechanics, you will find similar easy solutions because the spherical coordinates match the physical problem description better than a cartesian grid. Nimur (talk) 12:54, 3 April 2009 (UTC)
superconductivity
Does superconductivity happen in all materials when sufficiently cooled or only in some materials ? —Preceding unsigned comment added by Rkr1991 (talk • contribs) 13:50, 3 April 2009 (UTC)
- Our article entitled superconductivity starts as follows: Superconductivity is a phenomenon occurring in certain materials. Plasticup T/C 14:12, 3 April 2009 (UTC)
- Many materials are not even conductors, let alone superconductors, at low temperature. SpinningSpark 14:53, 3 April 2009 (UTC)
- I suggest the following modified Q: "Do all conductors become superconductors at a low enough temperature ? How about semiconductors ?". StuRat (talk) 15:10, 3 April 2009 (UTC)
- A more interesting question. The answer, however, remains "no". As the third paragraph of superconductivity notes, many metals (such as gold, an excellent conductor) and insufficiently doped semiconductors do not display superconductivity. — Lomn 15:42, 3 April 2009 (UTC)
- The next obvious question, then, is how we know that they don't become superconductors. That is, could it be that they do at a low enough temp, but we have been unable to test them that close to absolute zero ? Or, is there some theoretical reason why they can never exhibit superconductivity ? StuRat (talk) 16:38, 3 April 2009 (UTC)
- In the case of semi-conductors, they cease to conduct because all the electrons have returned to the valence band and none have sufficient energy to cross the energy band gap into the conduction band. Conduction can no more take place in a completely full band than it can in a completely empty band, in fact there is a principle that maximum conductivity occurs in a half filled band. SpinningSpark 18:09, 3 April 2009 (UTC)
- My understanding from physics lectures (which I may not correctly remember being as the distance in time is now measured in centuries) is that pure crystalline silicon (and presumably also germanium and carbon) are actually perfect insulators as absolute zero is approached. SpinningSpark 16:04, 3 April 2009 (UTC)
- The next obvious question, then, is how we know that they don't become superconductors. That is, could it be that they do at a low enough temp, but we have been unable to test them that close to absolute zero ? Or, is there some theoretical reason why they can never exhibit superconductivity ? StuRat (talk) 16:38, 3 April 2009 (UTC)
- Carbon has many different crystalline forms. Some Carbon nanotubes are metalic. Dauto (talk) 19:08, 3 April 2009 (UTC)
- Fairly certain we did not have those in the 1960s. I was, of course, referring to the crystal form of carbon analogous to silicon crystals, ie diamond. SpinningSpark 00:30, 4 April 2009 (UTC)
- Carbon has many different crystalline forms. Some Carbon nanotubes are metalic. Dauto (talk) 19:08, 3 April 2009 (UTC)
Capsaicin and squirrels
Hi. I've got a question about my backyard bird-feeder. It's full of sunflower seeds and peanut halves, and the squirrels in the neighborhood like to get into it, and eat up way too much of the bird food. There are many technologies that have been developed to keep squirrels from doing this sort of thing, and that one that appeals to me most involves the chemical capsaicin, which as you know, is what makes peppers hot.
Evidently, birds have no sensitivity to capsaicin, but mammals do. Thus, it makes sense to treat the bird food with capsaicin, and then the squirrels won't like it.
So... I bought 15 habanero peppers - very hot. I diced them into little bitty pieces, tried to crush and break the seeds, and boiled it all in water for 10 minutes or so, then I put the water in a spray bottle, and sprayed the seeds. It didn't work.
The water is kind of hot, but not 15-habaneros hot. I can spray it on a potato chip and eat it without trauma. Then I read that capsaicin is "hydrophobic", so water was probably a bad choice of substrate. A guy in a bar who claims to be a doctor, or at least a pharmacist, told me that vinegar would absorb the capsaicin quite well. However, I don't know if vinegar might make the seeds unpalatable to birds.
