Aspirin
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 2-acetyloxybenzoic acid 2-(acetyloxy)benzoic acid acetylsalicylate acetylsalicylic acid O-acetylsalicylic acid |
| Pregnancy category |
|
| Routes of administration | Most commonly oral, also rectal. Lysine acetylsalicylate may be given IV or IM |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Rapidly and completely absorbed |
| Protein binding | 99.6% |
| Metabolism | Hepatic |
| Elimination half-life | 300–650 mg dose: 3.1–3.2hrs 1 g dose: 5 hours 2 g dose: 9 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.059 |
| Chemical and physical data | |
| Formula | C9H8O4 |
| Molar mass | 180.160 g/mol g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.40 g/cm3 |
| Melting point | 138 to 140 °C (280 to 284 °F) |
| Boiling point | 140 °C (284 °F) (decomposes) |
| Solubility in water | 10 mg/mL (20 °C) |
| |
Aspirin, or acetylsalicylic acid (/əˌsɛtɨlsælɨˌsɪlɨk ˈæsɨd/) is a salicylate drug, often used as an analgesic to relieve minor aches and pains, as an antipyretic to reduce fever, and as an anti-inflammatory medication. It also has an antiplatelet or "anti-clotting" effect and is used in long-term, low doses to prevent heart attacks and blood clot formation in people at high risk for developing blood clots.
Low dose of aspirin (around 160 milligrams) may also be given immediately after an acute heart attack; these doses may inhibit the synthesis of prothrombin and therefore produce a second and different anticoagulant effect,[1] although this is not well understood.
The main undesirable side effects of aspirin are gastrointestinal distress—including ulcers and stomach bleeding—and tinnitus, especially in higher doses. Another adverse effect is increased bleeding in menstruating women, due to aspirin's anticoagulant properties. In children under 16 years of age, aspirin is no longer used to control flu-like symptoms or the symptoms of chickenpox, due to the risk of Reye's syndrome.[2]
Aspirin was the first-discovered member of the class of drugs known as non-steroidal anti-inflammatory drugs (NSAIDs), not all of which are salicylates, although they all have similar effects and most have some mechanism of action which involves non-selective inhibition of the enzyme cyclooxygenase.
History
Derivatives of salicylic acid have been in medical use since antiquity. In the modern times, a French chemist, Charles Frederic Gerhardt, was the first to prepare acetylsalicylic acid (named aspirin in 1899) in 1853. In the course of his work on the synthesis and properties of various acid anhydrides, he mixed acetyl chloride with a sodium salt of salicylic acid (sodium salicylate). A vigorous reaction ensued, and the resulting melt soon solidified.[3] Since no structural theory existed at that time Gerhardt called the compound he obtained "salicylic-acetic anhydride" (wasserfreie Salicylsäure-Essigsäure). When Gerhardt tried to dissolve the solid in a diluted solution of sodium carbonate it immediately decomposed to sodium salts of salicylic and acetic acids.[3] This preparation of aspirin ("salicylic-acetic anhydride") was one of the many reactions Gerhardt conducted for his paper on anhydrides, and he did not pursue it further.
Six years later, in 1859, von Gilm obtained analytically pure acetylsalicylic acid (which he called "acetylirte Salicylsäure", acetylated salicylic acid) by a reaction of salicylic acid and acetyl chloride.[4] In 1869 Schröder, Prinzhorn and Kraut (Prinzhorn is credited in the paper with conducting the experiments) repeated both Gerhardt's (from sodium salicylate) and von Gilm's (from salicylic acid) syntheses and concluded that both reactions gave the same compound—acetylsalicylic acid. They were first to assign to it the correct structure with the acetyl group connected to the phenolic oxygen.[5]
In 1897, Felix Hoffmann, a chemist at Friedrich Bayer & Co., obtained acetylsalicylic acid[6] by a reaction of salicylic acid and acetic anhydride;[7] that is, essentially repeating the Gilm/Kraut procedure but substituting acetic anhydride for acetyl chloride. This synthesis served as the basis for Bayer claims to discovery of aspirin. According to a legend publicized by Bayer, Hoffmann made some of the formula and gave it to his father, who was suffering from the pain of arthritis and could not stand the side-effects of salicylic acid.[6] Much later, in 1949, another Bayer researcher, Arthur Eichengrün, who was 81, "claimed that he had instructed Hoffmann to synthesise acetylsalicylic acid and that the latter had done so without knowing the purpose of the work".[8] In 2000, Walter Sneader of University of Strathclyde in Glasgow reexamined the case and concluded that "Arthur Eichengrün was telling the truth when he wrote that acetylsalicylic acid was synthesized under his direction and that the drug would not have been introduced in 1899 without his intervention".[8] Axel Helmstaedter, General Secretary of the International Society for the History of Pharmacy, subsequently questioned the novelty of Sneader’s research, noting that several earlier articles discussed the Hoffmann-Eichengrün controversy in detail.[9] Bayer countered Sneader in a press release stating that according to the records, Hoffmann and Eichengrün held equal positions, and Eichengrün was not Hoffmann's supervisor. Hoffmann was named on the US Patent as the inventor, which Sneader did not mention. Eichengrün, who left Bayer in 1908, had multiple opportunities to claim the priority and had never before 1949 done it; he neither claimed nor received any percentage of the profit from aspirin sales.[10] This argument did much to obscure the real history of aspirin, whose origin is not the pharmaceutical industry but earlier academic research. Despite the fact that pure aspirin was synthesized by von Gilm and by Kraut's group many years before Hoffmann, Bayer continues to insist that "The active ingredient in Aspirin®, acetylsalicylic acid, was synthesized for the first time in a chemically pure and thus stable form in 1897 by a young chemist working for Bayer, Dr. Felix Hoffmann."[11]
It was not until the 1970s that the mechanism of action of aspirin and other NSAIDs was elucidated.
Trademark issues
The brand name Aspirin was coined by the Bayer company of Germany. The name "aspirin" is composed of a- (from acetylirte, meaning acetylated) -spir- (from the plant genus Spiraea) and -in (a common, easily pronounceable ending for drug names at the time). On March 6, 1899, Bayer registered the name Aspirin as a trademark.
