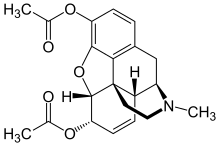
Heroin
 | |
 | |
| Clinical data | |
|---|---|
| Other names | Diamorphine, Diacetylmorphine, Acetomorphine, (Dual) Acetylated morphine, Morphine diacetate |
| Pregnancy category |
|
| Dependence liability | High |
| Routes of administration | Inhalation, Transmucosal, Intravenous, Oral, Intranasal, Rectal, Intramuscular |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | <35% (oral), 44–61% (inhaled)[1] |
| Protein binding | 0% (morphine metabolite 35%) |
| Metabolism | hepatic |
| Elimination half-life | <10 minutes [2] |
| Excretion | 90% renal as glucuronides, rest biliary |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.008.380 |
| Chemical and physical data | |
| Formula | C21H23NO5 |
| Molar mass | 369.41 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Heroin (diacetylmorphine (INN)), also known as diamorphine (BAN), is a semi-synthetic opioid drug synthesized from morphine, a derivative of the opium poppy. It is the 3,6-diacetyl ester of morphine (di (two)-acetyl-morphine). The white crystalline form is commonly the hydrochloride salt diacetylmorphine hydrochloride, though often adulterated thus dulling the sheen and consistency from that to a matte white powder, which diacetylmorphine freebase typically is.[3] 90% of diacetylmorphine is thought to be produced in Afghanistan.[4]
As with other opioids, diacetylmorphine is used as both an analgesic and a recreational drug. Frequent and regular administration is associated with tolerance and physical dependence, which may develop into addiction. Internationally, diacetylmorphine is controlled under Schedules I and IV of the Single Convention on Narcotic Drugs.[5] It is illegal to manufacture, possess, or sell diacetylmorphine without a license in Belgium, Denmark, Germany, Iran, India, the Netherlands, the United States, Australia, Canada, Ireland, Pakistan, the United Kingdom and Swaziland.
Under the chemical name diamorphine, diacetylmorphine is a legally prescribed controlled drug in the United Kingdom. It is available for prescription to long-term users in the Netherlands, United Kingdom, Switzerland, Germany and Denmark alongside psycho-social care,[6][7] and a similar programme is being campaigned for by liberal political parties in Norway. Some countries allow the government to sell or donate high-quality seizures of drugs and precursors which are otherwise legal for medicinal use to pharmaceutical manufacturers for use in preparing licit supplies of medical drugs and research chemicals; this was the case in Croatia prior to 2007.[8]
Etymology
In 1895, the German drug company Bayer marketed diacetylmorphine as an over the counter drug under the trademark name Heroin.[9] The name was derived from the German word "heroisch" (heroic) because of its perceived "heroic" effects upon a user.[9] It was chiefly developed as a morphine substitute for cough suppressants that did not have morphine's addictive side-effects. Morphine at the time was a popular recreational drug, and Bayer wished to find a similar but non-addictive substitute to market.[10] However, contrary to Bayer's advertising as a "non-addictive morphine substitute," Heroin would soon have one of the highest rates of dependence amongst its users.[11]
History

The opium poppy was cultivated in lower Mesopotamia as long ago as 3400 BCE.[12] The chemical analysis of opium in the 19th century revealed that most of its activity could be ascribed to two alkaloids, codeine and morphine.
Diacetylmorphine was first synthesized in 1874 by C. R. Alder Wright, an English chemist working at St. Mary's Hospital Medical School in London. He had been experimenting with combining morphine with various acids. He boiled anhydrous morphine alkaloid with acetic anhydride for several hours and produced a more potent, acetylated form of morphine, now called diacetylmorphine. The compound was sent to F. M. Pierce of Owens College in Manchester for analysis. Pierce told Wright:
Doses ... were subcutaneously injected into young dogs and rabbits ... with the following general results ... great prostration, fear, and sleepiness speedily following the administration, the eyes being sensitive, and pupils constrict, considerable salivation being produced in dogs, and slight tendency to vomiting in some cases, but no actual emesis. Respiration was at first quickened, but subsequently reduced, and the heart's action was diminished, and rendered irregular. Marked want of coordinating power over the muscular movements, and loss of power in the pelvis and hind limbs, together with a diminution of temperature in the rectum of about 4°.[13]

Wright's invention did not lead to any further developments, and diacetylmorphine only became popular after it was independently re-synthesized 23 years later by another chemist, Felix Hoffmann. Hoffmann, working at the Aktiengesellschaft Farbenfabriken (today the Bayer pharmaceutical company) in Elberfeld, Germany, was instructed by his supervisor Heinrich Dreser to acetylate morphine with the objective of producing codeine, a constituent of the opium poppy, pharmacologically similar to morphine but less potent and less addictive. Instead the experiment produced an acetylated form of morphine one and a half to two times more potent than morphine itself.
From 1898 through to 1910 diacetylmorphine was marketed under the trademark name Heroin as a non-addictive morphine substitute and cough suppressant. Bayer marketed the drug as a cure for morphine addiction before it was discovered that it rapidly metabolizes into morphine. As such, diacetylmorphine is essentially a quicker acting form of morphine. The company was embarrassed by the new finding, which became a historic blunder for Bayer.[14]
In the U.S.A. the Harrison Narcotics Tax Act was passed in 1914 to control the sale and distribution of diacetylmorphine and other opioids, which allowed the drug to be prescribed and sold for medical purposes. In 1924 the United States Congress banned its sale, importation or manufacture. It is now a Schedule I substance, which makes it illegal for non-medical use in signatory nations of the Single Convention on Narcotic Drugs treaty, including the United States.
The Health Committee of the League of Nations banned diacetylmorphine in 1925 although it took more than three years for this to be implemented. In the meantime, the first designer drugs, viz. 3,6 diesters and 6 monoesters of morphine and acetylated analogues of closely-related drugs like hydromorphone and dihydromorphine were produced in massive quantities to fill the worldwide demand for diacetylmorphine—this continued until 1930 when the Committee banned diacetylmorphine analogues with no therapeutic advantage over drugs already in use, the first major legislation of this type.[15]
Later, as with Aspirin, Bayer lost some of its trademark rights to Heroin under the 1919 Treaty of Versailles following the German defeat in World War I.[16]
Pharmacology

When taken orally, diacetylmorphine undergoes extensive first-pass metabolism via deacetylation, making it a prodrug for the systemic delivery of morphine.[17] When the drug is injected, however, it avoids this first-pass effect, very rapidly crossing the blood-brain barrier because of the presence of the acetyl groups, which render it much more fat soluble than morphine itself.[18] Once in the brain, it then is deacetylated variously into the inactive 3-monoacetylmorphine and the active 6-monoacetylmorphine (6-MAM), and then to morphine which bind to μ-opioid receptors, resulting in the drug's euphoric, analgesic (pain relief), and anxiolytic (anti-anxiety) effects; diacetylmorphine itself exhibits relatively low affinity for the μ receptor.[19] Unlike hydromorphone and oxymorphone, however, administered intravenously, diacetylmorphine creates a larger histamine release, similar to morphine, resulting in the feeling of a greater subjective "body high" to some, but also instances of pruritus (itching) when they first start using.[20]
Both morphine and 6-MAM are μ-opioid agonists which bind to receptors present throughout the brain, spinal cord and gut of all mammals. The μ-opioid receptor also binds endogenous opioid peptides such as β-endorphin, Leu-enkephalin, and Met-enkephalin. Repeated use of diacetylmorphine results in a number of physiological changes, including decreases in the number of μ-opioid receptors. [citation needed] These physiological alterations lead to tolerance and dependence, so that cessation of diacetylmorphine use results in a set of remarkably uncomfortable symptoms including pain, anxiety, muscle spasms, and insomnia called the opioid withdrawal syndrome. Depending on usage it has an onset four to 24 hours after the last dose of diacetylmorphine. Morphine also binds to δ- and κ-opioid receptors.