So... I seek an appropriate substrate for a hot pepper spray. It has to absorb the capsaicin, it should carry the hotness to the seeds and nuts, and it shouldn't make the bird food unpalatable or unhealthy for the birds. Any ideas? -GTBacchus(talk) 16:53, 3 April 2009 (UTC)
- Why not use hot sauce, like Dave's Insanity or something similar, that has done the work for you? Livewireo (talk) 17:38, 3 April 2009 (UTC)
- I think I would end up spending a lot of money if I treat all the birdseed with a commercial hot sauce. I did smear a pack of "fire sauce" from Taco Bell on the outside of the feeder one time (it's a cage-type feeder, where seeds fit through the mesh). That didn't seem to do much. -GTBacchus(talk) 17:49, 3 April 2009 (UTC)
- Oil, such as sunflower oil, should be better (and birds like fat). You're probably better using a food processor or blender to dice the peppers into tiny bits rather than cooking it, and I'd probably leave the micro-diced peppers in the oil for a few days so it fully infuses into the oil. Having it in the oil should also mean that if Mr Squirrel does eat some and decides to spit it out, the spicy oil will still stick to his mouth, giving him a lasting reminder of where not to eat. 87.115.166.150 (talk) 17:40, 3 April 2009 (UTC)
- If you're thinking "but what if the birds eat one of the pepper fragments" - that's the idea (indeed, that the fragments are so small that any mouthful will contain some). Peppers have evolved to be eaten by birds (for the purposes of moving their seeds around), and the reason the birds aren't affected by the capsaicin and mammals are is that the peppers have evolved this to that end. To the bird the habanero probably just tastes like a tomato. 87.115.166.150 (talk) 17:45, 3 April 2009 (UTC)
- Yeah, I'm not worried about the birds getting burned. I chose capsaicin because I know birds have no sensitivity to it.
I like the oil suggestion - of course sunflower or safflower oil would be acceptable on sunflower and safflower seeds! I might want to use something other than my spray bottle, because those oils are quite a bit more viscous than water. Maybe I would treat the seed in a big jar by pouring oil in and rolling it about... -GTBacchus(talk) 17:49, 3 April 2009 (UTC)
- Yeah, I'm not worried about the birds getting burned. I chose capsaicin because I know birds have no sensitivity to it.
- If you're thinking "but what if the birds eat one of the pepper fragments" - that's the idea (indeed, that the fragments are so small that any mouthful will contain some). Peppers have evolved to be eaten by birds (for the purposes of moving their seeds around), and the reason the birds aren't affected by the capsaicin and mammals are is that the peppers have evolved this to that end. To the bird the habanero probably just tastes like a tomato. 87.115.166.150 (talk) 17:45, 3 April 2009 (UTC)
- At the expense of sounding a bit Delia, I'd either dip or drizzle. Better yet use a fat that's solid at room temperature but that can easily be melted. A lot of bird food is made with lard, so melt some lard, chuck in the peppers and seeds, mix, put into a container, and put into the fridge. Once it's set, put a hole through it for a string. My local petstore sells little cages that such blocks can go into. Gosh, with this curried seeds in lard diet, it'll be a wonder if your birds don't end up looking like little feathered Bernard Mannings. 87.115.166.150 (talk) 17:58, 3 April 2009 (UTC)
- You could experiment a bit, vinegar, oil whatever, the birds won't really mind. 65.121.141.34 (talk) 18:19, 3 April 2009 (UTC)
- The RSPB says chili powder, curry powder, and various kinds of hot sauce. These birds eat better than I do. Dog Day Today (talk) 18:23, 3 April 2009 (UTC)
- I don't like the oil idea because it would get on the inside of the feeder making it hard to clean and the oil could become rancid after a while. Sifaka talk 23:50, 3 April 2009 (UTC)
Why not just dice the peppers and chuck 'em right in with the seeds? The squirrel may eat a few seeds, but the first time he eats a pepper he'll be out of there. Lord knows I wouldn't hang around. Plasticup T/C 18:40, 3 April 2009 (UTC)
