Stimulant
This article needs additional citations for verification. (December 2007) |
Stimulants (also referred to as psychostimulants) are psychoactive drugs that induce temporary improvements in either mental or physical functions or both. Examples of these kinds of effects may include enhanced alertness, wakefulness, and locomotion, among others. Due to their rendering a characteristic "up" feeling, stimulants are also occasionally referred to as "uppers". Depressants or "downers", which decrease mental and/or physical function, are in stark contrast to stimulants and are considered to be their functional opposites. Stimulants are widely used throughout the world as prescription medicines and without prescription both as legal substances and illicit substances of recreational use or abuse.
Effects
This section needs additional citations for verification. (January 2014) |
Stimulants produce a variety of different kinds of effects by enhancing the activity of the central and peripheral nervous systems. Common effects, which vary depending on the substance and dosage in question, may include enhanced alertness, awareness, wakefulness, endurance, productivity, and motivation, increased arousal, locomotion, heart rate, and blood pressure, and the perception of a diminished requirement for food and sleep. Many stimulants are also capable of improving mood and relieving anxiety, and some can even induce feelings of euphoria. However different effects are often dose related, such as amphetamine causing anxiety, dysthymia, hyperactivity and potentially heart failure at high doses, but relieving anxiety, producing euthymia or euphoria, reducing hyperactivity and being generally free of serious side effects at moderate doses used in clinical medicine. Stimulants exert their effects through a number of different pharmacological mechanisms, the most prominent of which include facilitation of norepinephrine (noradrenaline) and/or dopamine activity (e.g., via monoamine transporter inhibition or reversal),[1] adenosine receptor antagonism, and nicotinic acetylcholine receptor agonism.
Medical uses
Stimulants are used both individually and clinically for therapeutic purposes in the treatment of a number of indications, including the following:
- To counteract lethargy and fatigue throughout the day while at work or while doing other activities
- To reduce sleepiness and to keep the person awake when necessary, as well as to treat narcolepsy
- To decrease appetite and promote weight loss, as well as to treat obesity
- To improve concentration and focus, and reduce restlessness and hyperactivity, especially for those with attentional disorders such as ADHD
- Occasionally, used off-label to treat clinical depression and bipolar disorder, in particular, atypical depression and treatment-resistant depression[2][3][4][5][6]
- To relieve nasal congestion and to treat orthostatic hypotension and postural orthostatic tachycardia syndrome.
- To aid in smoking cessation. (bupropion, nicotine)
- To counteract fatigue and maintain alertness for extended periods and critical operations in military aviation and space flight.
- To offset sedative effects of opioids used long term at higher doses such as in cancer or AIDS patients. (May also have synergistic effect on opioids.)
- To relieve headache, in part by potentiating other drugs. (caffeine)
ADHD drugs
Stimulants are the most effective, most commonly prescribed medications for ADHD.[7] The most common stimulant medications are substituted phenethylamines: amphetamine, methylphenidate (Ritalin, Metadate, Concerta), dexmethylphenidate (Focalin), dextroamphetamine (Dexedrine, Zenzedi), mixed amphetamine salts (Adderall),[8] dextromethamphetamine (Desoxyn)[9] and lisdexamfetamine (Vyvanse).[10] Controlled-release formulations may allow once or twice daily administration of medication. Once daily morning administration is especially helpful for children preferring not to take their medication in the middle of the school day. Several controlled-release methods are used.
Ampakines
Ampakines are a class of compounds observed to enhance attention span and alertness, and facilitate learning and memory in clinical trials. They take their name from the glutamatergic AMPA receptor with which they strongly interact.
These stimulants tend to increase alertness without the peripheral (body) effects or addiction/tolerance/abuse potential of "traditional" stimulants (such as amphetamine),[medical citation needed] as they lack direct dopaminergic action. Their effect on sleep structure is not fully established and may reduce quality of sleep. The ampakine CX717, when administered at doses necessary to reduce the effects of sleep deprivation, reduced subsequent stage 4 and slow-wave recovery sleep.[11] Ampakines such as ampalex and CX717 have been developed but are awaiting further research before being commercially released. They have been investigated by DARPA for potential use in increasing military effectiveness.[12]
Eugeroics
A wakefulness-promoting agent (eugeroic) is a type of psychoactive drug that improves wakefulness and alertness, and reduces tiredness, drowsiness, and the need for sleep. They are used mainly in the treatment of sleeping disorders, excessive daytime sleepiness, and narcolepsy, though they are also used merely to counteract fatigue and lethargy and to enhance motivation and productivity. Wakefulness-promoting agents appear to function primarily by increasing catecholaminergic (adrenergic, dopaminergic) and histaminergic activity in the brain. Unlike many other stimulants, eugeroics are relatively non-addictive and non-dependence-forming.[medical citation needed]
The prototype drug in this class is modafinil, and other drugs include adrafinil, hydrafinil, 2C-NPH and armodafinil. The primary difference between these drugs and amphetamine-like stimulants is that wakefulness-promoting agents trigger activation of neurons in the hypothalamus-based wakefulness circuits, as opposed to producing diffuse neuronal activation.[13]
The functional opposites of wakefulness-promoting agents would be hypnotics/sedatives like antihistamines, opioids, and benzodiazepines.