There is also evidence that 6-MAM binds to a subtype of μ-opioid receptors which are also activated by the morphine metabolite morphine-6β-glucuronide but not morphine itself.[21] The third substype of third opioid type (mu-3) receptor. Which may be a commonality to other six position monoesters of morphine. The contribution of these receptors to the overall pharmacology of diacetylmorphine remains unknown.
A subclass of morphine derivatives, namely the 3,6 esters of morphine, with similar effects and uses includes the clinically-used strong analgesics nicomorphine (Vilan), and dipropanoylmorphine; there is also the latter's dihydromorphine analogue, diacetyldihydromorphine (Paralaudin). Two other 3,6 diesters of morphine invented in 1874-5 along with diacetylmorphine, dibenzoylmorphine and acetylpropionylmorphine, were made as substitutes after it was outlawed in 1925 and therefore sold as the first "designer drugs" until they were outlawed by the League of Nations in 1930.
Usage and effects


Worldwide, the UN estimates there are more than 50 million regular users of diacetylmorphine, cocaine and synthetic drugs.[23] Global users of diacetylmorphine are estimated at between 15.16 million and 21.13 million people aged 15–64.[24]
Medical use
The examples and perspective in this section may not represent a worldwide view of the subject. (February 2011) |
Under the chemical name diamorphine, diacetylmorphine is prescribed as a strong analgesic in the United Kingdom, where it is given via subcutaneous, intramuscular, intrathecal or intravenous route. Its use includes treatment for acute pain, such as in severe physical trauma, myocardial infarction, post-surgical pain, and chronic pain, including end-stage cancer and other terminal illnesses. In other countries it is more common to use morphine or other strong opioids in these situations.
In 2005, there was a shortage of diacetylmorphine in the UK, because of a problem at the main UK manufacturers.[25] Because of this, many hospitals changed to using morphine instead of diacetylmorphine. Although there is no longer a problem with the manufacturing of diacetylmorphine in the UK, many hospitals there have continued to use morphine.
Diacetylmorphine continues to be widely used in palliative care in the United Kingdom, where it is commonly given by the subcutaneous route, often via a syringe driver, if patients cannot easily swallow oral morphine solution. The advantage of diacetylmorphine over morphine is that diacetylmorphine is more fat soluble and therefore more potent, so smaller doses of it are needed for the same analgesic effect. Both of these factors are advantageous if giving high doses of opioids via the subcutaneous route, which is often necessary in palliative care.
The medical use of diacetylmorphine (in common with other strong opioids such as morphine, fentanyl and oxycodone) is controlled in the United Kingdom by the Misuse of Drugs Act 1971. In the UK, it is a class A controlled drug. Registers of its use are required to be kept in hospitals.
Diacetylmorphine is also used as a maintenance drug to treat addicts. Though this is somewhat controversial among proponents of a zero tolerance drug policy, it has proven superior to methadone in improving the social and health situation of addicts.[26] See: Heroin prescription for addicts
Recreational use


Diacetylmorphine is used as a recreational drug for the transcendent relaxation and intense euphoria it induces. Anthropologist Michael Agar once described diacetylmorphine as "the perfect whatever drug."[27] Tolerance quickly develops, and users need more of the drug to achieve the same effects. Its popularity with recreational drug users, compared to morphine, reportedly stems from its perceived different effects.[28] In particular, users report an intense rush, an acute transcendent state of euphoria, that occurs while the diacetylmorphine is being metabolized into 6-monoacetylmorphine (6-MAM) and morphine in the brain. Diacetylmorphine produces more euphoria than other opioids upon injection. One possible explanation is the presence of 6-monoacetylmorphine, a metabolite unique to diacetylmorphine. While other opioids of recreational use, such as codeine, produce only morphine, diacetylmorphine also leaves 6-MAM, also a psycho-active metabolite. However, this perception is not supported by the results of clinical studies comparing the physiological and subjective effects of injected diacetylmorphine and morphine in individuals formerly addicted to opioids; these subjects showed no preference for one drug over the other. Equipotent injected doses had comparable action courses, with no difference in subjects' self-rated feelings of euphoria, ambition, nervousness, relaxation, drowsiness, or sleepiness.[29]
Short-term addiction studies by the same researchers demonstrated that tolerance developed at a similar rate to both diacetylmorphine and morphine. When compared to the opioids hydromorphone, fentanyl, oxycodone, and pethidine/meperidine, former addicts showed a strong preference for diacetylmorphine and morphine, suggesting that diacetylmorphine and morphine are particularly susceptible to abuse and addiction. Morphine and diacetylmorphine were also much more likely to produce euphoria and other positive subjective effects when compared to these other opioids.[29]
Detection in biological fluids
The major metabolites of diacetylmorphine, 6-MAM, morphine, morphine-3-glucuronide and morphine-6-glucuronide, may be quantitated in blood, plasma or urine to monitor for abuse, confirm a diagnosis of poisoning or assist in a medicolegal death investigation. Most commercial opiate screening tests cross-react appreciably with these metabolites, as well as with other biotransformation products likely to be present following usage of street-grade diacetylmorphine such as 6-acetylcodeine and codeine. However, chromatographic techniques can easily distinguish and measure each of these substances. When interpreting the results of a test, it is important to consider the diacetylmorphine usage history of the individual, since a chronic user can develop tolerance to doses that would incapacitate an opiate-naive individual, and the chronic user often has high baseline values of these metabolites in his system. Furthermore, some testing procedures employ a hydrolysis step prior to quantitation that converts many of the metabolic products to morphine, yielding a result that may be many times larger than with a method that examines each product individually.[30]
Routes of administration
| Recreational uses:
Medicinal uses:
|
Contraindications:
|
| Central nervous system:
Neurological: Psychological: Cardiovascular & Respiratory:
Gastrointestinal:
Musculoskeletal: Skin:
Miscellaneous:
 |
The onset of diacetylmorphine's effects depends upon the route of administration. Studies have shown that the subjective pleasure of drug use (the reinforcing component of addiction) is proportional to the rate at which the blood level of the drug increases.[31] Intravenous injection is the fastest route of drug administration, causing blood concentrations to rise the most quickly, followed by smoking, suppository (anal or vaginal insertion), insufflation (snorting), and ingestion (swallowing).