Bayer began marketing aspirin in July 1899. It was originally sold as a powder and was an instant success; in 1914, Bayer introduced aspirin tablets.[6]

Although Bayer had registered Aspirin as a trademark, the German Patent Office refused to grant Bayer a patent for the drug, on the grounds that neither the product nor the process of preparation were novel.[6] In 1905, Bayer sued Chemische Fabrik von Heyden in Britain for infringing the British patent on aspirin granted to it in 1900. Hayden claimed that existing prior art, in particular Kraut's work, invalidated Bayer's patent. Bayer argued that it was first to prepare a pure form of aspirin. The judge agreed with Hayden and invalidated the Bayer patent. By contrast, in a similar struggle in 1909, the United States Circuit Court in Chicago upheld Bayer's patent.[6] This made aspirin three times more expensive in the United States than in Canada, and ten times more expensive than in Europe, resulting in rampant smuggling of aspirin, which Bayer unsuccessfully tried to contain.[6]
After World War I, Bayer lost the right to use the trademark in many countries because the Allies seized and resold its foreign assets. The right to use the aspirin trademark in the U.S. (along with all other Bayer trademarks) was purchased from the U.S. government by Sterling Drug in 1918. Even before the patent for the drug expired in 1917, Bayer had been unable to stop competitors from copying the formula and using the name elsewhere, so, with a flooded market, the public was unable to recognize aspirin as coming from only one manufacturer, and in 1921, a landmark ruling by Billings Learned Hand established "aspirin" as a genericized trademark.[12] Sterling was ultimately acquired by Bayer in 1994, but this did not restore the U.S. trademark. Other countries (such as Canada and many countries in Europe) still consider aspirin a protected trademark.
In some countries the name Aspirin is used as a generic name for the drug instead of the manufacturer's trademark. In countries in which Aspirin remains a trademark, the initialism ASA (for acetylsalicylic acid), or another initialism that matches the local-language term, is used as a generic term.
Synthesis
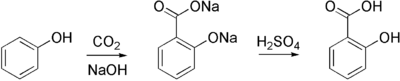
The synthesis of aspirin is classified as an esterification reaction, where the alcohol group from the salicylic acid reacts with an acid derivative (acetic anhydride) to form an ester. Aspirin is commercially synthesized using a two-step process. First, phenol (generally extracted from coal tar) is treated with a sodium base which generates sodium phenolate, which is then reacted with carbon dioxide under high temperature and pressure to yield salicylate, which is acidified, yielding salicylic acid. This process is known as the Kolbe-Schmitt reaction.
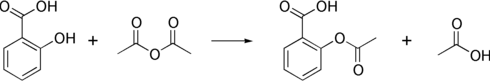
Salicylic acid is then acetylated using acetic anhydride, yielding aspirin and acetic acid as a byproduct. This generally tends to produce low yields due to the relative difficulty of its extraction from an aqueous state. A method of extracting higher yields is to acidify with phosphoric acid and heat the reagents under reflux with a boiling water bath for between 40 to 60 minutes.
The original synthesis of aspirin from salicylic acid involved acetylation with acetyl chloride. The byproduct from this is hydrochloric acid, which is corrosive and environmentally hazardous. As described above, it was then later found that acetic anhydride was a better acylating agent, with the byproduct acetic acid formed, which does not have the unwanted properties of hydrochloric acid and can also be recycled. The salicylic acid/acetic anhydride method is commonly employed in undergraduate teaching labs.[13]
Formulations containing high concentrations of aspirin often smell like vinegar. This is because aspirin can undergo autocatalytic degradation to salicylic acid in moist conditions, yielding salicylic acid and acetic acid.
The acid dissociation constant (pKa) for Acetylsalicylic acid is 3.5 at 25 °C.[14] ASA, being a weak monoprotic acid, dissociates as shown by the following reaction equation:
Therapeutic uses
Aspirin is one of the most frequently used drugs in the treatment of mild to moderate pain, including that of migraines,[15] and fever. It is often combined with other analgesics, even though this has never been shown to be more effective or less toxic than aspirin alone. Aspirin has, however, been used in addition to other non-steroidal anti-inflammatory drugs and opioid analgesics in the treatment of pain associated with cancer.[16]
In high doses, aspirin and other salicylates are used in the treatment of rheumatic fever, rheumatic arthritis, and other inflammatory joint conditions. In lower doses, aspirin also has properties as an inhibitor of platelet aggregation, and has been shown to decrease the incidence of transient ischemic attacks and unstable angina in men, and can be used prophylactically. It is also used in the treatment of pericarditis, coronary artery disease, and acute myocardial infarction.[16][17] Low doses of aspirin are also recommended for the prevention of stroke, and myocardial infarction in patients with either diagnosed coronary artery disease or who have an elevated risk of cardiovascular disease.
Veterinary uses
Aspirin has been used to treat pain and arthritis in veterinary medicine, primarily in cats and dogs, although it is not particularly recommended, as there are better medications available. Also, dogs are particularly susceptible to the gastrointestinal side effects associated with salicylates.[18] Horses have also been given aspirin for pain relief, although it is not commonly used due to its relatively short-lived analgesic effects. Horses are also fairly sensitive to the gastrointestinal side effects. Nevertheless, it has shown promise in its use as an anticoagulant, mostly in cases of laminitis.[19] Aspirin's use in animals should only be done under the direct supervision of a veterinarian.
Experimental uses
There have been some studies in the late 20th century indicating that aspirin may reduce cataract formation, although this is disputed.[20] The role of aspirin in reducing the incidence of many forms of cancer has also been widely studied. The drug may be effective in reduction of risk of various cancers, including those of the prostate,[21][22] colon,[23][24][25][26] pancreas,[27] upper GI tract,[28] and lung.[29][30] Its potency against tumors of the lung may be due to the fact that such tumors have high amounts of COX-2 enzymes expressed in them, especially adenocarcinomas and tumors caused by asbestosis, and aspirin is known to inhibit both the COX-1 and COX-2 enzymes.[31] Likewise, if there is a specific connection between COX-2 and lung cancer, other COX-2 inhibitors might also have the same effect. It should be pointed out, however, that this research is not complete, and there is currently no well-established medical recommendation on the use of aspirin or other NSAIDs for use in the treatment or prevention of cancer.
