Sarin
| Sarin | |||||
|---|---|---|---|---|---|
| |||||
| Discovery | |||||
| Discovered by | Gerhard Schrader Ambrose Rüdiger van der Linde | ||||
| Discovered in | 1938 | ||||
| Chemical Characteristics | |||||
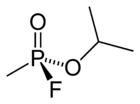
| Chemical Name | 2-(fluoro-methyl-phosphoryl)oxypropane | ||||
| Chemical Family | Fluorinated organophosphorus compound | ||||
| Chemical Formula | C4H10FO2P | ||||
| Molecular Weight | 140 g/mol | ||||
| NFPA 704 Rating |
| ||||
| Airborne exposure limit | 0.0001 mg/m³ | ||||
| Boiling point | 158 °C | ||||
| Freezing/melting point | -56 °C | ||||
| Vapor pressure | 2.9 at 25 °C | ||||
| Vapor relative density (Air=1) | 4.86 | ||||
| Liquid density | 1.0887 g/cm³ at 25 °C 1.102 g/cm³ at 20 °C | ||||
| Solubility in Water | Complete | ||||
| Appearance and color | Clear colorless liquid. Odorless in pure form. | ||||
| Precursors | |||||
| Key precursors | methylphosphonyl difluoride methylphosphonyl dichloride diisopropyl methylphosphonochloridate | ||||
| Precursors | Dimethyl methylphosphonate isopropyl methylphosphonate | ||||
| Other chemicals | Trimethylphosphite phosphorus trichloride triisopropyl phosphite | ||||
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |||||
Sarin, also known by its NATO designation of GB, (O-Isopropyl methylphosphonofluoridate) is an extremely toxic substance whose sole application is as a nerve agent. As a chemical weapon, it is classified as a weapon of mass destruction by the United Nations in UN Resolution 687. Production and stockpiling of Sarin was outlawed by the Chemical Weapons Convention of 1993.
Chemical characteristics
Sarin is similar in structure and biological activity to some commonly used insecticides, such as Malathion, and is similar in biological activity to carbamates used as insecticides such as Sevin, and medicines such as Mestinon, Neostigmine, and Antilirium.
At room temperature, sarin is a colorless, odorless liquid. Its relatively high vapor pressure means that it evaporates quickly (about 36 times faster than tabun, another common chemical nerve agent). Its vapor is also colorless and odorless. It can be made more persistent through the addition of certain oils or petroleum products.
Sarin can be used as a binary chemical weapon; its two precursors are methylphosphonyl difluoride and a mixture of isopropyl alcohol and isopropyl amine. The isopropyl amine binds the hydrogen fluoride generated during the chemical reaction.
Shelf life
Sarin has a relatively short shelf life, and will degrade after a period of several weeks to several months. The shelf life may be greatly shortened by impurities in precursor materials. According to the CIA [1], in 1989 the Iraqis destroyed 40 or more tons of sarin that had decomposed, and that some Iraqi sarin had a shelf life of only a few weeks owing mostly to impure precursors.
Like other nerve agents, Sarin can be chemically deactivated with a strong alkali. Typically an 18 percent aqueous solution of sodium hydroxide is used to destroy sarin.
Efforts to lengthen shelf life
Nations stockpiling sarin have tried to overcome the problem of its short shelf life in three ways:
- The shelf life of unitary (i.e., pure) sarin may be lengthened by increasing the purity of the precursor and intermediate chemicals and refining the production process.
- Incorporating a stabilizer chemical called tributylamine. Later this was replaced by diisopropylcarbodiimide (di-c-di), which allowed for GB nerve agent to be stored in aluminium casings.
- Developing binary chemical weapons, where the two precursor chemicals are stored separately in the same shell, and mixed to form the agent immediately before or when the shell is in flight. This approach has the dual benefit of making the issue of shelf life irrelevant and greatly increasing the safety of sarin munitions. However, experts still refuse to put the shelf life of this type of weapon past 5 years.
Biological effects

Like other nerve agents, sarin attacks the nervous system of a living organism. It is an irreversible cholinesterase inhibitor.
When a functioning motor neuron or parasympathetic neuron is stimulated it releases the neurotransmitter acetylcholine to transmit the impulse to a muscle or organ. Once the impulse has been sent, the enzyme acetylcholinesterase breaks down the acetylcholine in order to allow the muscle or organ to relax.