Ingestion does not produce a rush as forerunner to the high experienced with the use of diacetylmorphine, which is most pronounced with intravenous use. While the onset of the rush induced by injection can occur in as little as a few seconds, the oral route of administration requires approximately half an hour before the high sets in. Thus, with both higher the dosage of diacetylmorphine used and faster the route of administration used, the higher potential risk for psychological addiction.
Large doses of diacetylmorphine can cause fatal respiratory depression, and the drug has been used for suicide or as a murder weapon. The serial killer Dr Harold Shipman used it on his victims, as did Dr John Bodkin Adams (see his victim: Edith Alice Morrell).
Because significant tolerance to respiratory depression develops quickly with continued use and is lost just as quickly during withdrawal, it is often difficult to determine whether a diacetylmorphine lethal overdose was accidental, suicide or homicide. Examples include the overdose deaths of Sid Vicious, Janis Joplin, Tim Buckley, Layne Staley, Bradley Nowell, Ted Binion, and River Phoenix.[32]
Chronic use of diacetylmorphine and other opioids, has potentially been shown to be a cause of hyponatremia, resultant because of excess vasopressin secretion.
Oral
Oral use of heroin is less common than other methods of administration, mainly because there is little to no "rush", and the effects are less potent.[33] Diacetylmorphine is entirely converted to morphine by means of first-pass metabolism, resulting in deacetylation when ingested. Heroin's oral bioavailability is both dose-dependent (as is morphine's) and significantly higher than oral use of morphine itself, reaching up to 64.2% for high doses and 45.6% for low doses; opiate-naive users showed far less absorption of the drug at low doses, having bioavailabilities of only up to 22.9%. The maximum plasma concentration of morphine following oral administration of heroin was around twice as much as that of oral morphine.[34]
Injection
Injection, also known as "slamming", "banging", "shooting up" or "mainlining", is a popular method used by addicts which carries relatively greater risks than other methods of administration. Diacetylmorphine base (commonly found in Europe), when prepared for injection will only dissolve in water when mixed with an acid (most commonly citric acid powder or lemon juice) and heated. Diacetylmorphine in the United States is most commonly found in the hydrochloride salt form, requiring just water to dissolve. Users tend to initially inject in the easily accessible arm veins, but as these veins collapse over time, through damage caused by the acid, the user will often resort to injecting in other veins. Intravenous users can use a various single dose range using a hypodermic needle. The dose of diacetylmorphine used for recreational purposes is dependant on the frequency and level of use, thus a first-time user may use between 5 and 20 mg, while an addict may require several hundred mg per day.[35] As with the injection of any drug, if a group of users share a common needle without sterilization procedures, blood-borne diseases, such as HIV or hepatitis, can be transmitted.
Smoking
Smoking diacetylmorphine refers to vaporizing it to inhale the resulting fumes, not burning it to inhale the resulting smoke. It is commonly smoked in glass pipes made from glassblown Pyrex tubes and light bulbs. It can also be smoked off aluminium foil, which is heated underneath by a flame. This method is also known as "chasing the dragon" (whereas smoking methamphetamine is known as "chasing the white dragon").
Insufflation
Another popular route to intake diacetylmorphine is insufflation (snorting), where a user crushes the diacetylmorphine into a fine powder and then sharply inhales it (sometimes with a straw or a rolled up banknote, as with cocaine) into the nose where diacetylmorphine is absorbed through the soft tissue in the mucous membrane of the sinus cavity and straight into the bloodstream. This method of administration redirects first pass metabolism, with a quicker onset and higher bioavailability than oral administration, though the duration of action is shortened. This method is sometimes preferred by users who do not want to prepare and administer diacetylmorphine for injection or smoking, but still experience a fast onset with a rush.
Suppository
Little research has been focused on the suppository (anal or vaginal insertion) method of administration, also known as "plugging". This method of administration is commonly administered using an oral syringe. Diacetylmorphine can be dissolved and withdrawn into an oral syringe which may then be lubricated and inserted into the anus or vagina before the plunger is pushed. Anecdotal evidence of its effects are infrequently discussed, possibly due to social taboos in many cultures. The rectum and the vaginal canal is where the majority of the drug would likely be taken up, through the membranes lining its walls.
Regulation
In the Netherlands, diacetylmorphine is a List I drug of the Opium Law. It is available for prescription under tight regulation exclusively to long-term addicts for whom methadone maintenance treatment has failed. It cannot be used to treat severe pain or other illnesses.
In the United States, diacetylmorphine is a schedule I drug according to the Controlled Substances Act of 1970, making it illegal to possess without a DEA license. Possession of more than 100 grams of diacetylmorphine or a mixture containing diacetylmorphine is punishable with a minimum mandatory sentence of 5 years of imprisonment in a federal prison.
In Canada, diacetylmorphine is a controlled substance under Schedule I of the Controlled Drugs and Substances Act (CDSA). Any person who seeks or obtains diacetylmorphine without disclosing authorization 30 days prior to obtaining another prescription from a practitioner is guilty of an indictable offense and subject to imprisonment for a term not exceeding seven years. Possession of diacetylmorphine for the purpose of trafficking is guilty of an indictable offense and subject to imprisonment for life.
In Hong Kong, diacetylmorphine is regulated under Schedule 1 of Hong Kong's Chapter 134 Dangerous Drugs Ordinance. It is available by prescription. Anyone who supplies diacetylmorphine without a valid prescription can be fined $10,000 (HKD). The penalty for trafficking or manufacturing diacetylmorphine is a $5,000,000 (HKD) fine and life imprisonment. Possession of diacetylmorphine without a license from the Department of Health is illegal with a $1,000,000 (HKD) fine and/or 7 years of jail time.
In the United Kingdom, diacetylmorphine is available by prescription, though it is a restricted Class A drug. According to the 50th edition of the British National Formulary (BNF), diamorphine hydrochloride may be used in the treatment of acute pain, myocardial infarction, acute pulmonary oedema, and chronic pain. The treatment of chronic non-malignant pain must be supervised by a specialist. The BNF notes that all opioid analgesics cause dependence and tolerance but that this is "no deterrent in the control of pain in terminal illness". When used in the palliative care of cancer patients, diacetylmorphine is often injected using a syringe driver.
