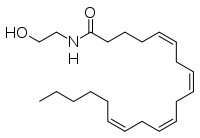
Anandamide
| |
| Names | |
|---|---|
| IUPAC name
(5Z,8Z,11Z,14Z)-N-(2-hydroxyethyl)icosa-5,8,11,14-tetraenamide
| |
| Other names
N-arachidonoylethanolamine
arachidonoylethanolamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | Anandamide |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C22H37NO2 | |
| Molar mass | 347.53 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Anandamide, also known as N-arachidonoylethanolamine or AEA, is an endogenous cannabinoid neurotransmitter. The name is taken from the Sanskrit word ananda, which means "bliss, delight", and amide.[1][2] It is synthesized from N-arachidonoyl phosphatidylethanolamine by multiple pathways.[3] It is degraded primarily by the fatty acid amide hydrolase (FAAH) enzyme, which converts anandamide into ethanolamine and arachidonic acid. As such, inhibitors of FAAH lead to elevated anandamide levels and are being pursued for therapeutic use.[4][5]
History
It was isolated and its structure first described in 1992 by Raphael Mechoulam of the Hebrew University.
Physiological functions
Anandamide's effects can be either central, in the brain, or peripheral, in other parts of the body. These distinct effects are mediated primarily by CB1 cannabinoid receptors in the central nervous system, and CB2 cannabinoid receptors in the periphery.[6] The latter are mainly involved in functions of the immune system. Cannabinoid receptors were originally discovered as being sensitive to Δ9-tetrahydrocannabinol (Δ9-THC, commonly called THC), which is the primary psychoactive cannabinoid found in cannabis. The discovery of anandamide came from research into CB1 and CB2, as it was inevitable that a naturally occurring (endogenous) chemical would be found to affect these receptors.
Anandamide has been shown to impair working memory in rats.[7] Studies are under way to explore what role anandamide plays in human behavior, such as eating and sleep patterns, and pain relief.
Anandamide is also important for implantation of the early stage embryo in its blastocyst form into the uterus. Therefore cannabinoids such as Δ9-THC might influence processes during the earliest stages of human pregnancy.[8] Peak plasma anandamide occurs at ovulation and positively correlates with peak estradiol and gonadotrophin levels, suggesting that these may be involved in the regulation of AEA levels.[9]
Anandamide plays a role in the regulation of feeding behavior, and the neural generation of motivation and pleasure. In addition, anandamide injected directly into the forebrain reward-related brain structure nucleus accumbens enhances the pleasurable responses of rats to a rewarding sucrose taste, and enhances food intake as well.[6][10]
A study published in 1998 shows that anandamide inhibits human breast cancer cell proliferation.[11] Some studies have linked anandamide release as a mechanism of analgesic effects induced by exercise, particularly by running.[12]
In 1996, researchers discovered anandamide in chocolate. They also detected the presence of two substances that might mimic the effects of anandamide, N-oleoylethanolamine and N-linoleoylethanolamine.[13]
Synthesis and degradation
The human body synthesizes anandamide from N-arachidonoyl phosphatidylethanolamine (NAPE), which is itself made by transferring arachidonic acid from lecithin to the free amine of cephalin through an N-acyltransferase enzyme.[14][15] Anandamide synthesis from NAPE occurs via multiple pathways and includes enzymes such as phospholipase A2, phospholipase C and NAPE-PLD.[3]
Endogenous anandamide is present at very low levels and has a very short half-life due to the action of the enzyme fatty acid amide hydrolase (FAAH), which breaks it down into free arachidonic acid and ethanolamine. Studies of piglets show that dietary levels of arachidonic acid and other essential fatty acids affect the levels of anandamide and other endocannabinoids in the brain.[16] High fat diet feeding in mice increases levels of anandamide in the liver and increases lipogenesis.[17] This suggests that anandamide may play a role in the development of obesity, at least in rodents.
Paracetamol (or acetaminophen in the U.S.A.) is metabolically combined with arachidonic acid by FAAH to form AM404.[18] This metabolite of paracetamol is a potent agonist at the TRPV1 vanilloid receptor, a weak agonist at both CB1 and CB2 receptors, and an inhibitor of anandamide reuptake. As a result, anandamide levels in the body and brain are elevated. In this fashion, paracetamol acts as a pro-drug for a cannabimimetic metabolite. This action may be partially or fully responsible for the analgesic effects of paracetamol.[19][20]
See also
- Cannabinoids
- Virodhamine
- Tetrahydrocannabinol (THC)
- 2-Arachidonoylglycerol
- Fatty acid amide hydrolase
- Raphael Mechoulam
References
- ^ Devane WA; et al. (1992). "Isolation and structure of a brain constituent that binds to the cannabinoid receptor". Science. 258 (5090): 1946–9. doi:10.1126/science.1470919. PMID 1470919.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - ^ Mechoulam R, Fride E (1995). "The unpaved road to the endogenous brain cannabinoid ligands, the anandamides". In Pertwee RG (ed.). Cannabinoid receptors. Boston: Academic Press. pp. 233–258. ISBN 0-12-551460-3.