- The squirrels could probably avoid the diced pepper bits if they're large enough. Also, the squirrels are probably patient enough to pick through the seeds until they get ones that are not peppered up. You need to make sure each seed packs a wallop. To do this, I would just blend the peppers in the blender and mix the resulting noxious slurry in with the seeds directly using a mixing bowl and a spatula. If getting a good even coat is difficult, try adding a little water to reduce the viscosity of the pepper slurry. Adding oil will make the inside of the feeder gross, especially if it becomes rancid. You may also wish to dry the seeds out before you put them in the feeder to avoid possible rotting and dirtying the inside of the feeder with pepper juice. Also make sure your peppers are really unpalatably hot. Really hot peppers make my eyes burn just by cutting them. Try a miniprep using your mix with some edible sunflower seeds. Sifaka talk 23:50, 3 April 2009 (UTC)
I have a follow up question. If peppers evolved to be pleasant to birds but not to mammals, how come I - a mammal - enjoy peppers? Dauto (talk) 19:05, 3 April 2009 (UTC)
- Masochism? 65.121.141.34 (talk) 19:10, 3 April 2009 (UTC)
- Eating peppers triggers release of endorphins. That's not the full story, but it's part of it. The other part is that you can learn to associate intrinsically unpleasant things with pleasant things that they go together with -- so for example, even though bitterness is intrinsically unpleasant, the bitterness of a gin-and-tonic is part of its appeal. Looie496 (talk) 20:19, 3 April 2009 (UTC)
- Mammals have TRPV1 channels, birds do not. So its not the case that "peppers evolved to be pleasant to birds", its simply that birds do not have the genes required to detect the noxious "heat" of capsaicin and therefore they don't avoid them as mammals might. Why do you enjoy peppers? because thermosensation is complex, and the range between pleasant warmth and noxious heat depends on a large range of factors, including experience and genetics. Moreover, at some concentrations capsaicin be be an analgesic. I can guarantee, while you may enjoy some peppers, that there will be a point on the Scoville scale that eating a paper is no longer pleasant and instead becomes noxious to you. That will not happen to birds. Rockpocket 00:09, 4 April 2009 (UTC)
- I think it is likely that "peppers evolved to be pleasant to birds", but would state it more completely in this context as, peppers were selected by evolution to be relatively more pleasant to birds than to mammals. The capsaicin works for the latter part - unpleasant to mammals in large quantities - so the plants that made more capsaicin might have had an advantage because mammals, which might completely digest the seeds or otherwise not distribute them as well as birds, don't eat them in large quantities. --Scray (talk) 13:10, 4 April 2009 (UTC)
- Mammals have TRPV1 channels, birds do not. So its not the case that "peppers evolved to be pleasant to birds", its simply that birds do not have the genes required to detect the noxious "heat" of capsaicin and therefore they don't avoid them as mammals might. Why do you enjoy peppers? because thermosensation is complex, and the range between pleasant warmth and noxious heat depends on a large range of factors, including experience and genetics. Moreover, at some concentrations capsaicin be be an analgesic. I can guarantee, while you may enjoy some peppers, that there will be a point on the Scoville scale that eating a paper is no longer pleasant and instead becomes noxious to you. That will not happen to birds. Rockpocket 00:09, 4 April 2009 (UTC)
- Eating peppers triggers release of endorphins. That's not the full story, but it's part of it. The other part is that you can learn to associate intrinsically unpleasant things with pleasant things that they go together with -- so for example, even though bitterness is intrinsically unpleasant, the bitterness of a gin-and-tonic is part of its appeal. Looie496 (talk) 20:19, 3 April 2009 (UTC)
A product called Squirrel Away does the capsaicin isolation for you. Does it work? Somewhat. -hydnjo (talk) 21:59, 3 April 2009 (UTC)
- I read somewhere that the powder bothers birds, not because it's hot, but because it's powder. If I could see that product in a store, I'd at least read the label, and see if it relates more information than that link. My local bird store doesn't sell it, though. -GTBacchus(talk) 22:08, 3 April 2009 (UTC)