Chemistry
Classifying stimulants is difficult, because of the large number of classes the drugs occupy, and the fact that they may belong to multiple classes; for example, ecstasy is a substituted methylenedioxyphenethylamine as well as a substituted amphetamine (and consequently, a substituted phenethylamine as well).
When referring to stimulants, the parent drug (e.g., amphetamine) will always be expressed in the singular; while the word "substituted" before the parent drug (substituted amphetamines).
Major stimulant classes include phenethylamines and their daughter class substituted amphetamines.
Amphetamines (class)
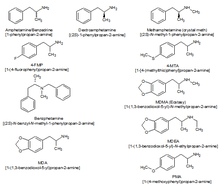
Substituted amphetamines are a group of phenylethylamine stimulants such as amphetamine and methamphetamine. With the exception of cathinones, many drugs in this class work primarily by activating trace amine-associated receptor 1 (TAAR1);[14] in turn, this causes reuptake inhibition and effluxion, or release, of dopamine, norepinephrine, and serotonin.[14] An additional mechanism of some substituted amphetamines is the release of neurotransmitters from synaptic vesicles into the cytosol, or intracellular fluid of the presynaptic neuron.[15]
Amphetamines-type stimulants are often used for their therapeutic effects. Physicians sometimes prescribe amphetamine to treat major depression, where subjects do not respond well to traditional SSRI medications,[citation needed] but evidence supporting this use is poor/mixed.[16] Notably, two recent large phase III studies of lisdexamfetamine (a prodrug to amphetamine) as an adjunct to an SSRI or SNRI in the treatment of major depressive disorder showed no further benefit relative to placebo in effectiveness.[17] Numerous studies have demonstrated the effectiveness of drugs such as Adderall (a mixture of salts of amphetamine and dextroamphetamine) in controlling symptoms associated with ADD/ADHD. Due to their availability and fast-acting effects, substituted amphetamines are prime candidates for abuse.[18]
Dopamine precursors
Dopamine is one of the principal neurotransmitters involved with stimulant activity in the brain, (others being norepinephrine and serotonin). Increase in its precursors may result in increased dopamine biosynthesis, especially in malnourished individuals. However levels of the enzyme tyrosine hydroxylase ultimately limit the biosynthesis regardless of increased tyrosine.
L-Tyrosine is the precursor that is 'closest' to being dopamine among those supplements legally available without prescription in most jurisdictions. It is converted by tyrosine hydroxylase into L-Dopa. Some of this L-Dopa is converted into dopamine and norepinephrine. Because tyrosine competes with other amino acids for entry into the brain supplement makers recommend tyrosine be taken on an empty stomach. However tyrosine hydroxylase is the rate limiting factor and even large dosages recommended by most supplement companies may not produce any noticeable effect. L-Phenylalanine is 'one step back' from L-Tyrosine—it must be converted into tyrosine before the tyrosine can be converted into L-Dopa, which in turn becomes dopamine. Dopamine is also the direct precursor of norepinephrine.
DBH: Dopamine β-hydroxylase; AADC:Aromatic L-amino acid decarboxylase; AAAH: (Biopterin-dependent) aromatic amino acid hydroxylase; COMT: Catechol O-methyltransferase; PNMT: Phenylethanolamine N-methyltransferase |
Notable stimulants
Amphetamine (drug)
Amphetamine is a potent central nervous system (CNS) stimulant of the phenethylamine class that is used in the treatment of attention deficit hyperactivity disorder (ADHD) and narcolepsy.[22] Amphetamine was discovered in 1887 and exists as two enantiomers: levoamphetamine and dextroamphetamine.[note 1][23] Amphetamine refers to equal parts of the enantiomers, i.e., 50% levoamphetamine and 50% dextroamphetamine.[24][25] Historically, it has been used to treat nasal congestion, depression, and obesity.[23][26] Amphetamine is also used as a performance and cognitive enhancer, and recreationally as an aphrodisiac and euphoriant.[27][28][29][30] Although it is a prescription medication in many countries, unauthorized possession and distribution of amphetamine is often tightly controlled due to the significant health risks associated with uncontrolled or heavy use.[31][32] As a consequence, amphetamine is illegally synthesized by clandestine chemists, trafficked, and sold.[33] Based upon drug and drug precursor seizures worldwide, illicit amphetamine production and trafficking is much less prevalent than that of methamphetamine.[33]
The first pharmaceutical amphetamine was Benzedrine, a brand of inhalers used to treat a variety of conditions.[23][26] Because the dextro isomer has greater stimulant properties, Benzedrine was gradually discontinued in favor of formulations containing all or mostly dextroamphetamine. Presently, it is typically prescribed as Adderall, dextroamphetamine (e.g., Dexedrine), or the inactive prodrug lisdexamfetamine (e.g., Vyvanse).[23][34] Amphetamine, through activation of a trace amine receptor, increases biogenic amine and excitatory neurotransmitter activity in the brain, with its most pronounced effects targeting the catecholamine neurotransmitters norepinephrine and dopamine.[14] At therapeutic doses, this causes emotional and cognitive effects such as euphoria, change in libido, increased arousal, and improved cognitive control.[28][29][35] Likewise, it induces physical effects such as decreased reaction time, fatigue resistance, and increased muscle strength.[27]