Street price
The European Monitoring Centre for Drugs and Drug Addiction reports that the retail price of brown diacetylmorphine varies from €14.5 per gram in Turkey to €110 per gram in Sweden, with most European countries reporting typical prices of €35-40 per gram. The price of white diacetylmorphine is reported only by a few European countries and ranged between €27 and €110 per gram.[36]
The United Nations Office on Drugs and Crime claims in its 2008 World Drug Report that typical US retail prices are US$172 per gram.[37]
Production and trafficking
Production
Diacetylmorphine is produced from acetylation of morphine derived from natural opium sources. Numerous mechanical and chemical means are used to purify the final product. The final products have a different appearance depending on purity and have different names.[38]
Trafficking

Traffic is heavy worldwide, with the biggest producer being Afghanistan.[39] According to a U.N. sponsored survey,[40] as of 2004[update], Afghanistan accounted for production of 87 percent of the world's diacetylmorphine.[41] Afghan opium kills 100,000 people every year worldwide.[42]
The cultivation of opium in Afghanistan reached its peak in 1999, when 350 square miles (910 km2) of poppies were sown. The following year the Taliban banned poppy cultivation, a move which cut production by 94 percent. By 2001 only 30 square miles (78 km2) of land were in use for growing opium poppies. A year later, after American and British troops had removed the Taliban and installed the interim government, the land under cultivation leapt back to 285 square miles (740 km2), with Afghanistan supplanting Burma to become the world's largest opium producer once more.[43] Opium production in that country has increased rapidly since, reaching an all-time high in 2006. War in Afghanistan once again appeared as a facilitator of the trade.[44] Some 3.3 million Afghans are involved in producing opium.[45]
At present, opium poppies are mostly grown in Afghanistan, and in Southeast Asia, especially in the region known as the Golden Triangle straddling Myanmar, Thailand, Vietnam, Laos and Yunnan province in the People's Republic of China. There is also cultivation of opium poppies in the Sinaloa region of Mexico and in Colombia. The majority of the diacetylmorphine consumed in the United States comes from Mexico and Colombia. Up until 2004, Pakistan was considered one of the biggest opium-growing countries.
Conviction for trafficking diacetylmorphine carries the death penalty in most Southeast Asian, some East Asian and Middle Eastern countries (see Use of death penalty worldwide for details), among which Malaysia, Singapore and Thailand are the most strict. The penalty applies even to citizens of countries where the penalty is not in place, sometimes causing controversy when foreign visitors are arrested for trafficking, for example the arrest of nine Australians in Bali, the death sentence given to Nola Blake in Thailand in 1987, or the hanging of an Australian citizen Van Tuong Nguyen in Singapore.
Trafficking history

The origins of the present international illegal diacetylmorphine trade can be traced back to laws passed in many countries in the early 1900s that closely regulated the production and sale of opium and its derivatives including diacetylmorphine. At first, diacetylmorphine flowed from countries where it was still legal into countries where it was no longer legal. By the mid-1920s, diacetylmorphine production had been made illegal in many parts of the world. An illegal trade developed at that time between diacetylmorphine labs in China (mostly in Shanghai and Tianjin) and other nations. The weakness of government in China and conditions of civil war enabled diacetylmorphine production to take root there. Chinese triad gangs eventually came to play a major role in the illicit diacetylmorphine trade. The French Connection route started in the 1930s.
Diacetylmorphine trafficking was virtually eliminated in the U.S. during World War II because of temporary trade disruptions caused by the war. Japan's war with China had cut the normal distribution routes for diacetylmorphine and the war had generally disrupted the movement of opium.
After World War II, the Mafia took advantage of the weakness of the postwar Italian government and set up diacetylmorphine labs in Sicily. The Mafia took advantage of Sicily's location along the historic route opium took westward into Europe and the United States.[46]
Large scale international diacetylmorphine production effectively ended in China with the victory of the communists in the civil war in the late 1940s.[citation needed] The elimination of Chinese production happened at the same time that Sicily's role in the trade developed.
Although it remained legal in some countries until after World War II, health risks, addiction, and widespread recreational use led most western countries to declare diacetylmorphine a controlled substance by the latter half of the 20th century.
In late 1960s and early 70s, the CIA supported anti-Communist Chinese Nationalists settled near Sino-Burmese border and Hmong tribesmen in Laos. This helped the development of the Golden Triangle opium production region, which supplied about one-third of diacetylmorphine consumed in US after 1973 American withdrawal from Vietnam. As of 1999, Myanmar (formerly Burma), the heartland of the Golden Triangle remained the second largest producer of diacetylmorphine, after Afghanistan.[47]
Soviet-Afghan war led to increased production in the Pakistani-Afghani border regions, as U.S.-backed mujaheddin militants raised money for arms from selling opium, contributing heavily to the modern Golden Crescent creation. By 1980, 60% of diacetylmorphine sold in the U.S. originated in Afghanistan.[47] It increased international production of diacetylmorphine at lower prices in the 1980s. The trade shifted away from Sicily in the late 1970s as various criminal organizations violently fought with each other over the trade. The fighting also led to a stepped up government law enforcement presence in Sicily.
Risks of use


- Intravenous use of diacetylmorphine (and any other substance) with non-sterile needles and syringes or other related equipment leads to several serious risks:
- The risk of contracting blood-borne pathogens such as HIV and hepatitis
- The risk of contracting bacterial or fungal endocarditis and possibly venous sclerosis
- Abscesses
- Poisoning from contaminants added to "cut" or dilute diacetylmorphine
- Chronic constipation
- Addiction
- Tolerance
- Physical dependence can result from prolonged use of all opioids, resulting in withdrawal symptoms on cessation of use
- Decreased kidney function (although it is not currently known if this is because of adulterants or infectious diseases)[48]
Many countries and local governments have begun funding programs that supply sterile needles to people who inject illegal drugs in an attempt to reduce these contingent risks and especially the contraction and spread of blood-borne diseases. The Drug Policy Alliance reports that up to 75% of new AIDS cases among women and children are directly or indirectly a consequence of drug use by injection. The United States federal government does not operate needle exchanges, although some state and local governments do support needle exchange programs.
Anthropologists Philippe Bourgois and Jeff Schonberg, who did a decade of field work among homeless diacetylmorphine and cocaine addicts in San Francisco, reported that the African-American addicts they observed were more inclined to "direct deposit" diacetylmorphine into a vein, rather than "skin-popping" their injections. (Skin-popping was a far more widespread practice among the white addicts: "By the midpoint of our fieldwork, most of the whites had given up searching for operable veins and skin-popped. They sank their needles perfunctorily, often through their clothing, into their fatty tissue.") Bourgois and Schonberg describes how the cultural difference between the African-Americans and the whites leads to this contrasting behavior, and also points out that the two different ways to inject diacetylmorphine comes with different health risks. Skin-popping more often results in abscesses, and direct injection more often leads to fatal overdose and also to hepatitis C and HIV infection.[49]
Diacetylmorphine overdose is usually treated with an opioid antagonist, such as naloxone (Narcan), or naltrexone, which has high affinity for opioid receptors but does not activate them. This reverses the effects of diacetylmorphine and other opioid agonists and causes an immediate return of consciousness but may precipitate withdrawal symptoms. The half-life of naloxone is much shorter than that of most opioid agonists, so that antagonist typically has to be administered multiple times until the opioid has been metabolized by the body.
Depending on drug interactions and numerous other factors, death from overdose can take anywhere from several minutes to several hours because of anoxia resulting from the breathing reflex being suppressed by µ-opioids. An overdose is immediately reversible with an opioid antagonist injection. Diacetylmorphine overdoses can occur because of an unexpected increase in the dose or purity or because of diminished opioid tolerance. However, many fatalities reported as overdoses are probably caused by interactions with other depressant drugs like alcohol or benzodiazepines.[50] It should also be noted that since diacetylmorphine can cause nausea and vomiting, a significant number of deaths attributed to diacetylmorphine overdose are caused by aspiration of vomit by an unconscious victim. Some sources quote the median lethal dose (for an average 75 kg opiate-naive individual) as being between 75 and 375 mg.[51] Illicit diacetylmorphine is of widely varying and unpredictable purity. This means that the user may prepare what they consider to be a moderate dose while actually taking far more than intended. Also, tolerance typically decreases after a period of abstinence. If this occurs and the user takes a dose comparable to their previous use, the user may experience drug effects that are much greater than expected, potentially resulting in a dangerous overdose.