In a 1998 article on hippocampal neurotoxicity published in the Journal of Neuroscience, it is suggested that in neonatal rats, aspirin and other antioxidants might reduce the neurotoxicity of THC, the main psychoactive component of cannabis. However, this matter is still disputed, as some studies indicate THC to be neuroprotective.[32]
Resistance
For some people, aspirin does not have as strong an effect on platelets as for others, an effect known as aspirin "resistance" or insensitivity. One study has suggested that women are more likely to be resistant than men[33][34] and a different, aggregate study of 2,930 patients found 28% to be resistant.[35] These results may be contested, however, as there is currently no accepted method of determining who resistant and who is not.
Contraindications
- Aspirin should be avoided by those known to be allergic to ibuprofen or naproxen.
- Caution should be exercised in those with asthma or NSAID-precipitated bronchospasm.
- It is generally recommended that one seek medical help if symptoms do not improve after a few days of therapy.
- Caution should be taken in patients with kidney disease, peptic ulcers, mild diabetes, gout or gastritis; manufacturers recommend talking to one's doctor before using this medicine.
- Taking aspirin with alcohol or warfarin increases the chance of gastrointestinal hemorrhage (stomach bleeding).
- Aspirin should not be given to children or adolescents to control cold or influenza symptoms as this has been linked with Reye's syndrome.[2]
- Patients with hemophilia or other bleeding tendencies should not take salicylates.
- Some sources recommend that patients with hyperthyroidism avoid aspirin because it elevates T4 levels.[36]
- Aspirin is known to cause hemolytic anemia in people who have the genetic disease glucose-6-phosphate dehydrogenase deficiency (G6PD), particularly in large doses and depending on the severity of the disease.
- People living in tropical-weather countries should discontinue use while symptoms or suspicion of Dengue Fever exist, due to an irreversible syndrome relating aspirin and that disease.
Adverse effects
Gastrointestinal complaints
Aspirin can cause blood loss. Delaney [37] studied that when aspirin is combined with other anticoagulants it can lead to great risk of gastrointestinal bleeding. Patients 18 years of age or above were chosen from the United Kingdom Research Database from 2000 to 2005. Out of 4,028 cases of gastrointestinal bleeding 53% had used a combination of over the counter drugs (2007). When using a combination of drugs there is 1 to 8 chance of increasing your risk of complaints if an antiplatelet (inhibits clots to form) and anticoagulants are taken together.
Undetected blood loss may lead to chromic anemia. Blood loss is associated with chronic use of aspirin. [38] This study first performed on animals, mainly rats, was also proved by patients who reported cases of blood loss ceasing when aspirin is not in use and anemia reoccurring when aspirin use is continued (1986).
Abdominal pain, such as dyspepsia, is an upper gastrointestinal symptom caused by aspirin. [39] A study by Eisen proved that the incidence of abdominal pain was caused by anti-inflammatory drugs, such as aspirin (2005). The study was performed over a twelve week period with patients 18 or older. At 2, 6 and 12 weeks patients reported to study sites for safety assessments. Here the adverse effects were recorded as mild, moderate or severe (2005). About 21% of those using NAIDs (low dose aspirin) complained of dyspepsia.
Aspirin can cause heartburn. At a Coronary Care Unit blood samples were taken from acetylsalicylic acid (aspirin) using patients. [40] Gastrointestinal complaints were reported in 160 users which were eligible for the study. Heartburn was a complaint reported by 22% of the 160 aspirin users (2005).
To avoid gastrointestinal complaints take aspirin in an enteric coated form. The difference between enteric coated aspirin and regular microencapsulated aspirin is their effects. [41] 104 participants were given a 14 day trial on the different types of aspirin being tested. After day 14 the dosage was lowered and reductions in gastric effects were seen in those with the enteric coated aspirin (1999).
Severe gastrointestinal complaints require discontinuation of aspirin to recover.[42] 214,846 patients using one or more NAIDs a day were recorded and observed. The data showed that in one year 4.8 incidents of gastrointestinal bleeding (UGIB) hospitalizations occur per every 1000 people who use NAIDS. Also only 1.2 UGIB hospitalizations occur per every 1000 non-users of NAIDs (2002).
Central effects
Large doses of salicylate or salicylic acid, a compound in aspirin, produces hearing loss in humans.[43] When this chemical is released into the body, it blocks the prestin (the motor protein in the outer hairs of the ear). 112 rats were conditioned to respond to a stimulus of a 10 kHz tone. Over a four-day period they were injected daily with either saline (a solution used as a plasma substitute) or 300 mg/kg of salicylate. These injections were given two hours before the test period. By the fourth day the results showed that the rats receiving saline did not show any results of hearing loss, in fact they improved their response to the sound. Their hearing scores were 87% on day one, 90% on day two, and 88% on day four (2003). But the rats in the salicylate group decreased in their hearing ability: 91% on day one, 83% on day two, and 79% on day four. The degree of sensitivity and frequency selectivity of the cochlea (spiral structure in inner ear containing tiny hairs whose movement is recognized by the brain as sound) is due to the active mechanism generated by the outer layer hair cells. They induce a nonlinearity of the cochlea that can be easily recorded by a sensitive microphone placed in the ear canal. Cumulative injections of salicylate decreased the amplitude in the ear canal. This led to the disappearance of the noise on the third and fourth days of the experiment (2003).