In contrast, much larger doses of amphetamine are likely to impair cognitive function and induce rapid muscle breakdown.[22][28][36] Substance dependence (i.e., addiction) is a serious risk of amphetamine abuse, but only rarely arises from proper medical use.[22][37] Very high doses can result in a psychosis (e.g., delusions and paranoia), which very rarely occurs at therapeutic doses even during long-term use.[38][39] As recreational doses are generally much larger than prescribed therapeutic doses, recreational use carries a far greater risk of serious side effects.[22][36]
Amphetamine is the parent compound of its own structural class, the (substituted) amphetamines, which includes prominent substances such as bupropion, cathinone, ecstasy, and methamphetamine.[40][41] It is chemically related to methamphetamine; however, unlike methamphetamine, its salts lack sufficient volatility to be smoked.[40] During long-term treatment in humans, amphetamine has been shown to normalize, or improve, brain function, in particular in the right caudate nucleus;[42][43] in contrast, methamphetamine induces permanent reductions in brain structure and function.[44][45] Amphetamine is also chemically related to the naturally occurring trace amines, to be specific phenethylamine and N-methylphenethylamine, both of which produced within the human body.[41]
Caffeine

Caffeine is a stimulant compound belonging to the xanthine class of chemicals naturally found in coffee, tea, and (to a lesser degree) cocoa or chocolate. It is included in many soft drinks, as well as a larger amount in energy drinks. Caffeine is the world's most widely used psychoactive drug and by far the most common stimulant. In North America, 90% of adults consume caffeine daily.[46] A few jurisdictions restrict its sale and use. Caffeine is also included in some medications, usually for the purpose of enhancing the effect of the primary ingredient, or reducing one of its side-effects (especially drowsiness). Tablets containing standardized doses of caffeine are also widely available.
Ephedrine
Ephedrine is a sympathomimetic amine similar in molecular structure to the well-known drugs phenylpropanolamine and methamphetamine, as well as to the important neurotransmitter epinephrine (adrenaline). Ephedrine is commonly used as a stimulant, appetite suppressant, concentration aid, and decongestant, and to treat hypotension associated with anaesthesia.
In chemical terms, it is an alkaloid with a phenethylamine skeleton found in various plants in the genus Ephedra (family Ephedraceae). It works mainly by increasing the activity of norepinephrine (noradrenaline) on adrenergic receptors.[47] It is most usually marketed as the hydrochloride or sulfate salt.
The herb má huáng (Ephedra sinica), used in traditional Chinese medicine (TCM), contains ephedrine and pseudoephedrine as its principal active constituents. The same may be true of other herbal products containing extracts from other Ephedra species.
MDMA

3,4-Methylenedioxymethamphetamine (MDMA or ecstasy), typically comes as tablets, capsules, and in powder/crystal form. Briefly used by some psychotherapists as an adjunct to therapy, the drug became popular recreationally and the DEA listed MDMA as a Schedule I controlled substance, prohibiting most medical studies and applications. MDMA is known for its entactogenic properties. The stimulant effects of MDMA include hypertension, anorexia (appetite loss), euphoria, social disinhibition, insomnia (enhanced wakefulness/inability to sleep), improved energy, increased arousal, and increased perspiration, among others.
MDMA differs from most stimulants in that its primary pharmacological effect is on the neurotransmitter serotonin rather than dopamine, epinephrine, or norepinephrine. Because of this, it is considered to be primarily an entactogen or an empathogen.
MDPV
Methylenedioxypyrovalerone (MDPV) is a psychoactive drug with stimulant properties that acts as a norepinephrine-dopamine reuptake inhibitor (NDRI).[48] It was first developed in the 1960s by a team at Boehringer Ingelheim.[49] MDPV remained an obscure stimulant until around 2004, when it was reported to be sold as a designer drug. Products labeled as bath salts containing MDPV were previously sold as recreational drugs in gas stations and convenience stores in the United States, similar to the marketing for Spice and K2 as incense.[50][51]
Incidents of psychological and physical harm have been attributed to MDPV use.[52][53]
Prolintane
Prolintane is a stimulant that has been around since the 1950s. It is stronger than caffeine and has a very good safety profile. Prolintane use isn't very common, though some people[who?] use it as a safer and more effective alternative to caffeine.
Mephedrone
Mephedrone is a synthetic stimulant drug of the amphetamine and cathinone classes. Slang names include drone[54] and MCAT.[55] It is reported to be manufactured in China and is chemically similar to the cathinone compounds found in the khat plant of eastern Africa. It comes in the form of tablets or a powder, which users can swallow, snort, or inject, producing similar effects to MDMA, amphetamines, and cocaine.
Mephedrone was first synthesized in 1929, but did not become widely known until it was rediscovered in 2003. By 2007, mephedrone was reported to be available for sale on the Internet; by 2008 law enforcement agencies had become aware of the compound; and, by 2010, it had been reported in most of Europe, becoming particularly prevalent in the United Kingdom. Mephedrone was first made illegal in Israel in 2008, followed by Sweden later that year. In 2010, it was made illegal in many European countries, and, in December 2010, the EU ruled it illegal. In Australia, New Zealand, and the USA, it is considered an analog of other illegal drugs and can be controlled by laws similar to the Federal Analog Act. In September 2011, the USA temporarily classified mephedrone as illegal, in effect from October 2011.