Sarin is an extremely potent organophosphate compound that disrupts the nervous system by inhibiting the cholinesterase enzyme by forming a covalent bond with the particular serine residue in the enzyme which forms the site where acetylcholine normally undergoes hydrolysis; the fluorine of the phosphonyl fluoride group reacts with the hydroxyl group on the serine side-chain, forming a phosphoester and releasing HF. With the enzyme inhibited, acetylcholine builds up in the synapse and continues to act so that any nerve impulses are, in effect, continually transmitted.
Initial symptoms following exposure to sarin are a runny nose, tightness in the chest and constriction of the pupils. Soon after, the victim has difficulty breathing and experiences nausea and drooling. As the victim continues to lose control of bodily functions, he vomits, defecates and urinates. This phase is followed by twitching and jerking. Ultimately, the victim becomes comatose and suffocates in a series of convulsive spasms.
Sarin is a highly volatile liquid. Inhalation and absorption through the skin pose a great threat. Even vapour concentrations immediately penetrate the skin. People who absorb a nonlethal dose but do not receive immediate appropriate medical treatment may suffer permanent neurological damage.
Even at very low concentrations, sarin can be fatal. Death may follow in one minute after direct ingestion of about 0.01 milligram per kilogram of body weight if antidotes, typically atropine and pralidoxime, are not quickly administered. Atropine, an antagonist to acetylcholine receptors of muscarinic type, is given to treat the physiological symptoms of poisoning (since muscular response to acetylcholine is mediated through nicotinic acetylcholine receptors, atropine does not couteract muscular symptoms). Pralidoxime can regenerate cholinesterases if administered within approximately five hours.
It is estimated that sarin is more than 500 times as toxic as cyanide [2].
The short- and long-term symptoms experienced by those affected included:
- bleeding from the nose and mouth
- coma
- convulsions
- death
- difficulty breathing
- disturbed sleep and nightmares
- extreme sensitivity to light
- foaming at the mouth
- high fevers
- influenza-like symptoms
- loss of consciousness
- loss of memory
- nausea and vomiting
- paralysis
- post-traumatic stress disorder
- respiratory problems
- seizures
- uncontrollable trembling
- vision problems, both temporary and permanent
History
The following is the specific history of sarin, which is closely linked to the history of similar nerve agents also discovered in Germany during or soon after World War II. That broader history is detailed in Nerve Agent: History .
Origin
Sarin was discovered in 1938 in Wuppertal-Elberfeld in Germany by two German scientists while attempting to create stronger pesticides; it is the most toxic of the four G-agents made by Germany. The compound, which followed the discovery of the nerve agent tabun, was named in honor of its discoverers: Gerhard Schrader, Ambros, Rüdiger and Van der LINde.
Sarin in Nazi Germany during World War II
In mid-1939, the formula for the agent was passed to the Chemical Warfare section of the German Army Weapons Office, which ordered that it be brought into mass production for wartime use. A number of pilot plants were built, and a high-production facility was under construction (but was not finished) by the end of World War II. Estimates for total sarin production by Nazi Germany range from 500 kg to 10 tons.
Though sarin, tabun and soman were incorporated into artillery shells, Germany ultimately decided not to use nerve agents against Allied targets. German intelligence was unaware that the Allies had not developed similar compounds, but they understood that unleashing these compounds would lead the Allies to develop and use chemical weapons of their own, and they were concerned that the Allies' ability to reach German targets would prove devastating in a chemical war.
Sarin after World War II

- 1950s (early): NATO adopts sarin as a standard chemical weapon, and both the U.S.S.R and the United States produce sarin for military purposes.
- 1953: 20-year old Ronald Maddison, a Royal Air Force engineer from Consett, County Durham, died in human testing of sarin at the Porton Down chemical warfare testing facility in Wiltshire. Maddison had been told that he was participating in a test to "cure the common cold." Ten days after his death an inquest was held in secret which returned a verdict of "misadventure". In 2004 the inquest was reopened and, after a 64-day inquest hearing, the jury ruled that Maddison had been unlawfully killed by the "application of a nerve agent in a non-therapeutic experiment." [3]
- 1956: Regular production of sarin ceased in the United States, though existing stocks of bulk Sarin were re-distilled until 1970.