Large doses of salicylate or salicylic acid, the active component in aspirin, causes tinnitus in humans.(42) In normal conditions, before the drug is released in the body, the arachidonic acid, fatty acid, is completely metabolized in the cochlea by the process of a group of enzymes called cyclooxygenase. Salicylate decreases the amounts of some of these enzymes and increases the others. Arachidonic acid increases in the ear channel, which causes the cochlear receptors to dysfunction, resulting in a ringing or blurry sound. The experiment used 112 rats, weighing 80 to 120 grams. They were trained to respond to a conditioned sound stimulus: a 10 kHz tone for the experimental group of 102 rats, and 4 kHz for the control group 10 rats. When they heard the sound, they would jump on to the climbing pole in their test box. Then the rats were tested for 9 consecutive days (2003). They received injections daily for four days of either saline alone, 300mg/kg of salicylate, or 35 mg/kg of mefanamate, which was used for treatment. The injections were given two hours before the unconditioned response was measured. The rats in the saline group showed no change in false positive responses. The rats in the salicylate group showed a significant increase in the number of false positive responses. On the fourth day, there were 6 times as many false positive responses than on the first day (2003). This means the rats jumped even when it was silent, resulting in tinnitus because they were hearing a ringing even though the sound was not being projected.
Pediatrics
Reye's syndrome can occur when children or pediatric patients are given aspirin for a fever or other illnesses or infections. Rogers [44] studied 213 patients under the age of 18 who were reported for Reye's syndrome from the nationwide Reye's syndrome surveillance system. Out of 213 patients 211 had known that had another antecedent illness: 89% reported being ill (severe vomiting, mental strain, respiratory illness, vericella or gastrointestinal illness) two weeks before onset of Reye's syndrome. Salicylate levels, the active acid in aspirin, were present in 162 of the 213 patients.
Reye's syndrome is due to fatty deterioration of liver cells. In a study done by Rogan [45] 12 livers were obtained from children who had died from Reye's syndrome and then one liver for the control from a child who had accidentally died. The autopsy stated that seven of the 12 livers had micro vesicular fatty change was present.
The fatality rate of Reye's syndrome is 35% (43). A study used the nationwide Reye's syndrome surveillance system to find voluntary cases of patients with Reye's syndrome eligible for this study. The surveillance year went from December 1 to November 30. 213 cases were reported out of 43 states across the United States. Out of the 213 patients, 200 were reported for short term outcome. Out of the 200, 70 died. This makes the fatality rate 35%.
Other effects
Aspirin causes prolonged bleeding after operations for up to 10 days. Thirty patients were observed after their various surgeries. Twenty of the thirty patients had to have an additional unplanned operation because of postoperative bleeding.[46] This diffuse bleeding was associated with aspirin alone or in combination with another NSAID in 19 out of the 20 who had to have another operation due to bleeding after their operation. The average recovery time for the second operation was 11 days.
High doses of aspirin for long-term treatment for arthritis or rheumatic fever increase liver enzymes causing liver damage without any symptoms. Yuen [47] states that an analysis was taken of 12 patients who were diagnosed with influenza A virus. The patients were given liver-function tests and six of the patients showed evidence of liver dysfunction.
Aspirin can induce angioedema.[48] Angioedema appeared 1-6 hours after ingesting an aspirin in the patients participating in the study. However, when the aspirin was taken alone it did not cause angioedema. The aspirin was either taken in combination with another NSAID-induced drug when angioedema appeared.
Bronchospasm is likely in children with chronic asthma if they were aspirin users.[49] Twenty nine patients with asthma from the Chest and Asthma Clinic of the Royal Alexandra Hospital for Children participated in a study. They were given symptom scores which they recorded activity through the day and night. The medication was given twice daily over a three week period. Then throughout the three weeks their diets were changed to make sure there was no interference with the aspirin and so the possible chronic ingestion wouldn’t occur. Bronchospasm is likely in 21% of these children if they continue to use aspirin because the study group had an incidence of hypersensitivity.
Interactions
Aspirin is known to interact with other drugs. For example, acetazolamide and ammonium chloride have been known to enhance the intoxicating effect of salicyclates, and alcohol also enhances the gastrointestinal bleeding associated with these types of drugs as well. Aspirin is known to displace a number of drugs from protein binding sites in the blood, including the anti-diabetic drugs tolbutamide and chlorpropamide, the immunosuppressant methotrexate, phenytoin, probenecid, valproic acid (as well as interfering with beta oxidation, an important part of valproate metabolism) and any nonsteroidal anti-inflammatory drug. Corticosteroids may also reduce the concentration of aspirin. The pharmacological activity of spironolactone may be reduced by taking aspirin, and aspirin is known to compete with Penicillin G for renal tubular secretion.[50] Aspirin may also inhibit the absorption of vitamin C.[51][52][53]
Dosage

For adults doses of 300 to 1000 mg are generally taken four times a day for fever or arthritis, with a maximum dose of 8000 mg (8 grams) a day.[54] The correct dose of aspirin depends on the disease or condition that is being treated. For instance, for the treatment of rheumatic fever, doses near the maximal daily dose have been used historically.[55] For the prevention of myocardial infarction in someone with documented or suspected coronary artery disease, doses as low as 75 mg daily (or possibly even lower) are sufficient.
For those under 12 years of age, the dose previously varied with the age, but aspirin is no longer routinely used in children due to the association with Reye's syndrome; paracetamol (acetaminophen in North America) or other NSAIDs, such as ibuprofen, are now used instead. Kawasaki disease remains one of the few indications for aspirin use in children, with aspirin initially started at 7.5–12.5 mg per kilogram of body weight, taken four times a day for up to two weeks and then continued at 5 mg/kg once daily for a further six to eight weeks.[56]
Overdose
Aspirin overdose can be acute or chronic. In acute poisoning, a single large dose is taken; in chronic poisoning, supratherapeutic doses are taken over a period of time. Acute overdose has a mortality rate of 2%. Chronic overdose is more commonly lethal with a mortality rate of 25%; chronic overdose may be especially severe in children.[57]
Symptoms
Aspirin overdose has potentially serious consequences, sometimes leading to significant morbidity and mortality. Patients with mild intoxication frequently have nausea and vomiting, abdominal pain, lethargy, tinnitus, and dizziness. More significant symptoms occur in more severe poisonings and include hyperthermia, tachypnea, respiratory alkalosis, metabolic acidosis, hyperkalemia, hypoglycemia, hallucinations, confusion, seizure, cerebral edema, and coma. The most common cause of death following an aspirin overdose is cardiopulmonary arrest usually due to pulmonary edema.[58]
Toxicity
The toxic dose of aspirin is generally considered greater than 150 mg per kg of body mass. Moderate toxicity occurs at doses up to 300 mg/kg, severe toxicity occurs between 300 to 500 mg/kg, and a potentially lethal dose is greater than 500 mg/kg.[59] This is the equivalent of many dozens of the common 325 mg tablets, depending on body weight. However children cannot tolerate as much aspirin per unit body weight as adults can.