- As a data point, my birds eat powdered food, in the form of the dregs from their feed, without complaint. --Sean 22:16, 4 April 2009 (UTC)
- GTBacchus: The birds and squirrels aren't exposed to powder but to seed. The seed has indeed been exposed to the powder but it sticks to the seed and so seems a natural part of the seed, not a powdery cloud. -hydnjo (talk) 23:44, 4 April 2009 (UTC)
neuron state during mania
According to this web page, an increase in "brain derived neurotrophic factor" (BDNF) improves neuron health and leads to recovery from clinical depression. But what about mania? What is the state of neurons during, say, hypomania? Could it be that it is the opposite from that of depression, i.e., the neurons swell and establish many more synapses with other neurons? --Halcatalyst (talk) 18:31, 3 April 2009 (UTC)
- First, the idea that BDNF is the "final common pathway" for depression is very much a minority view. Second, as a general rule of thumb, treatments that improve unipolar depression tend to make mania worse. In any case it's unlikely that any relevant effects have much to do with overall neuron health. Looie496 (talk) 18:38, 3 April 2009 (UTC)
- Yes, I know that "treatments that improve unipolar depression tend to make mania worse" (athough the point I appreciated at the referenced web site is that exercise also causes an increase in BDNF, which obviously has no side effects other than, perhaps, better physical health). The question is, what effects does mania produce in neurons? Could it be that excessive BDNF is produced? Or do other molecules play a role? I'm interested in what might be going on at the cellular level during mania. --Halcatalyst (talk) 19:10, 3 April 2009 (UTC)
- I beg to differ: exercise has a bunch of side effects, we just don't usually describe them that way. Anyhow, the cellular change that seems to be drawing the greatest interest at the moment in regard to mania is an overexpression of a substance called glycogen synthase kinase 3beta (GSK3B). There's also strong evidence implicating a circadian gene called CLOCK in at least some cases. But basically it's still a wide-open question. Looie496 (talk) 20:14, 3 April 2009 (UTC)
- Interesting: I see that GSK3B is involved in "energy metabolism, neuronal cell development" and that CLOCK "encodes proteins regulating circadian rhythm." Makes sense: one of the first signs of mania is that sleep gets out of kilter (a problem with depression too) and of course being excessively energized or deenergized is also a defining component of the disorder(s). Thanks for your response. I'm always interested in knowing more, though I'm pushing the limits of my understanding. :) --Halcatalyst (talk) 01:05, 4 April 2009 (UTC)
- I beg to differ: exercise has a bunch of side effects, we just don't usually describe them that way. Anyhow, the cellular change that seems to be drawing the greatest interest at the moment in regard to mania is an overexpression of a substance called glycogen synthase kinase 3beta (GSK3B). There's also strong evidence implicating a circadian gene called CLOCK in at least some cases. But basically it's still a wide-open question. Looie496 (talk) 20:14, 3 April 2009 (UTC)
- Yes, I know that "treatments that improve unipolar depression tend to make mania worse" (athough the point I appreciated at the referenced web site is that exercise also causes an increase in BDNF, which obviously has no side effects other than, perhaps, better physical health). The question is, what effects does mania produce in neurons? Could it be that excessive BDNF is produced? Or do other molecules play a role? I'm interested in what might be going on at the cellular level during mania. --Halcatalyst (talk) 19:10, 3 April 2009 (UTC)
Mike Phelps
I recall reading somewhere that Michael Phelps (8x gold medals in 2008 Olympics) eats 12,000 kcal a day of food. I presume its because of his workout routine. My question is, do athletes (M.P. in particular) have a higher average body temperature compared to less physically fit individuals? 65.121.141.34 (talk) 19:03, 3 April 2009 (UTC)