Methamphetamine
Methamphetamine (contracted from N-methyl-alpha-methylphenethylamine) is a neurotoxin and potent psychostimulant of the phenethylamine and amphetamine classes that is used to treat attention deficit hyperactivity disorder (ADHD) and obesity.[44][56][45] Methamphetamine exists as two enantiomers, dextrorotary and levorotary.[57][58] Dextromethamphetamine is a stronger CNS stimulant than levomethamphetamine;[36][57][58] however, both are addictive and produce the same toxicity symptoms at high doses.[58] Although rarely prescribed due to the potential risks, methamphetamine hydrochloride is approved by the United States Food and Drug Administration (USFDA) under the trade name Desoxyn.[56] Recreationally, methamphetamine is used to increase sexual desire, lift the mood, and increase energy, allowing some users to engage in sexual activity continuously for several days straight.[56][59]
Methamphetamine may be sold illicitly, either as pure dextromethamphetamine or in an equal parts mixture of the right- and left-handed molecules (i.e., 50% levomethamphetamine and 50% dextromethamphetamine).[59] Both dextromethamphetamine and racemic methamphetamine are schedule II controlled substances in the United States.[56] Also, the production, distribution, sale, and possession of methamphetamine is restricted or illegal in many other countries due to its placement in schedule II of the United Nations Convention on Psychotropic Substances treaty.[60][61] In contrast, levomethamphetamine is an over-the-counter drug in the United States.[note 2]
In low doses, methamphetamine can cause an elevated mood and increase alertness, concentration, and energy in fatigued individuals.[36][56] At higher doses, it can induce psychosis, rhabdomyolysis, and cerebral hemorrhage.[36][56] Methamphetamine is known to have a high potential for abuse and addiction.[36][56] Recreational use of methamphetamine may result in psychosis or lead to post-withdrawal syndrome, a withdrawal syndrome that can persist for months beyond the typical withdrawal period.[64] Unlike amphetamine and cocaine, methamphetamine is neurotoxic to humans, damaging both dopamine and serotonin neurons in the central nervous system (CNS).[44][45] Entirely opposite to the long-term use of amphetamine, there is evidence that methamphetamine causes brain damage from long-term use in humans;[44][45] this damage includes adverse changes in brain structure and function, such as reductions in gray matter volume in several brain regions and adverse changes in markers of metabolic integrity.[42][43][45]
Nicotine
This section needs expansion with: a connection between its addictive properties and stimulant effect. You can help by adding to it. (January 2014) |
Nicotine is the active chemical constituent in tobacco, which is available in many forms, including cigarettes, cigars, chewing tobacco, and smoking cessation aids such as nicotine patches, nicotine gum, and electronic cigarettes. Nicotine is used widely throughout the world for its stimulating and relaxing effects.
Phenylpropanolamine
Phenylpropanolamine (PPA; Accutrim; β-hydroxyamphetamine), also known as the stereoisomers norephedrine and norpseudoephedrine, is a psychoactive drug of the phenethylamine and amphetamine chemical classes that is used as a stimulant, decongestant, and anorectic agent.[65] It is commonly used in prescription and over-the-counter cough and cold preparations. In veterinary medicine, it is used to control urinary incontinence in dogs under trade names Propalin and Proin.
In the United States, PPA is no longer sold without a prescription due to a proposed increased risk of stroke in younger women. In a few countries in Europe, however, it is still available either by prescription or sometimes over-the-counter. In Canada, it was withdrawn from the market on 31 May 2001.[66] In India, human use of PPA and its formulations were banned on 10 February 2011.[67]
Propylhexedrine
Propylhexedrine (Hexahydromethamphetamine, Obesin) is a stimulant medication, sold over-the-counter in the United States as the cold medication Benzedrex.[68] The drug has also been used as an appetite suppressant in Europe. Propylhexedrine is not an amphetamine, though it is structurally similar; it is instead a cycloalkylamine, and thus has stimulant effects that are less potent than similarly structured amphetamines, such as methamphetamine.
The abuse potential of propylhexedrine is fairly limited, due its limited routes of administration: in the United States, Benzedrex is only available as an inhalant, mixed with lavender oil and menthol. These ingredients cause unpleasant tastes, and abusers of the drug have reported unpleasant "menthol burps." Injection of the drug has been found to cause transient diplopia and brain stem dysfunction.[69][70][71]
Dimethylamylamine
Dimethylamylamine is a stimulant drug, once sold in over-the-counter workout supplements and study aids in the United States as in the supplement Jack 3D, but it was later discontinued. Dimethylamylamine is not an amphetamine, though it is structurally similar, and thus has stimulant effects that are less potent than similarly structured amphetamines, such as amphetamine.
Pseudoephedrine
Pseudoephedrine is a sympathomimetic drug of the phenethylamine and amphetamine chemical classes. It may be used as a nasal/sinus decongestant, as a stimulant,[72] or as a wakefulness-promoting agent.[73]
The salts pseudoephedrine hydrochloride and pseudoephedrine sulfate are found in many over-the-counter preparations, either as a single ingredient or (more commonly) in combination with antihistamines, guaifenesin, dextromethorphan, and/or paracetamol (acetaminophen) or another NSAID (such as aspirin or ibuprofen).