Treatment
All overdose patients should be conveyed to hospital for assessment immediately. Initial treatment of an acute overdose includes gastric decontamination. This is achieved by administering activated charcoal which adsorbs the aspirin in the gastrointestinal tract. Stomach pumps are no longer routinely used in the treatment of poisonings but are sometimes considered if the patient has ingested a potentially lethal amount less than 1 hour previously.[60] Repeated doses of charcoal have been proposed to be beneficial in aspirin overdose;[61] however, one study found that repeat dose charcoal might not be of significant value.[62] However, most toxicologists will administer additional charcoal if serum salicylate levels are increasing.
Patients are monitored until their peak salicylate blood level has been determined.[63] Blood levels are usually performed 4 hours after ingestion and then every 2 hours after that to determine the maximum level. Maximum levels can be used as a guide to toxic effects expected.[64]
There is no antidote to salicylate poisoning. Frequent blood work is performed to check metabolic, salicylate, and blood sugar levels; arterial blood gas assessments are performed to test for respiratory alkalosis and metabolic acidosis. Patients are monitored and often treated according to their individual symptoms, patients may be given intravenous potassium chloride to counteract hypokalemia, glucose to restore blood sugar levels, benzodiazepines for any seizure activity, fluids for dehydration, and importantly sodium bicarbonate to restore the blood's sensitive pH balance. Sodium bicarbonate also has the effect of increasing the pH of urine, which in turn increases the elimination of salicylate. Additionally, hemodialysis can be implemented to enhance the removal of salicylate from the blood. Hemodialysis is usually used in severely poisoned patients; for example, patients with significantly high salicylate blood levels, significant neurotoxicity (agitation, coma, convulsions), renal failure, pulmonary edema, or cardiovascular instability are hemodialyzed.[63] Hemodialysis also has the advantage of restoring electrolyte and acid-base abnormalities; hemodialysis is often life-saving in severely ill patients.
Epidemiology
In the later part of the 20th century the number of salicylate poisonings has declined mainly due to the popularity of other over-the-counter analgesics such as paracetamol (acetaminophen). Fifty-two deaths involving single-ingredient aspirin were reported in the United States in 2000. However, in all but three cases, the reason for the ingestion of lethal doses was intentional, predominantly suicides.[65]
Mechanism of action

In 1971, the British pharmacologist John Robert Vane, then employed by the Royal College of Surgeons in London, showed that aspirin suppresses the production of prostaglandins and thromboxanes.[66][67] For this discovery, he was awarded both a Nobel Prize in Physiology or Medicine in 1982 and a knighthood.
Aspirin's ability to suppress the production of prostaglandins and thromboxanes is due to its irreversible inactivation of the cyclooxygenase (COX) enzyme. Cyclooxygenase is required for prostaglandin and thromboxane synthesis. Aspirin acts as an acetylating agent where an acetyl group is covalently attached to a serine residue in the active site of the COX enzyme. This makes aspirin different from other NSAIDs (such as diclofenac and ibuprofen), which are reversible inhibitors.
Low-dose, long-term aspirin use irreversibly blocks the formation of thromboxane A2 in platelets, producing an inhibitory effect on platelet aggregation. This anticoagulant property makes aspirin useful for reducing the incidence of heart attacks.[68] 40 mg of aspirin a day is able to inhibit a large proportion of maximum thromboxane A2 release provoked acutely, with the prostaglandin I2 synthesis being little affected; however, higher doses of aspirin are required to attain further inhibition.[69]
Prostaglandins are local hormones (paracrine) produced in the body and have diverse effects in the body, including but not limited to transmission of pain information to the brain, modulation of the hypothalamic thermostat, and inflammation. Thromboxanes are responsible for the aggregation of platelets that form blood clots. Heart attacks are primarily caused by blood clots, and their reduction with the introduction of small amounts of aspirin has been seen to be an effective medical intervention. The side-effect of this is that the ability of the blood in general to clot is reduced, and excessive bleeding may result from the use of aspirin.

There are at least two different types of cyclooxygenase: COX-1 and COX-2. Aspirin irreversibly inhibits COX-1 and modifies the enzymatic activity of COX-2. Normally COX-2 produces prostanoids, most of which are pro-inflammatory. Aspirin-modified COX-2 produces lipoxins, most of which are anti-inflammatory. Newer NSAID drugs called COX-2 selective inhibitors have been developed that inhibit only COX-2, with the hope for reduction of gastrointestinal side-effects.
However, several of the new COX-2 selective inhibitors have been recently withdrawn (see Vioxx), after evidence emerged that COX-2 inhibitors increase the risk of heart attack. It is proposed that endothelial cells lining the microvasculature in the body express COX-2, and, by selectively inhibiting COX-2, prostaglandins (specifically PGI2; prostacyclin) are downregulated with respect to thromboxane levels, as COX-1 in platelets is unaffected. Thus, the protective anti-coagulative effect of PGI2 is decreased, increasing the risk of thrombus and associated heart attacks and other circulatory problems. Since platelets have no DNA, they are unable to synthesize new COX once aspirin has irreversibly inhibited the enzyme, an important difference with reversible inhibitors.
Furthermore, aspirin has two additional modes of actions, contributing to its strong analgesic, antipyretic and anti-inflammatory properties:
- It uncouples oxidative phosphorylation in cartilaginous (and hepatic) mitochondria, by diffusing from the inner membrane space as a proton carrier back into the mitochondrial matrix, where it ionizes once again to release protons. In short, aspirin buffers and transports the protons. (Note: This effect in high doses of aspirin actually causes fever due to the heat released from the electron transport chain, instead of its normal antipyretic action.)