- ^ a b Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.prostaglandins.2008.12.002, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.prostaglandins.2008.12.002instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/S0074-7742(09)85005-8, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/S0074-7742(09)85005-8instead. - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1016/j.lfs.2009.06.003, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1016/j.lfs.2009.06.003instead. - ^ a b Pacher P, Batkai S, Kunos G (2006). "The Endocannabinoid System as an Emerging Target of Pharmacotherapy". Pharmacol Rev. 58 (3): 389–462. doi:10.1124/pr.58.3.2. PMC 2241751. PMID 16968947.
{{cite journal}}: Unknown parameter|unused_data=ignored (help)CS1 maint: multiple names: authors list (link) - ^ allet PE, Beninger RJ (1996). "The endogenous cannabinoid receptor agonist anandamide impairs memory in rats". Behavioural Pharmacology. 7 (3): 276–284.
- ^ Piomelli D (2004). "THC: moderation during implantation". Nat. Med. 10 (1): 19–20. doi:10.1038/nm0104-19. PMID 14702623.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ El-Talatini MR, Taylor AH, Konje JC (2010). "The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle". Fertil. Steril. 93 (6): 1989–96. doi:10.1016/j.fertnstert.2008.12.033. PMID 19200965.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Mahler SV, Smith KS, Berridge KC (2007). "Endocannabinoid hedonic hotspot for sensory pleasure: anandamide in nucleus accumbens shell enhances 'liking' of a sweet reward". Neuropsychopharmacology. 32 (11): 2267–78. doi:10.1038/sj.npp.1301376. PMID 17406653.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ De Petrocellis L; et al. (1998). "The endogenous cannabinoid anandamide inhibits human breast cancer cell proliferation". Proc. Natl. Acad. Sci. U.S.A. 95 (14): 8375–80. doi:10.1073/pnas.95.14.8375. PMC 20983. PMID 9653194.
{{cite journal}}: Cite has empty unknown parameter:|1=(help); Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - ^ http://www.harford.de/arne/articles/NeuroReport.pdf
- ^ di Tomaso E, Beltramo M, Piomelli D. (1996). "Brain cannabinoids in chocolate". Nature. 382 (6593): 677–8. doi:10.1038/382677a0. PMID 8751435.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Natarajan V, Reddy PV, Schmid PC, Schmid HH (1982). "N-Acylation of ethanolamine phospholipids in canine myocardium". Biochim. Biophys. Acta. 712 (2): 342–55. PMID 7126608.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Cadas H, di Tomaso E, Piomelli D (1997). "Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain". J. Neurosci. 17 (4): 1226–42. PMID 9006968.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Berger A; et al. (2001). "Anandamide and diet: Inclusion of dietary arachidonate and docosahexaenoate leads to increased brain levels of the corresponding N-acylethanolamines in piglets". Proc. Natl. Acad. Sci. U.S.A. 98 (11): 6402–6. doi:10.1073/pnas.101119098. PMC 33480. PMID 11353819.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - ^ Osei-Hyiaman D; et al. (2005). "Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity". J. Clin. Invest. 115 (5): 1298–305. doi:10.1172/JCI23057. PMC 1087161. PMID 15864349.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help) - ^ Attention: This template ({{cite doi}}) is deprecated. To cite the publication identified by doi:10.1074/jbc.M501489200, please use {{cite journal}} (if it was published in a bona fide academic journal, otherwise {{cite report}} with
|doi=10.1074/jbc.M501489200instead. - ^ Bertolini A; et al. (2006). "Paracetamol: new vistas of an old drug". CNS Drug Rev. 12 (3–4): 250–75. doi:10.1111/j.1527-3458.2006.00250.x. PMID 17227290.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Sinning C; et al. (2008). "New analgesics synthetically derived from the paracetamol metabolite N-(4-hydroxyphenyl)-(5Z,8Z,11Z,14Z)-icosatetra-5,8,11,14-enamide". J. Med. Chem. 51 (24): 7800–5. doi:10.1021/jm800807k. PMID 19053765.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)
External links
- Could anandamide be the missing link to "runner's high"? Accessed 2008-10-18