Catha edulis (Khat)

Khat is a flowering plant native to the Horn of Africa and the Arabian Peninsula.[74][75]
Khat contains a monoamine alkaloid called cathinone, a "keto-amphetamine", that is said to cause excitement, loss of appetite, and euphoria. In 1980, the World Health Organization (WHO) classified it as a drug of abuse that can produce mild to moderate psychological dependence (less than tobacco or alcohol),[76] although the WHO does not consider khat to be seriously addictive.[75] It is a controlled substance in some countries, such as the United States, Canada, and Germany, while its production, sale, and consumption are legal in other nations, including Djibouti, Ethiopia, Somalia, and Yemen.[77]
Cocaine
Cocaine is an SNDRI. Cocaine is made from the leaves of the coca shrub, which grows in the mountain regions of South American countries such as Bolivia, Colombia, and Peru. In Europe, North America, and some parts of Asia, the most common form of cocaine is a white crystalline powder. Cocaine is a stimulant but is not normally prescribed therapeutically for its stimulant properties, although it sees clinical use as a local anesthetic, in particular in ophthalmology. Most cocaine use is recreational and its abuse potential is high(albeit higher than amphetamine), and so its sale and possession are strictly controlled in most jurisdictions. Other tropane derivative drugs related to cocaine are also known such as troparil and lometopane but have not been widely sold or used recreationally.[78]
Abuse
Abuse of central nervous system (CNS) stimulants is common. Addiction to some CNS stimulants can quickly lead to medical, psychiatric, and psychosocial deterioration. Drug tolerance, dependence, and sensitization as well as a withdrawal syndrome can occur.[79]
Stimulants enhance the activity of the central and peripheral nervous systems. Common effects may include increased alertness, awareness, wakefulness, endurance, productivity, and motivation, arousal, locomotion, heart rate, and blood pressure, and a diminished desire for food and sleep.
Use of stimulants may cause the body to reduce significantly its production of natural body chemicals that fulfill similar functions. Until the body reestablishes its normal state, once the effect of the ingested stimulant has worn off the user may feel depressed, lethargic, confused, and miserable. This is referred to as a "crash", and may provoke reuse of the stimulant.
Testing
The presence of stimulants in the body may be tested by a variety of procedures. Serum and urine are the common sources of testing material although saliva is sometimes used. Commonly used tests include chromatography, immunologic assay, and mass spectrometry.[80] Patients taking ADHD-prescribed, Adderall-type amphetamine compounds are commonly surprised upon being tested as "positive" for "meth", or methamphetamine (Desoxyn—its licit, FDA-licensed, medicinal form) in forensically unsophisticated urinalysis, as methamphetamine is the active ingredient of the drug Desoxyn, and is chemically similar to the active ingredients of other ADHD medications.
See also
Notes
- ^ Enantiomers are molecules that are mirror images of one another; they are structurally identical, but of the opposite orientation.
Levoamphetamine and dextroamphetamine are also known as L-amph or levamfetamine (INN) and D-amph or dexamfetamine (INN) respectively. - ^ The active ingredient in Vicks VapoInhaler is listed as Levmetamfetamine, the INN of Levomethamphetamine.[62][63]
References
- ^ Riddle EL, Fleckenstein AE, Hanson GR (2005). "Role of monoamine transporters in mediating psychostimulant effects". The AAPS journal. 7 (4): E847–51. doi:10.1208/aapsj070481. PMC 2750953. PMID 16594636.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Stotz, Gabriele; Woggon, Brigitte; Angst, Jules (1 December 1999). "Psychostimulants in the therapy of treatment-resistant depression Review of the literature and findings from a retrospective study in 65 depressed patients". Dialogues in Clinical Neuroscience. 1 (3): 165–174. ISSN 1294-8322. PMC 3181580. PMID 22034135.
- ^ Stewart, Jonathan W.; Deliyannides, Deborah A.; McGrath, Patrick J. (1 January 2014). "How treatable is refractory depression?". Journal of Affective Disorders. 167: 148–152. doi:10.1016/j.jad.2014.05.047. ISSN 1573-2517. PMID 24972362.
- ^ Dell'Osso, Bernardo; Ketter, Terence A.; Cremaschi, Laura; Spagnolin, Gregorio; Altamura, A. Carlo (1 August 2013). "Assessing the roles of stimulants/stimulant-like drugs and dopamine-agonists in the treatment of bipolar depression". Current Psychiatry Reports. 15 (8): 378. doi:10.1007/s11920-013-0378-z. ISSN 1535-1645. PMID 23881710.
- ^ Corp, Stephanie A.; Gitlin, Michael J.; Altshuler, Lori L. (1 September 2014). "A review of the use of stimulants and stimulant alternatives in treating bipolar depression and major depressive disorder". The Journal of Clinical Psychiatry. 75 (9): 1010–1018. doi:10.4088/JCP.13r08851. ISSN 1555-2101. PMID 25295426.
- ^ Huang, Chang-Chih; Shiah, I.-Shin; Chen, Hsing-Kang; Mao, Wei-Chung; Yeh, Yi-Wei (1 August 2008). "Adjunctive use of methylphenidate in the treatment of psychotic unipolar depression". Clinical Neuropharmacology. 31 (4): 245–247. doi:10.1097/WNF.0b013e318157d998. ISSN 1537-162X. PMID 18670250.
- ^ Kolar, Dusan; Keller, Amanda; Golfinopoulos, Maria; Cumyn, Lucy; Syer, Cassidy; Hechtman, Lily (1 April 2008). "Treatment of adults with attention-deficit/hyperactivity disorder". Neuropsychiatric Disease and Treatment. 4 (2): 389–403. ISSN 1176-6328. PMC 2518387. PMID 18728745.