It has been speculated that an unknown portion of diacetylmorphine related deaths are the result of an overdose or allergic reaction to quinine, which may sometimes be used as a cutting agent.[52]
A final factor contributing to overdoses is place conditioning. Diacetylmorphine use is a highly ritualized behavior. While the mechanism has yet to be clearly elucidated, longtime diacetylmorphine users display increased tolerance to the drug in locations where they have repeatedly administered. When the user injects in a different location, this environment-conditioned tolerance does not occur, resulting in a greater drug effect. The user's typical dose of the drug, in the face of decreased tolerance, becomes far too high and can be toxic, leading to overdose.[53]
A small percentage of diacetylmorphine smokers, and occasionally IV users, may develop symptoms of toxic leukoencephalopathy. The cause has yet to be identified, but one speculation is that the disorder is caused by an uncommon adulterant that is only active when heated.[54][55][56] Symptoms include slurred speech and difficulty walking.
Cocaine is sometimes used in combination with diacetylmorphine, and is referred to as a speedball when injected or moonrocks when smoked together. Cocaine acts as a stimulant, whereas diacetylmorphine acts as a depressant. Coadministration provides an intense rush of euphoria with a high that combines both effects of the drugs, while excluding the negative effects, such as anxiety and sedation. The effects of cocaine wear off far more quickly than diacetylmorphine, thus if an overdose of diacetylmorphine was used to compensate for cocaine, the end result is fatal respiratory depression.[citation needed]
Harm reduction

Harm reduction is a public health philosophy that seeks to reduce the harms associated with the use of diacetylmorphine. One aspect of harm reduction initiatives focuses on the behaviour of individual users. This includes promoting safer means of taking the drug, such as smoking, nasal use, oral or rectal insertion. This attempts to avoid the higher risks of overdose, infections and blood-borne viruses associated with injecting the drug. Other measures include using a small amount of the drug first to gauge the strength, and minimize the risks of overdose. For the same reason, poly drug use (the use of two or more drugs at the same time) is discouraged. Injecting diacetylmorphine users are encouraged to use new needles, syringes, spoons/steri-cups and filters every time they inject and not share these with other users. Users are also encouraged to not use it on their own, as others can assist in the event of an overdose.
Governments that support a harm reduction approach usually fund needle & syringe exchange programmes, which supply new needles and syringes on a confidential basis, as well as education on proper filtering prior to injection, safer injection techniques, safe disposal of used injecting gear and other equipment used when preparing diacetylmorphine for injection may also be supplied including citric acid sachets/vitamin C sachets, steri-cups, filters, alcohol pre-injection swabs, sterile water ampules and tourniquets (to stop use of shoe laces or belts).
Another harm reduction measure employed for example in Europe, Canada and Australia are safe injection sites where users can inject diacetylmorphine and cocaine under the supervision of medically trained staff. Safe injection sites are low threshold and allow social services to approach problem users that would otherwise be hard to reach.[58]
Withdrawal
The withdrawal syndrome from diacetylmorphine (the so-called cold turkey) may begin within 6 to 24 hours of discontinuation of the drug; however, this time frame can fluctuate with the degree of tolerance as well as the amount of the last consumed dose. Symptoms may include: sweating, malaise, anxiety, depression, akathisia, priapism, extra sensitivity of the genitals in females, general feeling of heaviness, cramp-like pains in the limbs, excessive yawning or sneezing, tears, rhinorrhea, sleep difficulties (insomnia), cold sweats, chills, severe muscle and bone aches; nausea and vomiting, diarrhea, cramps, and fever.[59]
Prescription for addicts
The UK Department of Health's Rolleston Committee Report[60] in 1926 established the British approach to diacetylmorphine prescription to users, which was maintained for the next 40 years: dealers were prosecuted, but doctors could prescribe diacetylmorphine to users when withdrawing from it would cause harm or severe distress to the patient. This "policing and prescribing" policy effectively controlled the perceived diacetylmorphine problem in the UK until 1959 when the number of diacetylmorphine addicts doubled every 16th month during a period of ten years, 1959–1968.[61] In 1964 the Brain Committee recommended that only selected approved doctors working at approved specialised centres be allowed to prescribe diacetylmorphine and benzoylmethylecgonine to users. The law was made more restrictive in 1968. Beginning in the 1970s, the emphasis shifted to abstinence and the use of methadone, until now only a small number of users in the UK are prescribed diacetylmorphine.[62]
In 1994 Switzerland began a trial diacetylmorphine maintenance program for users that had failed multiple withdrawal programs. The aim of this program is to maintain the health of the user to avoid medical problems stemming from the illicit use of diacetylmorphine. Reducing drug-related crime and preventing overdoses were two other goals. The first trial in 1994 involved 340 users, although enrollment was later expanded to 1000 based on the apparent success of the program. Participants are allowed to inject diacetylmorphine in specially designed pharmacies for 15 Swiss francs per day.[63] A national referendum in November 2008 showed 68% of voters supported the plan,[64] introducing diacetylmorphine prescription into federal law. The trials before were based on time-limited executive ordinances.
The success of the Swiss trials led German, Dutch,[65] and Canadian[66] cities to try out their own diacetylmorphine prescription programs.[67] Some Australian cities (such as Sydney) have instituted legal diacetylmorphine supervised injecting centers, in line with other wider harm minimization programs.
Since January 2009 Denmark has prescribed diacetylmorphine to a few addicts that have tried methadone and subutex without success.[68] Beginning in February 2010, addicts in Copenhagen and Odense will be eligible to receive free diacetylmorphine. Later in 2010 other cities including Århus and Esbjerg will join the scheme. In total, around 230 addicts will be able to receive free diacetylmorphine.[69] However, Danish addicts will only be able to inject heroin according to the policy set by Danish National Board of Health.[70] Of the estimated 1500 drug users who do not benefit from the current oral substitution treatment, approximately 900 will not be in the target group for treatment with injectable diacetylmorphine, either because of "massive multiple drug abuse of non-opioids" or "not wanting treatment with injectable diacetylmorphine".[71]
In July 2009, the German Bundestag passed a law allowing diacetylmorphine prescription as a standard treatment for addicts; while diacetylmorphine prescription was started in 2002, it was only authorized as a large-scale trial.[72]
Popular culture
Actors and comedians
- John Belushi (Article)
- Robert Downey, Jr. (Article)
- MacKenzie Phillips (Article)
- Tom Sizemore (Article)
- Billie Holiday (Article)
Film and television
- The film, American Gangster, is loosely based on real life drug dealer Frank Lucas, who sold diacetylmorphine.[73]
- The character Nicholas Black in Brookside died of a diacetylmorphine overdose in 1986. This was the first time diacetylmorphine addiction had been portrayed on a British television soap-opera.
