EIF5A
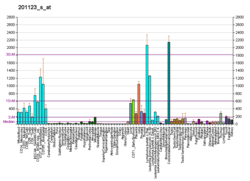
Appearance
Eukaryotic translation initiation factor 5A-1 is a protein that in humans is encoded by the EIF5A gene.[5]
It is the only known protein to contain the unusual amino acid hypusine [N (ε)- (4-amino-2-hydroxybutyl)-lysine], which is synthesized on eIF5A at a specific lysine residue from the polyamine spermidine by two catalytic steps.[6]
EF-P is the prokaryotic homolog of eIF5A, which is also modified post-translationally in a similar but distinct way.[7][8] Template:PBB Summary
References
- ^ a b c ENSG00000288145 GRCh38: Ensembl release 89: ENSG00000132507, ENSG00000288145 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000078812 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Steinkasserer A, Jones T, Sheer D, Koettnitz K, Hauber J, Bevec D (Jun 1995). "The eukaryotic cofactor for the human immunodeficiency virus type 1 (HIV-1) rev protein, eIF-5A, maps to chromosome 17p12-p13: three eIF-5A pseudogenes map to 10q23.3, 17q25, and 19q13.2". Genomics. 25 (3): 749–52. doi:10.1016/0888-7543(95)80025-H. PMID 7759117.
- ^ Wolff EC, Kang KR, Kim YS, Park MH (May 2007). "Posttranslational synthesis of hypusine: evolutionary progression and specificity of the hypusine modification". Amino Acids. 33 (2): 341–350. doi:10.1007/s00726-007-0525-0. PMC 2572820. PMID 17476569.
- ^ Park JH, Johansson HE, Aoki H, Huang BX, Kim HY, Ganoza MC, Park MH (Nov 2011). "Post-translational modification by β-lysylation is required for activity of Escherichia coli elongation factor P (EF-P)". Journal of Biological Chemistry. 287 (4): 2579–2590. doi:10.1074/jbc.M111.309633. PMC 3268417. PMID 22128152.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Peil L, Starosta AL, Virumäe K, Atkinson GC, Tenson T, Remme J, Wilson DN (2012). "Lys34 of translation elongation factor EF-P is hydroxylated by YfcM". Nature Chemical Biology. 8 (8): 695–7. doi:10.1038/nchembio.1001. PMID 22706199.
Further reading