- ^ Sulzer D, Sonders MS, Poulsen NW, Galli A (April 2005). "Mechanisms of neurotransmitter release by amphetamines: a review". Progress in Neurobiology. 75 (6): 406–33. doi:10.1016/j.pneurobio.2005.04.003. PMID 15955613.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ National Toxicology, Program (July 2005). "NTP-CERHR monograph on the potential human reproductive and developmental effects of amphetamines". Ntp Cerhr Mon (16): vii–III1. PMID 16130031.
- ^ Howland RH (August 2008). "Lisdexamfetamine: a prodrug stimulant for ADHD". Journal of Psychosocial Nursing and Mental Health Services. 46 (8): 19–22. doi:10.3928/02793695-20080801-05. PMID 18777964.
- ^ Boyle, J.; Stanley, N.; James, L.M.; Wright, N.; Johnsen, S.; Arbon, E.L.; Dijk, D.J. (August 2012). "Acute sleep deprivation: the effects of the AMPAKINE compound CX717 on human cognitive performance, alertness and recovery sleep". Journal of psychopharmacology (Oxford, England). 26 (8): 1047–57. doi:10.1177/0269881111405353. PMID 21940760.
- ^ Saletan, W. (16 July 2008). "Night of the Living Meds – The U.S. military's sleep-reduction program". Slate Magazine. Retrieved 5 April 2012.
- ^ Ballon, D.D.; Feifel, D. (2006). "A systematic review of modafinil: potential clinical uses and mechanisms of action". Journal of Clinical Psychiatry. 67 (4): 554–66. doi:10.4088/JCP.v67n0406. PMID 16669720.
- ^ a b c d Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". J. Neurochem. 116 (2): 164–176. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ^ Eiden LE, Weihe E (January 2011). "VMAT2: a dynamic regulator of brain monoaminergic neuronal function interacting with drugs of abuse". Ann. N. Y. Acad. Sci. 1216: 86–98. doi:10.1111/j.1749-6632.2010.05906.x. PMID 21272013.
- ^ Orr K, Taylor D (2007). "Psychostimulants in the Treatment of Depression". CNS Drugs. 21 (3): 239–57. doi:10.2165/00023210-200721030-00004. PMID 17338594.
- ^ Dale, Elena; Bang-Andersen, Benny; Sánchez, Connie (2015). "Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs". Biochemical Pharmacology. 95 (2): 81–97. doi:10.1016/j.bcp.2015.03.011. ISSN 0006-2952.
- ^ Efforts of the National Institute on Drug Abuse to Prevent and Treat Prescription Drug Abuse, Testimony Before the Subcommittee on Criminal Justice, Drug Policy, and Human Resources Committee on Government Reform, United States House of Representatives, 26 July 2006
- ^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ a b c d "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. June 2013. p. 11. Retrieved 7 January 2014.
- ^ a b c d Heal DJ, Smith SL, Gosden J, Nutt DJ (June 2013). "Amphetamine, past and present – a pharmacological and clinical perspective". J. Psychopharmacol. 27 (6): 479–496. doi:10.1177/0269881113482532. PMC 3666194. PMID 23539642.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Amphetamine". DrugBank. University of Alberta. 7 January 2014. Retrieved 13 October 2013.
{{cite web}}:|section=ignored (help) - ^ "Amphetamine". National Library of Medicine – Medical Subject Headings. National Institutes of Health. Retrieved 7 January 2014.
- ^ a b Rasmussen N (July 2006). "Making the first anti-depressant: amphetamine in American medicine, 1929–1950". J . Hist. Med. Allied Sci. 61 (3): 288–323. doi:10.1093/jhmas/jrj039. PMID 16492800.
- ^ a b Liddle DG, Connor DJ (June 2013). "Nutritional supplements and ergogenic AIDS". Prim. Care. 40 (2): 487–505. doi:10.1016/j.pop.2013.02.009. PMID 23668655.
Amphetamines and caffeine are stimulants that increase alertness, improve focus, decrease reaction time, and delay fatigue, allowing for an increased intensity and duration of training...
Physiologic and performance effects
• Amphetamines increase dopamine/norepinephrine release and inhibit their reuptake, leading to central nervous system (CNS) stimulation
• Amphetamines seem to enhance athletic performance in anaerobic conditions 39 40
• Improved reaction time
• Increased muscle strength and delayed muscle fatigue
• Increased acceleration
• Increased alertness and attention to task - ^ a b c Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 13: Higher Cognitive Function and Behavioral Control". In Sydor A, Brown RY (ed.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 318. ISBN 978-0-07-148127-4.
Therapeutic (relatively low) doses of psychostimulants, such as methylphenidate and amphetamine, improve performance on working memory tasks both in individuals with ADHD and in normal subjects...it is now believed that dopamine and norepinephrine, but not serotonin, produce the beneficial effects of stimulants on working memory. At abused (relatively high) doses, stimulants can interfere with working memory and cognitive control, as will be discussed below. It is important to recognize, however, that stimulants act not only on working memory function, but also on general levels of arousal and, within the nucleus accumbens, improve the saliency of tasks. Thus, stimulants improve performance on effortful but tedious tasks...through indirect stimulation of dopamine and norepinephrine receptors.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b Montgomery KA (June 2008). "Sexual desire disorders". Psychiatry (Edgmont). 5 (6): 50–55. PMC 2695750. PMID 19727285.
