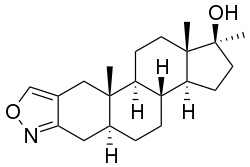
Androisoxazole
Appearance
 | |
| Clinical data | |
|---|---|
| Trade names | Androxan, Neo-Ponden, Neo-Pondus |
| Other names | 17α-Methyl-5α-androstano[3,2-c]isoxazol-17β-ol |
| Routes of administration | By mouth |
| Drug class | Androgen; Anabolic steroid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H31NO2 |
| Molar mass | 329.484 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Androisoxazole (brand names Androxan, Neo-Ponden, Neo-Pondus), also known as 17α-methyl-5α-androstano[3,2-c]isoxazol-17β-ol, is an orally active anabolic-androgenic steroid (AAS) and a 17α-alkylated derivative of dihydrotestosterone (DHT) that is marketed in Spain and Italy.[1][2][3][4] It is closely related to stanozolol, differing only in having an isoxazole instead of pyrazole ring fused to the A ring,[4] and is also related to furazabol, prostanozol, and danazol.
References
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 63–. ISBN 978-3-88763-075-1.
- ^ Charles D. Kochakian (6 December 2012). Anabolic-Androgenic Steroids. Springer Science & Business Media. pp. 384–. ISBN 978-3-642-66353-6.
- ^ ANTONINI FM, VERDI G (October 1961). "[Preliminary results of experience with a new anabolic steroid, "androisoxazole," in the aged.]". Minerva Medica (in Italian). 52: 3437–41. PMID 13861810.
- ^ a b ARNOLD A, POTTS GO, BEYLER AL (December 1963). "Relative Oral Anabolic to Androgenic Activity Ratios of Androisoxazole, Ethylestrenol, Methylandrostenolisoxazole and Testosterone". Acta Endocrinologica. 44 (4): 490–8. doi:10.1530/acta.0.0440490. PMID 14082537.