- The film Gia, based on a true story of model Gia Carangi, is about her addiction and use of diacetylmorphine and how it affected her.[74]
- The 2000 American film, Requiem for a Dream, depicts, among other things, the diacetylmorphine addiction of young couple.
- Many characters on The Sopranos (#5 of TV Guide's 50 Greatest TV Shows of All Time) use or sell heroin. Most notable are Big Pussy Bonpensiero - who became an FBI informant after getting nabbed "pushing H" and paid the ultimate price) - and heroin-addicted Christopher Moltisanti - whose abuse of heroin and other substances, the effects of his addictions, storylines involving fellow substance abusers (e.g., Corky Caporale, J.T. Dolan, Brendan Filone, Adriana La Cerva, and Julianna Skiff), and Mafia-organized drug intervention are prominent plot points throughout the series.
- The 1996 British film, Trainspotting, follows a group of heroin addicts and their passage through life.
- The 1955 American film, The Man with the Golden Arm, follows the story of a heroin addict who gets clean while in prison, but struggles to stay that way in the outside world.
- The 1986 British film Sid and Nancy, portrays the life of Sid Vicious, bassist of the seminal punk rock band the Sex Pistols, and his relationship with girlfriend Nancy Spungen.
- the 1995 American drama The Basketball Diaries.
Musicians
- B.G., a rap artist from New Orleans, raps about his previous addiction to diacetylmorphine (via injection) in numerous songs.[75]
- David Bowie's first single, "Space Oddity", was seemingly about his experience with diacetylmorphine, as his 1980 single "Ashes to Ashes" included lines that refer to Major Tom as "...a junkie/strung out on heaven's high/hitting an all-time low."[76]
- Boy George[77]
- Kurt Cobain[78]
- Miles Davis[79]
- Jerry Garcia, guitarist for the Grateful Dead, was a diacetylmorphine user for many years. He died of heart failure while at the Serenity Knolls drug treatment center in San Francisco, undergoing treatment for his diacetylmorphine addiction after a recent relapse.[80][81]
- Mitch Hedberg was arrested for diacetylmorphine possession in 2003 and died of an accidental speedball overdose in 2005.[82]
- Billie Holiday[83]
- Janis Joplin[84][85]
- Courtney Love[86]
- Jim Morrison[85][87]
- Charlie Parker[88]
- John Phillips[89]
- Keith Richards[90]
- Michael Rudetsky[91]
- Nikki Sixx of Mötley Crüe released diaries from his time as a diacetylmorphine addict titled: The Heroin Diaries: A Year in the Life of a Shattered Rock Star.[92] An album was also produced based on the book.[92]
- Slash[citation needed]
- James Taylor[93]
- Sid Vicious of the Sex Pistols died of a diacetylmorphine overdose, and allegedly stabbed his girlfriend to death while both were strung out on diacetylmorphine.[94]
- Rozz Williams's final album before his suicide, The Whorse's Mouth, dealt with his diacetylmorphine addiction.[95]
- Trent Reznor
Writers
- Claude Brown, in Manchild in the Promised Land[96]
- William S. Burroughs [97]
- Christiane F.
- Jim Carroll
See also
- Alphamethylfentanyl (another opioid analgesic with the nickname "China White")
- Black Tar Heroin
- Cheese (recreational drug)
- Diacetyldihydromorphine
- Dipropanoylmorphine
- Drug injection
- Drugs and prostitution
- HIV in Yunnan
- Heroin chic
- Ibogaine
- Illegal drug trade
- Illicit drug use in Australia
- Monoacetylmorphine
- Morphine
- Opioids
- Opium
- Opium poppy
- Polish heroin
References
- ^ Rook, Elisabeth J.; Van Ree, Jan M.; Van Den Brink, Wim; Hillebrand, Michel J. X.; Huitema, Alwin D. R.; Hendriks, Vincent M.; Beijnen, Jos H. (2006). "Pharmacokinetics and Pharmacodynamics of High Doses of Pharmaceutically Prepared Heroin, by Intravenous or by Inhalation Route in Opioid-Dependent Patients". Basic <html_ent glyph="@amp;" ascii="&"/> Clinical Pharmacology <html_ent glyph="@amp;" ascii="&"/> Toxicology. 98: 86–96. doi:10.1111/j.1742-7843.2006.pto_233.x.
- ^ "Chemical Sampling Information: Heroin". Osha.gov. Retrieved 2010-10-20.
- ^ "Basic Facts About Heroin". Alcoholism.about.com. Retrieved 2010-10-20.
- ^ "Russia blames Nato for drug surge". BBC News. 2010-02-27. Retrieved 2010-05-04.
- ^ "Yellow List: List of Narcotic Drugs Under International Control" (PDF). International Narcotics Control Board. 2004. Retrieved May 5, 2006.
{{cite web}}: Unknown parameter|month=ignored (help) Referring URL = http://www.incb.org/incb/yellow_list.html - ^ "Europe: German Parliament Approves Heroin Maintenance". StoptheDrugWar.org. Retrieved 2010-10-20.
- ^ "Europe: Denmark Parliament Approves Heroin Maintenance Pilot Project". StoptheDrugWar.org. Retrieved 2010-10-20.
- ^ "ELDD | Country profiles". Eldd.emcdda.europa.eu. Retrieved 2010-10-20.
- ^ a b "Online Etymology Dictionary". Etymonline.com. Retrieved 2010-10-20.
- ^ "Heroin History Timeline, Heroin & Methadone Addiction Treatment By Narconon Arrowhead - Heroin Addiction Drug Rehab". Heroinaddiction.com. Retrieved 2010-10-20.
- ^ [1][dead link]
- ^ "Opium Throughout History". PBS Frontline. Retrieved 2006-10-22.
- ^ Wright, C.R.A. (2003-08-12). "On the Action of Organic Acids and their Anhydrides on the Natural Alkaloids". Archived from the original on 2004-06-06. Note: this is an annotated excerpt of Wright, C.R.A. (1874). "On the Action of Organic Acids and their Anhydrides on the Natural Alkaloids". Journal of the Chemical Society. 27: 1031–1043. doi:10.1039/js8742701031.
- ^ "''How aspirin turned hero'', Sunday Times article, reproduced on a BLTC website, accessed 18 March 2009". Opioids.com. 1998-09-13. Retrieved 2010-10-20.
- ^ dibenzoylmorphine
- ^ "Treaty of Versailles, Part X, Section IV, Article 298". 1919-06-28. pp. Annex, Paragraph 5. Retrieved 2008-10-25.
- ^ Sawynok J (1986). "The therapeutic use of heroin: a review of the pharmacological literature". Can. J. Physiol. Pharmacol. 64 (1): 1–6. PMID 2420426.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Klous, M; Brink, W; Ree, J; Beijnen, J (2005). "Development of pharmaceutical heroin preparations for medical co-prescription to opioid dependent patients". Drug and Alcohol Dependence. 80 (3): 283–95. doi:10.1016/j.drugalcdep.2005.04.008. PMID 15916865.
