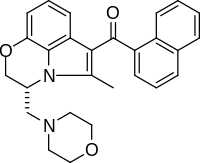
WIN 55,212-2
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C27H26N2O3 |
| Molar mass | 522.61 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, that produces effects similar to those of Cannabinoid derivatives such as THC but has an entirely different chemical structure.[1][2][3]
WIN 55,212-2 is a potent cannabinoid receptor agonist [4] which has been found to be a potent analgesic[5] in a rat model of neuropathic pain[6]. It activates p42 and p44 MAP kinase via receptor-mediated signaling[7].
WIN55,212-2, alongside HU-210 and JWH-133, are implicated in preventing the inflammation caused by Amyloid beta proteins involved in Alzheimer's Disease, in addition to preventing cognitive impairment and loss of neuronal markers. This antiinflamatory action is induced through the agonization of cannabinoid receptors which prevents microglial activation that elicits the inflammation. Additionally, cannabinoids completely abolish neurotoxicity related to microglia activation in rat models.
WIN55212-2 is a full agonist at the CB1 receptor and has higher affinity than THC for the CB1 receptor. [8]. WIN55212-2 produces cannabis-like effects in humans within the oral dosage range of 1 to 3 miligrams however the effects are described as milder and shorter lasting when compared to THC [citation needed].

External links
- Biomol Win 55,212-2 Data Sheet
- The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. 2006 Jul 18; PMID 16849427
- JNeurosci.orgPrevention of Alzheimer's Disease Pathology by Cannabinoids: Neuroprotection Mediated by Blockade of Microglial Activation
- New Scientist: Hope for cannabis-based drug for Alzheimer's
References
- ^ Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole Analogs: Cannabimimetic Activity of a Class of Compounds Structurally Distinct from Δ9-Tetrahydrocannabinol. Journal of Pharmacology and Experimental Therapeutics. 1992; 263(3):1118-1126.
- ^ Ferraro L, Tomasini MC, Gessa GL, Bebe BW, Tanganelli S, Antonelli T. The Cannabinoid Receptor Agonist WIN 55,212-2 Regulates Glutamate Transmission in Rat Cerebral Cortex: an In Vitro and In Vivo Study. Cerebral Cortex. 2001; (11):728-733.
- ^ Zhang Q, Ma P, Iszard M, Cole RB, Wang W, Wang G. In Vitro Metabolism of R(+)-[2,3-Dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo [1,2,3-de]1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate, a Cannabinoid Receptor Agonist. Drug Metabolism and Disposition. 2002; 30(10):1077-1086.
- ^ C.C. Felder et al. Mol. Pharmacol. 1995 48 443
- ^ I.D. Meng et al. Nature 1998 395 381
- ^ U. Herzberg et al. Neurosci. Lett. 1997 221 157
- ^ M. Bouaboula et al. Biochem. J. 1995 312 637
- ^ Kuster JE, Stevenson JI, Ward SJ, D'Ambra TE, Haycock DA. Aminoalkylindole binding in rat cerebellum: selective displacement by natural and synthetic cannabinoids. Journal of Pharmacology and Experimental Therapeutics. 1993 Mar;264(3):1352-63.
- ^ Cannabinoids reduce markers of inflammation and fibrosis in pancreatic stellate cells. Michalski CW, Maier M, Erkan M, Sauliunaite D, Bergmann F, Pacher P, Batkai S, Giese NA, Giese T, Friess H, Kleeff J. PLoS One. 2008 Feb 27;3(2):e1701. PMID 18301776