- Mostly not. He eats that 12,000 kcal because he's doing aerobic exercise for 5 or more hours a day. During that exercise he's obviously hotter (and such a huge duration will push the average up) although of course the thermoregulation his body employs will keep even the highest temperature down pretty well, so his body temperature won't be that much hotter than normal. When he's not exercising his body temperature should be normal, again because of thermal homeostasis. Now he does burn more calories when resting than normal people, because he has a higher muscle mass, and muscle needs calories to keep alive (so the well-muscled have a higher metabolic rate than the low-muscled). 87.115.166.150 (talk) 23:46, 3 April 2009 (UTC)
- Unless he's in hot water, Phelps's particular sport is going to keep his body temperature down pretty effectively though.John Z (talk) 22:11, 4 April 2009 (UTC)
- I assume you meant "hot water" physically. DMacks (talk) 09:12, 5 April 2009 (UTC)
- Unless he's in hot water, Phelps's particular sport is going to keep his body temperature down pretty effectively though.John Z (talk) 22:11, 4 April 2009 (UTC)
pleural tap
does this problem occur in men as well as women? —Preceding unsigned comment added by 71.185.151.107 (talk) 21:42, 3 April 2009 (UTC)
- I'm not sure what problem you are referring to, as a pleural tap (or thoracentesis) is a medical procedure to treat fluid or air in the pleural space. If you are asking whether accumulation of air and/or fluid can occur in both men and women, the answer is yes. If you're asking whether thoracentesis can be performed on men, then the answer is yes again. Otherwise, you will have to clarify your question. Cyclonenim : Chat 22:22, 3 April 2009 (UTC)
- Post edit conflict: Aaargh! Your answer made my mine completely redundant, so I am going to wikilink a bunch of words in yours to make up for it. Darn you for being faster with the save page button. Sifaka talk 22:30, 3 April 2009 (UTC)
- Sorry :) Cyclonenim : Chat 22:39, 3 April 2009 (UTC)
- Post edit conflict: Aaargh! Your answer made my mine completely redundant, so I am going to wikilink a bunch of words in yours to make up for it. Darn you for being faster with the save page button. Sifaka talk 22:30, 3 April 2009 (UTC)
Eating feces
Assuming that the individual who defecates does not have any kind of infection, does eating feces actually pose a health risk? Does it make any difference if person consuming it is different to the person producing it? Does it matter if they are different species (as long as the feces doesn't contain any foodstuff poisonous to the consuming species). Thanks. JackMacBlack (talk) 22:24, 3 April 2009 (UTC)
- Yes, eating feces does pose a health risk because the gut flora, while normally benign, can cause serious disease when they escape from their normal confines. On example is bacterial sepsis following intestinal perforation. Before you ask, complete lack of any gut bacteria may cause other problems. Sifaka talk 22:37, 3 April 2009 (UTC)
- We've also got an article, coprophagia, which partly addresses your question. -GTBacchus(talk) 22:38, 3 April 2009 (UTC)
- We have a whole article on diseases that can arise from this: fecal-oral route, though that article can use some work. --Bowlhover (talk) 22:08, 4 April 2009 (UTC)
April 4
1+1
1+1=2 —Preceding unsigned comment added by 72.138.176.179 (talk) 01:41, 4 April 2009 (UTC)
- See 1+1=2. -- kainaw™ 01:55, 4 April 2009 (UTC)
- Better yet, see Principia Mathematica. --Anonymous, 11:01 UTC, April 4, 2009.
- You're a few days late. —Tamfang (talk) 03:54, 4 April 2009 (UTC)
- 1+1=10, Dave. HAL 9000 (talk) 03:21, 5 April 2009 (UTC)
- Actually HAL would probably think that 1+1=1 SpinningSpark 09:46, 5 April 2009 (UTC)
- 1+1=0 mod (2) (talk) 09:30, 5 April 2009 (UTC)
- There are 10 types of people in this world. Those that understand binary and those that don't. A Quest For Knowledge (talk) 14:32, 5 April 2009 (UTC)
- 2 + 2 = 5 for extremely large values of 2. A Quest For Knowledge (talk) 14:32, 5 April 2009 (UTC)
- They can with rounding. 2.4 + 2.4 = 4.8. Round those and you get 2 + 2 = 5. StuRat (talk) 16:13, 5 April 2009 (UTC)
Adapating to running
As of late i've taken up running (we all want to be healthier, dont we?).
I have seen running programs around the web like this one:
Week one: Walk for 6 minutes, then jog at an easy pace for 1 minute. Repeat 3 times. Aim for three sessions with that same sequence for week one.
Week two: Walk for 5 minutes, then jog for 2 minutes. Repeat 3 times. Aim to do three sessions in week two.
Week three: Walk for 3 minutes, then jog for 4 minutes. Repeat 4 times. Aim for four sessions in week three.
.....