- ^ Wilens TE, Adler LA, Adams J, Sgambati S, Rotrosen J, Sawtelle R, Utzinger L, Fusillo S (January 2008). "Misuse and diversion of stimulants prescribed for ADHD: a systematic review of the literature". J. Am. Acad. Child Adolesc. Psychiatry. 47 (1): 21–31. doi:10.1097/chi.0b013e31815a56f1. PMID 18174822.
Stimulant misuse appears to occur both for performance enhancement and their euphorogenic effects, the latter being related to the intrinsic properties of the stimulants (e.g., IR versus ER profile)...
Although useful in the treatment of ADHD, stimulants are controlled II substances with a history of preclinical and human studies showing potential abuse liability.{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Convention on psychotropic substances". United Nations Treaty Collection. United Nations. Retrieved 7 January 2014.
- ^ "Methamphetamine facts". DrugPolicy.org. Retrieved 7 January 2014.
- ^ a b Chawla S, Le Pichon T (2006). "World Drug Report 2006" (PDF). United Nations Office on Drugs and Crime. pp. 128–135. Retrieved 7 January 2014.
- ^ "Adderall IR Prescribing Information" (PDF). United States Food and Drug Administration. March 2007. p. 5. Retrieved 2 November 2013.
- ^ "Adderall XR Prescribing Information" (PDF). United States Food and Drug Administration. June 2013. pp. 4–8. Retrieved 7 October 2013.
- ^ a b c d e f Westfall DP, Westfall TC (2010). "Miscellaneous Sympathomimetic Agonists". In Brunton LL, Chabner BA, Knollmann BC (ed.). Goodman & Gilman's Pharmacological Basis of Therapeutics (12th ed.). New York: McGraw-Hill. ISBN 978-0-07-162442-8.
{{cite book}}: External link in|sectionurl=|sectionurl=ignored (|section-url=suggested) (help)CS1 maint: multiple names: editors list (link) - ^ Stolerman IP (2010). Stolerman IP (ed.). Encyclopedia of Psychopharmacology. Berlin; London: Springer. p. 78. ISBN 978-3-540-68698-9.
Although [substituted amphetamines] are also used as recreational drugs, with important neurotoxic consequences when abused, addiction is not a high risk when therapeutic doses are used as directed.
- ^ Shoptaw SJ, Kao U, Ling W (2009). "Treatment for amphetamine psychosis (Review)". Cochrane Database of Systematic Reviews (1): CD003026. doi:10.1002/14651858.CD003026.pub3. PMID 19160215.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Greydanus D. "Stimulant Misuse: Strategies to Manage a Growing Problem" (PDF). American College Health Association (Review Article). ACHA Professional Development Program. p. 20. Retrieved 2 November 2013.
- ^ a b "Amphetamine". European Monitoring Centre for Drugs and Drug Addiction. Retrieved 19 October 2013.
- ^ a b Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacol. Ther. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
Fig. 2. Synthetic and metabolic pathways for endogenous and exogenously administered trace amines and sympathomimetic amines...
Trace amines are metabolized in the mammalian body via monoamine oxidase (MAO; EC 1.4.3.4) (Berry, 2004) (Fig. 2)...It deaminates primary and secondary amines that are free in the neuronal cytoplasm but not those bound in storage vesicles of the sympathetic neurone...
Thus, MAO inhibitors potentiate the peripheral effects of indirectly acting sympathomimetic amines. It is not often realized, however, that this potentiation occurs irrespective of whether the amine is a substrate for MAO. An α-methyl group on the side chain, as in amphetamine and ephedrine, renders the amine immune to deamination so that they are not metabolized in the gut. Similarly, β-PEA would not be deaminated in the gut as it is a selective substrate for MAO-B which is not found in the gut...
Brain levels of endogenous trace amines are several hundred-fold below those for the classical neurotransmitters noradrenaline, dopamine and serotonin but their rates of synthesis are equivalent to those of noradrenaline and dopamine and they have a very rapid turnover rate (Berry, 2004). Endogenous extracellular tissue levels of trace amines measured in the brain are in the low nanomolar range. These low concentrations arise because of their very short half-life,... - ^ a b Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K (February 2013). "Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects". JAMA Psychiatry. 70 (2): 185–198. doi:10.1001/jamapsychiatry.2013.277. PMID 23247506.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Spencer TJ, Brown A, Seidman LJ, Valera EM, Makris N, Lomedico A, Faraone SV, Biederman J (September 2013). "Effect of psychostimulants on brain structure and function in ADHD: a qualitative literature review of magnetic resonance imaging-based neuroimaging studies". J. Clin. Psychiatry. 74 (9): 902–917. doi:10.4088/JCP.12r08287. PMC 3801446. PMID 24107764.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d Malenka RC, Nestler EJ, Hyman SE (2009). "15". In Sydor A, Brown RY (ed.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. p. 370. ISBN 9780071481274.
Unlike cocaine and amphetamine, methamphetamine is directly toxic to midbrain dopamine neurons.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e Krasnova IN, Cadet JL (May 2009). "Methamphetamine toxicity and messengers of death". Brain Res. Rev. 60 (2): 379–407. doi:10.1016/j.brainresrev.2009.03.002. PMC 2731235. PMID 19328213.