- ^ Inturrisi, C; Schultz, M; Shin, S; Umans, JG; Angel, L; Simon, EJ (1983). "Evidence from opiate binding studies that heroin acts through its metabolites". Life Sciences. 33: 773–6. doi:10.1016/0024-3205(83)90616-1. PMID 6319928.
- ^ Histamine release by morphine and diamorphine in man. & Cutaneous Complications of Intravenous Drug Abuse
- ^ Brown, G; Yang, K; King, MA; Rossi, GC; Leventhal, L; Chang, A; Pasternak, GW (1997). "3-Methoxynaltrexone, a selective heroin/morphine-6β-glucuronide antagonist". FEBS Letters. 412 (1): 35–8. doi:10.1016/S0014-5793(97)00710-2. PMID 9257684.
- ^ a b Office of National Drug Control Policy (ONDCP): Heroin Facts & Figures. Retrieved 11 February 2009.
- ^ "Drug Trade". BBC News.
- ^ "Illegal drugs: Canada's growing international market". CBC News. June 24, 2009.
- ^ "Diamorphine shortage: UKHRA statement on the UK diamorphine shortage". Ukhra.org. 2005-09-14. Retrieved 2010-10-20.
- ^ Haasen, C.; Verthein, U.; Degkwitz, P.; Berger, J.; Krausz, M.; Naber, D. (2007). "Heroin-assisted treatment for opioid dependence: Randomised controlled trial". The British Journal of Psychiatry. 191: 55–62. doi:10.1192/bjp.bp.106.026112. PMID 17602126.
- ^ Agar, Michael (2007). Dope Double Agent: The Naked Emperor on Drugs. Lulu.com. ISBN 1411681037. Retrieved 2009-10-22.
What a great New York drug heroin was, I thought. Like any city, but more than most, New York is an information overload, a constant perceptual tornado that surrounds you most places you walk on the streets. Heroin is the audio-visual technology that helps manage that overload by dampening it in general and allowing a focus on some part of it that the human perceptual equipment was, in fact, designed to handle.
- ^ : 188–93. doi:10.1007/s00213-002-1271-3.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help) - ^ a b Martin WR, Fraser HF (1961). "A comparative study of physiological and subjective effects of diacetylmorphine and morphine administered intravenously in postaddicts". Journal of Pharmacology and Experimental Therapeutics. 133: 388–99. PMID 13767429.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Baselt, R. (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 730–5. ISBN 093189008X.
- ^ Journal of Pharmacology and Experimental Therapeutics (JPET) | Onset of Action and Drug Reinforcement
- ^ "First murder charge over heroin mix that killed 400 - World - Times Online", TimesOnline.co.uk.
- ^ Sepulfreak. "Erowid Experience Vaults: Heroin - Catching the Waves - 41495." Erowid. 08 July 2005. Web. 09 Jan. 2011.<http://www.erowid.org/experiences/exp.php?ID=41495>.
- ^ "Oral Diacetylmorphine (heroin) Yields Greater Morphine Bioavailability than Oral Morphine: Bioavailability Related to Dosage and Prior Opioid Exposure." PubMed. Dec. 2008. Web. 09 Jan. 2011. <http://www.ncbi.nlm.nih.gov/pubmed/18945270>.
- ^ Notes on heroin dosage & tolerance. Erowid's Vault, 2001.
- ^ European Monitoring Centre for Drugs and Drug Addiction (2008). Annual report: the state of the drugs problem in Europe (PDF). Luxembourg: Office for Official Publications of the European Communities. p. 70. ISBN 978-92-9168-324-6.
- ^ United Nations Office on Drugs and Crime (2008). World drug report (PDF). United Nations Publications. p. 49. ISBN 978-92-1-148229-4.
- ^ "Documentation of a heroin manufacturing process in Afghanistan. BULLETIN ON NARCOTICS, Volume LVII, Nos. 1 and 2, 2005" (PDF). United Nations Office on Drugs and Crime. Retrieved 2010-10-20.
- ^ Nazemroaya, Mahdi Darius (2006). "The War in Afghanistan: Drugs, Money Laundering and the Banking System". GlobalResearch.ca. Retrieved 2006-10-22.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "Afghanistan opium survey - 2004" (PDF). Retrieved 2006-10-22.
- ^ McGirk, Tim (2004). "Terrorism's Harvest: How al-Qaeda is tapping into the opium trade to finance its operations and destabilize Afghanistan". Time Magazine Asia. Retrieved 2006-10-22.
{{cite news}}: Unknown parameter|month=ignored (help) - ^ "World failing to dent heroin trade, U.N. warns". CNN.com. October 21, 2009.
- ^ OPIUM WARS WITHIN, Jackie Jura ~ an independent researcher monitoring local, national and international events ~ http://www.orwelltoday.com/afghanheroin.shtml
- ^ Gall, Carolotta (2006). "Opium Harvest at Record Level in Afghanistan". New York Times - Asia Pacific. Retrieved 2006-10-22.
{{cite news}}: Unknown parameter|month=ignored (help) - ^ "UN horrified by surge in opium trade in Helmand". The Guardian.
- ^ Eric C. Schneider, Smack: Heroin and the American City, University of Pennsylvania Press, 2008, chapter one
- ^ a b "War Views: Afghan heroin trade will live on". Richard Davenport-Hines. BBC. October 200q. Retrieved 2008-10-30.
{{cite news}}: Check date values in:|date=(help) - ^ Dettmeyer, Reinhard B; Preuß, Johanna; Wollersen, Heike; Madea, Burkhard (2005). "Heroin-associated nephropathy". Expert Opinion on Drug Safety. 4 (1): 19–28. doi:10.1517/14740338.4.1.19. PMID 15709895.
- ^ Bourgois, Philippe (2009). Righteous Dopefiend. Berkeley and Los Angeles: University of California Press. ISBN 0520254988. Retrieved 2009-10-13.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Darke, Shane; Zador, Deborah (1996). "Fatal heroin 'overdose': a review". Addiction. 91 (12): 1765–1772. doi:10.1046/j.1360-0443.1996.911217652.x. PMID 8997759.
- ^ "Toxic Substances in water". Lincoln.pps.k12.or.us. Retrieved 2010-10-20.
- ^ The "heroin overdose" mystery and other occupational hazards of addiction, Schaffer Library of Drug Policy
- ^ Gerevich, JóZsef; Bácskai, Erika; Farkas, Lajos; Danics, Zoltán (2005). "A case report: Pavlovian conditioning as a risk factor of heroin 'overdose' death". Harm Reduction Journal. 2: 11. doi:10.1186/1477-7517-2-11. PMC 1196296. PMID 16042795.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Hill MD, Cooper PW, Perry JR (2000). "Chasing the dragon--neurological toxicity associated with inhalation of heroin vapour: case report". CMAJ. 162 (2): 236–8. PMC 1232277. PMID 10674060.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Halloran, O.; Ifthikharuddin, S; Samkoff, L (2005). "Leukoencephalopathy from "chasing the dragon"". Neurology. 64 (10): 1755. doi:10.1212/01.WNL.0000149907.63410.DA. PMID 15911804.