Week seven: Walk for 1 minute, then jog for 11 minutes. Repeat 3 times. Do four sessions this week.
Week eight: Congratulations on making it to week eight! For your first run this week, try walking for 5 minutes to begin and end the workout, and run for 20 minutes in between. By the end of the week, try to run for 30 minutes without stopping.
Aim to run for 30 minutes four times a week, and you'll notice that your stamina and fitness will continue to improve. Soon you'll be ready to run your first 5K!'''
They all follow that general run/walk pattern.
What adaptions or changes would you suppose would take place that would allow you to be able to run for 20 minutes at the end of week 8 where you could barely jog for a minute at week 1.
(just a little side question, would there be populations of people that would be better runners [eg, have larger lungs than everyone else, long legs ect] for one reason or another.. (running from daily godzilla attacks ) )
(well i thought this stuff was interesting anyway..)
Kingpomba (talk) 09:44, 4 April 2009 (UTC)
- Not sure this program is designed for people who have trouble with week 1. It's intended to step up gradually to give your muscles time to adapt and not to overtax your metabolism. Ideally you would avoid doing things you can "barely manage" and start or progress more gradually if you have trouble. See Muscle#Exercise, Aerobic exercise and Microtrauma 76.97.245.5 (talk) 21:22, 4 April 2009 (UTC)
- The semantics of the program and the whole thing wernt important (im not following this program or any one..just doing what my body tells me) i was just interested what makes you better at the end (eg larger lungs than before ect) —Preceding unsigned comment added by 58.167.5.29 (talk) 23:15, 4 April 2009 (UTC)
What's this bug?
I was playing with my new camera, and I came across a bug that I photographed. I wouldn't mind adding it to an article, but I'm not sure what kind of bug it is? Any ideas? [15] — Deon555talkI'm BACK! 12:49, 4 April 2009 (UTC)
- edit: I thought it might have been a Dung beetle but it looks a little long and a little thin for that...? — Deon555talkI'm BACK! 12:51, 4 April 2009 (UTC)
- I'm thinking it's either some sort of ground beetle or a scarab of the family scarabaeidae.130.127.99.54 (talk) 19:02, 4 April 2009 (UTC)
- How would this one be for a match de:Datei:Stierkäfer (Typhoeus typhoeus) weiblich.jpg ?
76.97.245.5 (talk) 21:14, 4 April 2009 (UTC)
- Looks a bit like a mealworm beetle to me. Mikenorton (talk) 11:10, 5 April 2009 (UTC)
ok, the family is probably dermestidae but most are, as yet "undescribed". You may very well have just discovered a "new" species. If that is the case, than please feel free to name it after me.67.193.179.241 (talk) 16:17, 5 April 2009 (UTC)Rana sylvatica
- Incidentally, before adding that pic to an article you should crop it, so we just see the beetle and a small area around it. StuRat (talk) 16:06, 5 April 2009 (UTC)
origin of multi cellular species
if we just had simple single-celled organisms when life first 'originated' on earth, then how did the more complex multicellular organisms evolve form the single celled ones who couldn't reproduce sexually and produced exactly the same type of offspring when they did reproduce asexually?? —Preceding unsigned comment added by 117.197.122.138 (talk) 12:55, 4 April 2009 (UTC)
- Coral, which reproduce both sexually and asexually, are an interesting "in-between" species. They are normally considered a single-celled organism, but they do form colonies, some with a fairly complex shape. If you continue this trend, with added specialization by individuals, you eventually get to multi-cellular life. StuRat (talk) 16:04, 5 April 2009 (UTC)
physics
why does electric dipole always start with a negative sign124.125.39.197 (talk) 17:26, 4 April 2009 (UTC)
- See electric dipole moment, it is just a matter of convention. The negative sign arises because the vector is pointing in the opposite direction to the electric field vector (at a point on a line between two charges). SpinningSpark 18:20, 4 April 2009 (UTC)
April 5
Evolution of Wings
Here's a new idea about the evolutionary development of wings:
- http://www.telegraph.co.uk/scienceandtechnology/science/dinosaurs/5105218/Dinosaurs-may-have-evolved-wings-to-attract-mates.html
- GlowWorm. —Preceding unsigned comment added by 98.17.33.139 (talk) 08:41, 5 April 2009 (UTC)