- It induces the formation of NO-radicals in the body that enable the white blood cells (leukocytes) to fight infections more effectively. This has been found recently by Dr. Derek W. Gilroy, winning Bayer's International Aspirin Award 2005.[70]
More recent data suggest that salicylic acid and its derivatives will modulate signaling through NF-κB.[71] NF-κB is a transcription factor complex that plays a central role in many biological processes, including inflammation.
Polymorphism
Polymorphism, or the ability of a substance to form more than one crystal structure, is important in the development of pharmaceutical ingredients. Many drugs are receiving regulatory approval for only a single crystal form or polymorph. For a long time, only one crystal structure for aspirin was known, although there had been indications that aspirin might have a second crystalline form since the 1960s. The elusive 2nd polymorph was first discovered by Vishweshwar and coworkers in 2005. [72], fine structural details were given by Bond et al. [73] A new crystal type was found after attempted co-crystallization of aspirin and levetiracetam from hot acetonitrile. The form II is only stable at 100 K and reverts back to form I at ambient temperature. In the (unambiguous) form I two salicylic molecules form centrosymmetric dimers through the acetyl groups with the (acidic) methyl proton to carbonyl hydrogen bonds and in the newly claimed form II each salicylic molecule forms the same hydrogen bonds but then with two neighboring molecules instead of one. With respect to the hydrogen bonds formed by the carboxylic acid groups both polymorphs form identical dimer structures.
See also
References
- ^ Julian, D G (1996-09-24). "A comparison of aspirin and anticoagulation following thrombolysis for myocardial infarction (the AFTER study): a multicentre unblinded randomised clinical trial". BMJ. 313 (7070). British Medical Journal: 1429–1431. Retrieved 2007-10-04.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ a b Macdonald S (2002). "Aspirin use to be banned in under 16 year olds". BMJ. 325 (7371): 988. PMID 12411346. PMC 1169585.
- ^ a b Template:De icon Gerhardt C (1853). "Untersuchungen über die wasserfreien organischen Säuren". Annalen der Chemie und Pharmacie. 87: 149–179. doi:10.1002/jlac.18530870107.
- ^ Template:De icon von Gilm H (1859). "Acetylderivate der Phloretin- und Salicylsäure". Annalen der Chemie und Pharmacie. 112 (2): 180–185. doi:10.1002/jlac.18591120207.
- ^ Template:De icon Schröder, Prinzhorn, Kraut K (1869). "Uber Salicylverbindungen". Annalen der Chemie und Pharmacie. 150 (1): 1–20. doi:10.1002/jlac.18691500102.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Charles C. Mann and Mark L. Plummer (1991). The aspirin wars: money, medicine, and 100 years of rampant competition. Boston, Mass: Harvard Business School Press. pp. 25–36. ISBN 0-87584-401-4.
- ^ Hoffmann H. "US Patent 644,077 dated February 27, 1900". US Patent Office. Retrieved 2007-05-20.
- ^ a b Sneader W (2000). "The discovery of aspirin: a reappraisal". BMJ. 321 (7276): 1591–4. doi:10.1136/bmj.321.7276.1591. PMID 11124191. PMC 1119266.
- ^ Helmstaedter A (2001). "Aspirin history: Is there a need for a reappraisal ?". Rapid Responses to: "The discovery of aspirin: a reappraisal". bmj.com. Retrieved 2007-05-20.
- ^ "Bayer AG: Zum Vortrag von Dr. Walter Sneader über die Entwicklung der Acetylsalicylsäure" (in German). Retrieved 2007-05-20.
- ^ "Aspirin FAQ's". Bayer Healthcare. Retrieved 2007-05-20.
{{cite web}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Bayer Co. v. United Drug Co., 272 F. 505 (S.D.N.Y. 1921). Retrieved on 2007-09-07.
- ^ Palleros, Daniel R. (2000). Experimental Organic Chemistry. New York: John Wiley & Sons. p. 494. ISBN 0-471-28250-2.
- ^ "Acetylsalicylic acid". Jinno Laboratory, School of Materials Science, Toyohashi University of Technology. March 1 1996. Retrieved 2007-09-07.
{{cite web}}: Check date values in:|date=(help) - ^ Aukerman G, Knutson D, Miser WF (2002). "Management of the acute migraine headache". Am Fam Phys. 66 (11): 2123–30. PMID 12484694.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Katzung, Bertram G. (1998). Basic and Clinical Pharmacolog (7th ed. ed.). Stamford, Connecticut: Appleton & Lange. ISBN -8385-0565-1.
{{cite book}}:|edition=has extra text (help); Check|isbn=value: length (help) - ^ ISIS-2 Collaborative group (1988). "Randomized trial of intravenous streptokinase, oral aspirin, both, or neither among 17,187 cases of suspected acute myocardial infarction: ISIS-2". Lancet (2): 349–60. PMID 2899772.
{{cite journal}}: CS1 maint: numeric names: authors list (link) - ^ Crosby, Janet Tobiassen (2006). "Veterinary Questions and Answers". About.com. Retrieved 2007-09-05.