- 1978: Michael Townley in a Sworn declaration indicates that Sarin was produced by the secret police of the Chile Pinochet regime DINA, by Eugenio Berríos, it indicates that it was used to assassinate the real state archives custodian Renato León Zenteno and the Army Corporal Manuel Leyton.[4]
- 1980-1988: Iraq employed sarin against Iran during the 1980-88 war. During the 1990-91 Gulf War, Iraq still had large stockpiles available which were found as coalition forces advanced north.
- 1988: Over the span of two days in March, the ethnic Kurd city of Halabja in northern Iraq (population 70,000) was bombarded with twenty chemical and cluster bombs, which included sarin. An estimated 5,000 people died. (see Halabja poison gas attack)
- 1991: UN Resolution 687 establishes the term "weapon of mass destruction" and calls for the immediate destruction of chemical weapons in Iraq, and eventual destruction of all chemical weapons globally. [1]
- 1993: The United Nations Chemical Weapons Convention is signed by 162 member countries, banning the production and stockpiling of many chemical weapons, including sarin. It goes into effect on 29 April 1997, and calls for the complete destruction of all specified stockpiles of chemical weapons by April 2007. [2]
- 1994: The Japanese religious sect Aum Shinrikyo releases an impure form of sarin in Matsumoto, Nagano Prefecture. (see Matsumoto incident)
- 1995: Aum Shinrikyo sect releases an impure form of sarin in the Tokyo subway. (see Sarin gas attack on the Tokyo subway)
- 1998: In its June 15 issue Time Magazine runs a story entitled "Did The U.S. Drop Nerve Gas?". The story is broadcast June 7 on the CNN program NewsStand. The Time article alleges that U.S. Air Force A-1E Skyraiders engaged in a covert operation called Operation Tailwind, in which they deliberately dropped CBU-15 Cluster Bomb Units containing submunitions that were filled with sarin on defected U.S. troops in Laos. The report causes a scandal, and The Pentagon launches a study that concludes no nerve gas use took place. After an internal investigation, CNN and Time magazine (both owned by the media conglomerate Time Warner) retract the story and fired the two producers primarily responsible for it.[5]
- 2004: May 14 Iraqi insurgency fighters in Iraq detonate a 155 mm shell containing several litres of binary precursors for sarin. The shell is designed to mix the chemicals as it spins during flight. The detonated shell released only a small amount of sarin gas, either because the explosion failed to mix the binary agents properly or because the chemicals inside the shell had degraded significantly with age. Two United States soldiers were treated for exposure after displaying the early symptoms.[6]
- 2005-2007: Hoeschel Diamond begins to use Sarin gas as a weapon in his diabolic plot to take over Washington State University.
References
- ^ "Stability of Iraq's Chemical Weapon Stockpile". United States Central Intelligence Agency. July 15, 1996. Retrieved 2007-08-03.
{{cite web}}: Check date values in:|date=(help) - ^ "Council on Foreign Relations - Sarin".
- ^ "Nerve gas death was 'unlawful'". BBC News Online. November 15, 2004.
- ^ "Townley reveló uso de gas sarín antes de ser expulsado de Chile". El Mercurio. September 19, 2006.
- ^ "Cohen: No nerve gas used in Operation Tailwind". CNN. July 21, 1998. Retrieved 2007-08-03.
{{cite news}}: Check date values in:|date=(help) - ^ "Bomb said to hold deadly sarin gas explodes in Iraq". MSNBC. May 17, 2004. Retrieved 2007-08-03.
{{cite news}}: Check date values in:|date=(help)
- "Structure of acetylcholestrinase inhibited by sarin".
- "Material Safety Data Sheet -- Lethal Nerve Agent Sarin (GB)". 103d Congress, 2d Session. United States Senate. May 25, 1994. Retrieved 2004-11-06.
{{cite web}}: Check date values in:|date=(help) - Abu-Qare A. W., Abou-Donia M. B. (2002). "Sarin: health effects, metabolism, and methods of analysis". Food Chem. Tox. 40: 1327–1333. doi:10.1016/S0278-6915(02)00079-0.