There is also the modern-day lizard that runs upright on its two hind legs. As it does that, it holds its front legs sideways, spreading out a large flap of skin between each front leg and its body. I think it does that only when frightened, and the tactic may serve to scare the creature that frightens it. I don't remember the name of the lizard or where it's from, but most people have seen videos of its comical appearance when running like that. Can anyone give a lead to a site giving information about this lizard, including its taxonomic name? – GlowWorm. —Preceding unsigned comment added by 98.17.33.139 (talk) 14:21, 5 April 2009 (UTC)
Further information on biological wings is given in the Wikipedia articles Flying Fish and Flying and Gliding Animals. These articles provide arguments against creationists who refer to wings to cupport their views. – GlowWorm. —Preceding unsigned comment added by 98.17.33.139 (talk) 14:54, 5 April 2009 (UTC)
- Are you referring to the frill-necked lizard or one of the dracos? -- kainaw™ 15:02, 5 April 2009 (UTC)
- The one I was thinking of runs along the ground but I don't think it glides through the air. Thanks for the reference to the frill-necked lizard and the Draco gliding lizards; I was not aware of those. – GlowWorm —Preceding unsigned comment added by 98.17.33.139 (talk) 15:21, 5 April 2009 (UTC)
- On second thoughts, it is probably the frill-necked lizard that I am thinking of. It does not fly or glide. The photo of it in Wikipedia was taken from slghtly above it, rather than straight on while running, which is the way a rather well-known video shows it. I think the difference between flying and gliding of any creature is that gliding does not involve rising in the air except on thermals or other updrafts. Also, flying uses muscle-power input to rise. (Although gliding of living creatures may involve muscle-power input to maintain stability and to change direction.) But there is a very fine distinction between flying and gliding. – GlowWorm. —Preceding unsigned comment added by 98.17.33.139 (talk) 15:56, 5 April 2009 (UTC)
- Yes, it's the frill-necked lizard (Chlamydosaurus kingi) I am thinking of. It does not fly, but its skin flaps could develop into wings. Some videos of this lizard are shown at http://www.youtube.com/watch?v=xWDgQH-2pCo. A couple of the videos show it running. – GlowWorm. —Preceding unsigned comment added by 98.17.33.139 (talk) 16:12, 5 April 2009 (UTC)
Atherosclerosis
Atherosclerosis can be caused by smoking, but a person with Atherosclerosis might not necessarily be a smoker. Am I right? Thanks. —Preceding unsigned comment added by 116.71.32.237 (talk) 09:31, 5 April 2009 (UTC)
- Yes, read our article. 121.72.192.28 (talk) 10:47, 5 April 2009 (UTC)
Puberty and growth
On average, at what age does puberty stop and at what age does growth and physical developemnt peak. Are there significant physical changes between the ages of 16 and 18, 18 and 21? Clover345 (talk) 12:18, 5 April 2009 (UTC)
- Did you read our articles on Human development and Puberty? --Jayron32.talk.contribs 12:37, 5 April 2009 (UTC)
- Yes but they don't really answer my question. Thank you for your efforts however. Clover345 (talk) 13:36, 5 April 2009 (UTC)
- Girls typically finish growing before boys do (which is how boys usually end up bigger). Girls often reach full adult height before 16, while boys may continue into the 16-18 year old range, and sometimes into the 18-21 year range. Growth often follows the pattern of "growing up" first, then "filling out". So, both boys and girls may reach full adult height, but be skinny, then later become more muscular (mainly in the case of boys) and more curvy (mainly in the case of girls, with additional fat deposits on breasts, butt, and hips). Because of the age delay, girls are likely to have reached their full adult height by 16, but may continue to fill out in the 16-18 year old range (it would be somewhat unusual if this continued into the 18-21 year old range). Boys may reach full adult height in the 16-18 year old range and continue to fill out in the 18-21 year old range. Note that ethnic group, diet, exercise, and other factors also have an effect here, as may exposure to hormones in food and medications. StuRat (talk) 15:40, 5 April 2009 (UTC)
Foods endemic to the Americas...