- ^ Cambridge H, Lees P, Hooke RE, Russell CS (1991). "Antithrombotic actions of aspirin in the horse". Equine Vet J. 23 (2): 123–7. PMID 1904347.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Chew EY, Williams GA, Burton TC, Barton FB, Remaley NA, Ferris FL (1992). "Aspirin effects on the development of cataracts in patients with diabetes mellitus. Early treatment diabetic retinopathy study report 16". Arch Ophthalmol. 110 (3): 339–42. PMID 1543449.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Bosetti; et al. (2006). "Aspirin and the risk of prostate cancer". Eur J Cancer Prev. 15 (1): 43–5. PMID 16374228.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Menezes; et al. (2006). "Regular use of aspirin and prostate cancer risk (United States)". Cancer Causes & Control. 17 (3): 251–6. doi:10.1007/s10552-005-0450-z. PMID 16489532.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Thun MJ, Namboodiri MM, Heath CW (1991). "Aspirin use and reduced risk of fatal colon cancer". N Engl J Med. 325 (23): 1593–6. PMID 1669840.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Baron; et al. (2003). "A randomized trial of aspirin to prevent colorectal adenomas". N Engl J Med. 348 (10): 891–9. PMID 12621133.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Chan; et al. (2004). "A Prospective Study of Aspirin Use and the Risk for Colorectal Adenoma". Ann Intern Med. 140 (3): 157–66. PMID 14757613.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Chan; et al. (2005). "Long-term Use of Aspirin and Nonsteroidal Anti-inflammatory Drugs and Risk of Colorectal Cancer". JAMA. 294 (8): 914–23. PMID 16118381.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Schernhammer; et al. (2004). "A Prospective Study of Aspirin Use and the Risk of Pancreatic Cancer in Women". J Natl Cancer Inst. 96 (1): 22–28. PMID 14709735.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Bosetti; et al. (2003). "Aspirin use and cancers of the upper aerodigestive tract". Br J Cancer. 88 (5): 672–74. PMID 12618872.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Akhmedkhanov; et al. (2002). "Aspirin and lung cancer in women". Br J cancer. 87 (11): 1337–8. PMID 12085255.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Moysich KB, Menezes RJ, Ronsani A; et al. (2002). "Regular aspirin use and lung cancer risk". BMC Cancer. 2: 31. PMID 12453317.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) Free full text - ^ Wolff; et al. (1998). "Expression of cyclooxygenase-2 in human lung carcinoma". Cancer Research. 58 (22): 4997–5001.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Chan GC, Hinds TR, Impey S, Storm DR (1998). "Hippocampal neurotoxicity of Δ9-tetrahydrocannabinol". J Neurosci. 18 (14): 5322–32. PMID 9651215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) Free full text - ^ "Aspirin may be less effective heart treatment for women than men". University of Michigan. April 26, 2007. Retrieved 2007-09-09.
{{cite news}}: Check date values in:|date=(help) - ^ Dorsch MP, Lee JS, Lynch DR, Dunn SP, Rodgers JE, Schwartz T, Colby E, Montague D, Smyth SS (2007). "Aspirin Resistance in Patients with Stable Coronary Artery Disease with and without a History of Myocardial Infarction". Ann Pharmacother (May). doi:10.1345/aph.1H621. PMID 17456544.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Increased risk of heart attack or stroke for patients who are resistant to aspirin". 2008-01-17. Retrieved 2008-01-20.
- ^ "www.patient.co.uk/showdoc/40001339/".
- ^ Delaney JA, Opatrny L, Brophy JM & Suissa S (2007). "Drug drug interactions between antithrombotic medications and the risk of gastrointestinal bleeding". CMAJ. 177 (4): 347–51. doi:10.1503/cmaj.070186. PMID 17698822. PMC 1942107.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Graham DY & Smith JL (1986). "Aspirin and the stomach". Ann Intern Med. 104 (3): 390–398. PMID 3511824.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Eisen GM, Goldstein JL, Hanna DB & Rublee DA (2005). "Meta-analysis: upper gastrointestinal tolerability of valdecoxib, a cyclooxygenase-2-specific inhibitor, compared with nonspecific nonsteroidal anti-inflammatory drugs among patients with osteoarthritis and rheumatoid arthritis". Aliment Pharmacol Ther. 21 (5): 591–598. doi:10.1111/j.1365-2036.2005.02383.x.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ van Oijen MG, Huybers S, Peters WH, Drenth JP, Laheij RJ, Verheugt FW & Jansen JB (2005). "Polymorphisms in genes encoding acetylsalicylic acid metabolizing enzymes are unrelated to upper gastrointestinal health in cardiovascular patients on acetylsalicylic acid". Br J Clin Pharmacol. 60 (6): 623–8. doi:10.1111/j.1365-2125.2005.02495.x. PMID 16305586. PMC 1884887.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Brown N, May JA, Wilcox RG, Allan LM, Wilson AM, Kiff PS & Heptinstall S (1999). "Comparison of antiplatelet activity of microencapsulated aspirin 162.5 milligrams with enteric coated aspirin 75 milligrams and 150 milligrams in patients with atherosclerosis". Br J Clin Pharmacol. 48 (1): 57–62. doi:10.1046/j.1365-2125.1999.00947.x. PMID 10383561. PMC 2014875.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mellemkjaer L, Blot WJ, Sørensen HT, Thomassen L, McLaughlin JK, Nielsen GL & Olsen JH (2002). "Upper gastrointestinal bleeding among users of NAIDs: a population-based cohort study in Denmark". Br J Clin Pharmacol. 53 (2): 173–81. doi:10.1046/j.0306-5251.2001.01220.x. PMID 11851641. PMC 1874281.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Guitton, M.J., & Caston, J., & Ruel, J., & Johnson, R.M., & Pujol, R., & Puel J. (2003). Salicylate induces tinnitus through activation of cochlear NMDA receptors. The Journal of Neuroscience, 23(9). Retrieved on December 6, 2007, from http://www.jneurosci.org/cgi/content/full/23/9/3944
- ^ Rogers MF, Schonberger LB, Hurwitz ES & Rowley DL (1985). "National Reye syndrome surveillance, 1982". Pediatrics. 75 (2): 260–4. PMID 3969325.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Rogan WJ, Yang GC & Kimbrough RD (1985). "Aflatoxin and Reye's syndrome: a study of livers from deceased cases". ArchEnviron Health. 40 (2): 91–5. PMID 4004347.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Scher, K.S. (1996). "Unplanned reoperation for bleeding". Am Surg. 62 (1): 52–55. PMID 8540646.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Yuen KY, Chan PK, Peiris M, Tsang DN, Que TL, Shortridge KF, Cheung PT, To WK, Ho ET, Sung R & Cheng AF (1996). "Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus". Lancet. 351 (9101): 467–71. doi:10.1016/S0140-6736(98)01182-9. PMID 9482437.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Berges-Gimeno MP & Stevenson DD (2004). "Nonsteroidal anti-inflammatory drug-induced reactions and desensitization". J Asthma. 41 (4): 375–84. doi:10.1081/JAS-120037650. PMID 15281324.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Towns SJ & Mellis CM (1984). "Role of acetyl salicylic acid and sodium metabisulfite in chronic childhood asthma". Pediatrics. 73 (5): 631–7. PMID 6718119.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Katzung (1998), p. 584.
