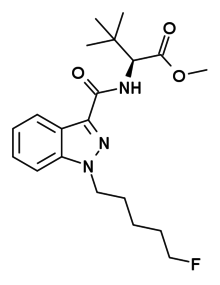
5F-ADB
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.257.468 |
| Chemical and physical data | |
| Formula | C20H28FN3O3 |
| Molar mass | 377.460 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
5F-ADB (also known as 5F-MDMB-PINACA) is an indazole-based synthetic cannabinoid from the indazole-3-carboxamide family, which has been used as an active ingredient in synthetic cannabis products and has been sold online as a designer drug.[1] 5F-ADB is a potent agonist of the CB1 receptor,[2] though it is unclear whether it is selective for this target. 5F-ADB was first identified in November 2014 from post-mortem samples taken from an individual who had died after using a product containing this substance. Subsequent testing identified 5F-ADB to have been present in a total of ten people who had died from unexplained drug overdoses in Japan between September 2014 and December 2014, and it was added to the Japanese banned drug list in December 2014. 5F-ADB is believed to be extremely potent based on the very low levels detected in tissue samples, and appears to be significantly more toxic than earlier synthetic cannabinoid drugs that had previously been sold.[3]
In the United States, 5F-ADB is a Schedule I controlled substance.[4]
See also
References
- ^ Shevyrin V, Melkozerov V, Nevero A, Eltsov O, Shafran Y, Morzherin Y, Lebedev AT (Apr 2015). "Identification and analytical characteristics of synthetic cannabinoids with an indazole-3-carboxamide structure bearing a N-1-methoxycarbonylalkyl group". Analytical and Bioanalytical Chemistry. 407: 6301–15. doi:10.1007/s00216-015-8612-7. PMID 25893797.
- ^ Samuel D Banister; et al. (July 2016). "The pharmacology of valinate and tert-leucinate synthetic cannabinoids 5F-AMBICA, 5F-AMB, 5F-ADB, AMB-FUBINACA, MDMB-FUBINACA, MDMB-CHMICA, and their analogues". ACS Chemical Neuroscience. 7: 1241–54. doi:10.1021/acschemneuro.6b00137. PMID 27421060.
{{cite journal}}: Cite has empty unknown parameter:|1=(help) - ^ Hasegawa, Koutaro; Wurita, Amin; Minakata, Kayoko; Gonmori, Kunio; Yamagishi, Itaru; Nozawa, Hideki; Watanabe, Kanako; Suzuki, Osamu (2014). "Identification and quantitation of 5-fluoro-ADB, one of the most dangerous synthetic cannabinoids, in the stomach contents and solid tissues of a human cadaver and in some herbal products". Forensic Toxicology. 33: 112–121. doi:10.1007/s11419-014-0259-0.
- ^ "Schedules of Controlled Substances: Temporary Placement of Six Synthetic Cannabinoids (5F-ADB, 5F-AMB, 5F-APINACA, ADB-FUBINACA, MDMB-CHMICA and MDMB-FUBINACA) Into Schedule I". Drug Enforcement Administration.