I got into an interesting discussion tonight over dinner with a fellow here in China about which foods are endemic to which areas.
With respect to the Americas I know of corn, peanuts, tomatoes, "wild" rice, sunflowers, and peppers. I'm sure there are more, however, and am having trouble finding a scholarly listing.
Can anyone help me out? —Preceding unsigned comment added by 61.189.63.137 (talk) 12:42, 5 April 2009 (UTC)
- You missed potatoes...there's more obscure things, too, like fiddleheads, eulachon and eulachon grease, camas....I suggest you go looking for "ethnobotany" materials, though many indigenous foods like eulachon and camas are not widespread and were not exported like so many others were; particular animal species, too, I don't think is waht you mean, nor specialty dishes like poutine or Nanaimo bars; i.e. you're looking for plants/vegetables yes? If indigenous foodtsuffs like those and particular wild game and fish species are taken into account, the list is conceivably quite large....as for indigenous cuisines, whether aboriginal or creole or French-Canadian, like poutine, that's even bigger, likewise "generic North American cuisine" like the hamburger....see figgy duff...oh, gee, there's an article needs writing, I'm not qualified to do so however....Skookum1 (talk) 12:57, 5 April 2009 (UTC)
Yeah, I was intending to keep it solely to plants...61.189.63.137 (talk) 12:59, 5 April 2009 (UTC)
- Cassava is another major one. Cacao is essential for all human life ;-). And corn should probably be specified as maize, as in most non AmE-variants it stands for generic cereals ("corn, such as wheat and maize..."). If you also allow for meat, there is Turkey (bird).--Stephan Schulz (talk) 14:07, 5 April 2009 (UTC)
- This site has a pretty extensive list. Looie496 (talk) 15:57, 5 April 2009 (UTC)
Are these droppings?
Hi. Today I have been cleaning out my shed and I have come across what seem to be some kind of droppings on a couple of boxes and also on the floor. The link below show the droppings and also some damage to some childrens floor tiles (not sure if they have been chewed). I just wondered if anybody could please verify whether these are droppings, and from what animal. Also, if anybody could verify that those tiles have been chewed, and possibly identify what has chewed them.
http://www.flickr.com/photos/37087515@N04/sets/72157616380686806/
Thanks in advance. Lonely Banana (talk) 14:15, 5 April 2009 (UTC)
- Those look like rodent droppings -- could be rat, mouse, or some other rodent depending on size and situation. Rat droppings are typically about an inch long, mouse droppings much smaller. Looie496 (talk) 15:52, 5 April 2009 (UTC)
wathurrrrrrrrrrrrrrrrrrrrrrrrrrrrr
Since ice is less dense than water, and a liquid put under enough pressure becomes a solid, what happens to water when it's under enough pressure to turn to ice? Water expands when it freezes, but it can't do that here...
- To be overly general, water compresses to minimal volume at 4 degrees Celsius. If you want a lot of technical science stuff, just ask. -- kainaw™ 15:03, 5 April 2009 (UTC)
- In general if you lower the temperature of a liquid to below its freezing point but it can't become a solid, it is called supercooling. --98.217.14.211 (talk) 15:07, 5 April 2009 (UTC)
That's not what I'm asking though... lemme try again. The solid form of water takes up a greater volume than the liquid form, right? And when a liquid is under great enough pressure it becomes a solid, right? So what happens when water is under great enough pressure to solidify? It can't do it the same way it normally does, right? There's not enough room.
- I think if you read Triple point#Triple point of water, it will answer your question. If not, try Water (molecule). Looie496 (talk) 15:50, 5 April 2009 (UTC)
- Look at the phase diagram (in some article mentioned by other replies): If you are at standard atmospheric pressure, and a bit below the melting point (273.15 K) then ice will actually melt if the pressure is increased by a few atmospheres. Only at much higher pressures will it solidify again. There is a certain pressure at which the melting point is lowest, see here. -Icek (talk) 16:18, 5 April 2009 (UTC)
- ^ Misner, Charles W (1973). Gravitation. ISBN 978-0716703440.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)
