Pirandamine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
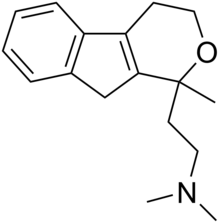
| Formula | C17H23NO |
| Molar mass | 257.377 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Pirandamine (AY-23,713) is a tricyclic derivative which acts as a selective serotonin reuptake inhibitor (SSRI).[1][2][3] It was investigated in the 1970s as a potential antidepressant but clinical development was not commenced and it was never marketed.[1] Pirandamine is structurally related to tandamine, which, in contrast, is a selective norepinephrine reuptake inhibitor.[1][3]
Synthesis

Condensation of 1-indanone (1) with ethyl bromoacetate and zinc affords Reformatsky reaction product 2; then reduction with LiAlH4 gives diol 3. Dehydration with H2SO4 gives the indene ethanol 4. Acid catalyzed condensation of 4 with ethyl acetoacetate then gives the fused tetrahydropyran derivative 5 (no doubt by a scheme quite analogous to prodolic acid). The ester is then saponified to the corresponding acid, which is then converted to the dimethylamide (6). Reduction with LiAlH4 completes the synthesis of the antidepressant agent pirandamine (7).
See also
- Tandamine
- Prindamine (21489-22-5)[5]
References
- ^ a b c Pugsley T, Lippmann W (May 1976). "Effects of tandamine and pirandamine, new potential antidepressants, on the brain uptake of norepinephrine and 5-hydroxytryptamine and related activities". Psychopharmacology. 47 (1): 33–41. doi:10.1007/BF00428698. PMID 1085452.
- ^ Lippmann W, Pugsley TA (August 1976). "Pirandamine, a relatively selective 5-hydroxytryptamine uptake inhibitor". Pharmacological Research Communications. 8 (4): 387–405. doi:10.1016/0031-6989(76)90039-4. PMID 1088377.
- ^ a b Lippmann W, Seethaler K (April 1977). "Effects of tandamine and pirandamine, selective blockers of biogenic amine uptake mechanisms, on gastric acid secretion and ulcer formation in the rat". Life Sciences. 20 (8): 1393–400. doi:10.1016/0024-3205(77)90367-8. PMID 853871.
- ^ I. Jirkovsky, L. G. Humber and R. Noureldin,Eur. J. Med. Chem., 11, 571 (1976).
- ^ http://www.chemicalbook.com/ProductChemicalPropertiesCB71179577_EN.htm