- ^ Offiah, C; Hall, E (2008). "Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy". Clinical Radiology. 63 (2): 146–52. doi:10.1016/j.crad.2007.07.021. PMID 18194689.
- ^ Nutt, D; King, LA; Saulsbury, W; Blakemore, C (2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet. 369 (9566): 1047–53. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831.
- ^ Kimber, Jo; Dolan, Kate; Wodak, Alex (2005). "Survey of drug consumption rooms: service delivery and perceived public health and amenity impact". Drug and Alcohol Review. 24 (1): 21–4. doi:10.1080/09595230500125047. PMID 16191717.
- ^ Adult Health Advisor 2005.4: Narcotic Drug Withdrawal[dead link]
- ^ "Rolleston Report". Departmental Commission on Morphine and Heroin Addiction, United Kingdom. 1926. Retrieved 28 January 2011.
- ^ Nils Bejerot: The Swedish Addiction Epidemic in global perspective[dead link]
- ^ Goldacre, Ben (1998). "Methadone and Heroin: An Exercise in Medical Scepticism". Retrieved 2006-12-18.
- ^ Nadelmann, Ethan (1995). "Switzerland's Heroin Experiment". Drug Policy Alliance. Retrieved 2006-10-22.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "Swiss approve prescription heroin". BBC News Online. 30 November 2008. Retrieved 30 November 2008.
{{cite news}}: Unknown parameter|curly=ignored (help) - ^ "Heroin prescription 'cuts costs'". BBC News. 2005-06-05. Retrieved 2006-10-22.
{{cite news}}: Unknown parameter|month=ignored (help) - ^ "About the study". North American Opiate Medication Initiative. Retrieved 2006-10-22.
- ^ Nordt, C; Stohler, R (2006). "Incidence of heroin use in Zurich, Switzerland: a treatment case register analysis". The Lancet. 367: 1830–4. doi:10.1016/S0140-6736(06)68804-1. PMID 16753485.
- ^
"Danmark redo för skattebetalt heroin" (in Swedish). 2008. Retrieved 2008-11-30.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "Gratis heroin klar til danske narkomaner" (in Danish). Information. 2010. Retrieved 2010-02-14.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "Heroin-behandling bliver kun i kanyler" (in Danish). Information. 2009. Retrieved 2010-02-14.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "Prescription of injectable heroin for drug users". Danish National Boad of Health. 2007. Retrieved 2010-02-14.
{{cite journal}}: Cite journal requires|journal=(help); Unknown parameter|month=ignored (help) - ^ "Durchbruch für die Behandlung von Schwerstopiatabhängigen ("Breakthrough for the treatment of heavily addicted opiate users", German)". Bundesministerium für Gesundheit (German ministry of health). 2009. Retrieved 2009-08-23.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ Janelle Oswald (2007-12-09). "The Real American Gangster". voice-online. Retrieved 2008-03-08.
She spent five years in prison for aiding her husband's narcotic smuggling trade. Having to get used to the public life again after living like a 'ghost' since her release, the making of her partner's life on the big screen has brought back many memories, some good and some bad.
- ^ Vallely, Paul (2005-09-10). "Gia: The tragic tale of the world's first supermodel". The Independent. London. Retrieved 2009-05-12.
- ^ Koslow, Jessica. "B.G.: Heroin & Cash". Hip Hop DX. Retrieved 26 July 2010.
- ^ Matthew Bates (2008-12). "Loaded - Great heroin songs of the rock era" (PDF). pp. 26–27. Archived from the original (PDF) on 2008-04-09. Retrieved 2008-01-17.
{{cite web}}: Check date values in:|date=(help) - ^ People Magazine
- ^ Burnt Out
- ^ Miles Davis
- ^ Lesh, Phil (2005). Searching for the Sound: My Life with the Grateful Dead. Little, Brown, and Company. ISBN 0-316-00998-9.
- ^ Dougherty, Steve (21). "What a Long, Strange Trip". People Magazine. 44. Retrieved 26 July 2010.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ "Report: Mitch Hedberg died of drug overdose". MSNBC
- ^ Billie Holiday
- ^ New York Times
- ^ a b Times Online
- ^ Orlando Sentinel
- ^ Daily Mail
- ^ VH1
- ^ Telegraph
- ^ Daily Mail
- ^ People Magazine, "Long Island musician found dead of a heroin overdose at Boy George's home in London"
- ^ a b "Nikki Sixx's The Heroin Diaries #7 on The New York Times Best Seller List".
- ^ Classic Bands
- ^ "Sid Vicious dies from drugs overdose". BBC News. 1979-02-02. Retrieved 2010-01-05.
- ^ Ankeny, Jason. "Rozz Williams". Allmusic. Retrieved 2008-04-11.
- ^ Race Matters
- ^ Independent
Further reading
- Diary Of A Drug Fiend by Aleister Crowley (1922)
- Junkie (novel) by William S. Burroughs (1953) ISBN 0-14-200316-6
- Heroin (1998) ISBN 1-56838-153-0
- Heroin Century (2002) ISBN 0-415-27899-6
- This is Heroin (2002) ISBN 1-86074-424-9
- The Heroin User's Handbook by Francis Moraes (paperback 2004) ISBN 1-55950-216-9
- The Little Book of Heroin by Francis Moraes (paperback 2000) ISBN 0-914171-98-4
- Heroin: A True Story of Addiction, Hope and Triumph by Julie O'Toole (paperback 2005) ISBN 1-905379-01-3
- The Heroin Diaries: A Year in the Life of a Shattered Rockstar by Nikki Sixx (2007) ISBN 978-0-7434-8628-6
- Heroin: The Myths and the Facts by Richard Ashley (1972), St. Martin's Press, Library of Congress No. 72-89417
- "The Death Proclamation of Generation X: A Self-Fulfilling Prophesy of Goth, Grunge and Heroin" by Maxim W. Furek, M. (2008), i-Universe. ISBN 978-0-595-46319-0
- Seeds of Terror: How Heroin is Bankrolling the Taliban and Al Qaeda, by Gretchen Peters, publ. Thomas Dunne Books (2009)
External links
- UNODC - United Nations Office on Drugs and Crime - Afghan Opium Survey 2009
- Geopium: Geopolitics of Illicit Drugs in Asia, especially opium and heroin production and trafficking in and around Afghanistan and Burma (Articles and maps and French and English)
- Drugs Factfile what you really need to know
- The mismanagement of methadone
- Harrowing Heroin by Geoff Morton
- National Alliance of Advocates for Buprenorphine Treatment - non-profit education website for treatment of Heroin addiction
- NIDA InfoFacts on Heroin
- ONDCP Drug Facts
- United States Department of State fact sheet: anti-narcotics efforts in Pakistan - dated June 7, 2002
- BBC Article entitled 'When Heroin Was Legal'. References to the United Kingdom and the United States
- Harm reduction strategies in relation to heroin and other illicit drugs
- Heroin news page - Alcohol and Drugs History Society
- U.S. National Library of Medicine: Drug Information Portal - Heroin
: Related navpages:
|