- ^ Loh HS, Watters K & Wilson CW (1973). J Clin Pharmacol. 13 (11): 480–6. PMID 4490672 http://jcp.sagepub.com/cgi/content/abstract/13/11/480.
{{cite journal}}: Missing or empty|title=(help) - ^ Basu TK (1982). "Vitamin C-aspirin interactions". Int J Vitam Nutr Res Suppl. 23: 83–90. PMID 6811490.
{{cite journal}}: Cite has empty unknown parameter:|month=(help) - ^ Ioannides C, Stone AN, Breacker PJ & Basu TK (1982). "Impairment of absorption of ascorbic acid following ingestion of aspirin in guinea pigs". Biochem Pharmacol. 31 (24): 4035–8. doi:10.1016/0006-2952(82)90652-9. PMID 6818974.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ British National Formulary (45 ed.). British Medical Journal and Royal Pharmaceutical Society of Great Britain. March 2003.
- ^ Aspirin monograph: dosages, etc
- ^ British National Formulary for Children. British Medical Journal and Royal Pharmaceutical Society of Great Britain. 2006.
- ^ Gaudreault P, Temple AR, Lovejoy FH Jr. (1982). "The relative severity of acute versus chronic salicylate poisoning in children: a clinical comparison". Pediatrics. 70 (4): 566–9. PMID 7122154.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Thisted B, Krantz T, Stroom J, Sorensen MB. (1987). "Acute salicylate self-poisoning in 177 consecutive patients treated in ICU". Acta Anaesthesiol Scand. 31 (4): 312–6. PMID 3591255.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Temple AR. (1981). "Acute and chronic effects of aspirin toxicity and their treatment". Arch Intern Med. 141 (3 Spec No): 364–9. PMID 7469627.
- ^ Vale JA, Kulig K; American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. (2004). "Position paper: gastric lavage". J Toxicol Clin Toxicol. 42 (7): 933–43. PMID 15641639.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hillman RJ, Prescott LF. (1985). "Treatment of salicylate poisoning with repeated oral charcoal". Br Med J (Clin Res Ed). 291 (6507): 1472. PMID 3933714.
- ^ Kirshenbaum LA, Mathews SC, Sitar DS, Tenenbein M. (1990). "Does multiple-dose charcoal therapy enhance salicylate excretion?". Arch Intern Med. 150 (6): 1281–3. PMID 2191636.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Dargan PI, Wallace CI, Jones AL. (2002). "An evidenced based flowchart to guide the management of acute salicylate (aspirin) overdose". Emerg Med J. 19 (3): 206–9. doi:10.1136/emj.19.3.206. PMID 11971828.
{{cite journal}}: CS1 maint: multiple names: authors list (link)PMC 1725844 - ^ Meredith TJ, Vale JA. (1986). "Non-narcotic analgesics. Problems of overdosage". Drugs. 32 (Suppl 4): 117–205. PMID 3552583.
- ^ Litovitz TL, Klein-Schwartz W, White S, Cobaugh DJ, Youniss J, Omslaer JC, Drab A, Benson BE (2001). "2000 Annual report of the American Association of Poison Control Centers Toxic Exposure Surveillance System". Am J Emerg Med. 19 (5): 337–95. doi:10.1053/ajem.2001.25272. PMID 11555795.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ John Robert Vane (1971). "Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs". Nature - New Biology. 231 (25): 232–5. PMID 5284360.
- ^ Vane JR, Botting RM (2003). "The mechanism of action of aspirin" (PDF). Thromb Res. 110 (5–6): 255–8. doi:10.1016/S0049-3848(03)00379-7. PMID 14592543.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ [1] American Heart Association: Aspirin in Heart Attack and Stroke Prevention "The American Heart Association recommends aspirin use for patients who've had a myocardial infarction (heart attack), unstable angina, ischemic stroke (caused by blood clot) or transient ischemic attacks (TIAs or "little strokes"), if not contraindicated. This recommendation is based on sound evidence from clinical trials showing that aspirin helps prevent the recurrence of such events as heart attack, hospitalization for recurrent angina, second strokes, etc. (secondary prevention). Studies show aspirin also helps prevent these events from occurring in people at high risk (primary prevention)."
- ^ Tohgi, H (1992). "Effects of low-to-high doses of aspirin on platelet aggregability and metabolites of thromboxane A2 and prostacyclin". Stroke. Vol 23: 1400–1403. PMID 1412574.
{{cite journal}}:|volume=has extra text (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ "New mechanism of action of Aspirin discovered," in Medical News Today, October 2, 2005.
- ^ McCarty, MF (2006). "Preadministration of high-dose salicylates, suppressors of NF-kappaB activation, may increase the chemosensitivity of many cancers: an example of proapoptotic signal modulation therapy". Integr Cancer Ther. Vol 5 (3): 252–268. doi:10.1177/1534735406291499. PMID 16880431.
{{cite journal}}:|volume=has extra text (help); Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Peddy Vishweshwar, Jennifer A. McMahon, Mark Oliveira, Matthew L. Peterson, and Michael J. Zaworotko (2005). "The Predictably Elusive Form II of Aspirin". J. Am. Chem. Soc. 127 (48): 16802–16803. doi:10.1021/ja056455b.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Andrew D. Bond, Roland Boese, Gautam R. Desiraju (2007). "On the Polymorphism of Aspirin: Crystalline Aspirin as Intergrowths of Two "Polymorphic" Domains". Angewandte Chemie International Edition. 46 (4): 618–622. doi:10.1002/anie.200603373.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
External links
- DrugBank Aspirin Entry
- Reappraisal
- An aspirin a day keeps the doctor away
- Colour-enhanced scanning electron micrograph of aspirin crystals
- Aspirin research in the 1990s
- The History of Aspirin
- Aspirin and heart disease
- How Aspirin works
- Molview from bluerhinos.co.uk See Aspirin in 3D
- History of Aspro
- The science behind aspirin
- Take two: Aspirin, New uses and new dangers are still being discovered as aspirin enters its second century. Shauna Roberts, American Chemical Society
- Ling, Greg (2005). "Aspirin". How Products are Made. Vol. 1. Thomson Gale.