Neuroimaging studies have revealed that METH can indeed cause neurodegenerative changes in the brains of human addicts (Aron and Paulus, 2007; Chang et al., 2007). These abnormalities include persistent decreases in the levels of dopamine transporters (DAT) in the orbitofrontal cortex, dorsolateral prefrontal cortex, and the caudate-putamen (McCann et al., 1998, 2008; Sekine et al., 2003; Volkow et al., 2001a, 2001c). The density of serotonin transporters (5-HTT) is also decreased in the midbrain, caudate, putamen, hypothalamus, thalamus, the orbitofrontal, temporal, and cingulate cortices of METH-dependent individuals (Sekine et al., 2006) ...
Neuropsychological studies have detected deficits in attention, working memory, and decision-making in chronic METH addicts ...
There is compelling evidence that the negative neuropsychiatric consequences of METH abuse are due, at least in part, to drug-induced neuropathological changes in the brains of these METH-exposed individuals ...
Structural magnetic resonance imaging (MRI) studies in METH addicts have revealed substantial morphological changes in their brains. These include loss of gray matter in the cingulate, limbic, and paralimbic cortices, significant shrinkage of hippocampi, and hypertrophy of white matter (Thompson et al., 2004). In addition, the brains of METH abusers show evidence of hyperintensities in white matter (Bae et al., 2006; Ernst et al., 2000), decreases in the neuronal marker, N-acetylaspartate (Ernst et al., 2000; Sung et al., 2007), reductions in a marker of metabolic integrity, creatine (Sekine et al., 2002) and increases in a marker of glial activation, myoinositol (Chang et al., 2002; Ernst et al., 2000; Sung et al., 2007; Yen et al., 1994). Elevated choline levels, which are indicative of increased cellular membrane synthesis and turnover are also evident in the frontal gray matter of METH abusers (Ernst et al., 2000; Salo et al., 2007; Taylor et al., 2007). - ^ Lovett R (24 September 2005). "Coffee: The demon drink?". New Scientist (2518). Retrieved 3 August 2009. (subscription required)
- ^ Merck Manuals EPHEDrine Last full review/revision January 2010
- ^ Simmler, L. D.; Buser, T. A.; Donzelli, M.; Schramm, Y; Dieu, L-H.; Huwyler, J.; Chaboz, S.; Hoener, M. C.; Liechti, M. E. (2012). "Pharmacological characterization of designer cathinones in vitro". British Journal of Pharmacology. 168 (2): 458–470. doi:10.1111/j.1476-5381.2012.02145.x. ISSN 0007-1188.
- ^ US Patent 3478050 – 1-(3,4-Methylenedioxy Phenyl-2-pyrrolidino-Alkanones
- ^ "Abuse Of Fake 'Bath Salts' Sends Dozens To ER". KMBC.com. 23 December 2010.
{{cite journal}}: Cite journal requires|journal=(help) - ^ "MDPV Bath Salts Drug Over The Counter".
- ^ Samantha Morgan (9 November 2010). "Parents cautioned against over the counter synthetic speed". NBC 33 News. Retrieved 16 May 2011.
- ^ Kelsey Scram (6 January 2011). "Bath Salts Used to Get High". NBC 33 News. Retrieved 16 May 2011.
- ^ Cumming, E. (22 April 2010). "Mephedrone: Chemistry lessons". London: The Daily Telegraph. Archived from the original on 7 January 2014. Retrieved 14 September 2010.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Drugs crackdown hailed a success". BBC News. 8 March 2010. Archived from the original on 7 January 2014. Retrieved 31 March 2010.
{{cite news}}:|archive-date=/|archive-url=timestamp mismatch; 26 August 2012 suggested (help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d e f g "Desoxyn Prescribing Information" (PDF). United States Food and Drug Administration. December 2013. Retrieved 6 January 2014.
- ^ a b Kuczenski R, Segal DS, Cho AK, Melega W (February 1995). "Hippocampus norepinephrine, caudate dopamine and serotonin, and behavioral responses to the stereoisomers of amphetamine and methamphetamine". J. Neurosci. 15 (2): 1308–1317. PMID 7869099.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Mendelson J, Uemura N, Harris D, Nath RP, Fernandez E, Jacob P, Everhart ET, Jones RT (October 2006). "Human pharmacology of the methamphetamine stereoisomers". Clin. Pharmacol. Ther. 80 (4): 403–420. doi:10.1016/j.clpt.2006.06.013. PMID 17015058.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b San Francisco Meth Zombies (TV documentary). National Geographic Channel. August 2013. ASIN B00EHAOBAO.
{{cite AV media}}:|access-date=requires|url=(help) - ^ United Nations Office on Drugs and Crime (2007). Preventing Amphetamine-type Stimulant Use Among Young People: A Policy and Programming Guide (PDF). New York: United Nations. ISBN 9789211482232. Retrieved 11 November 2013.
- ^ "List of psychotropic substances under international control" (PDF). International Narcotics Control Board. United Nations. August 2003. Archived from the original (PDF) on 5 December 2005. Retrieved 19 November 2005.
- ^ "Vicks Vapoinhaler". Vicks. Retrieved 2 January 2014.
{{cite web}}:|section=ignored (help) - ^ "Levomethamphetamine". Pubchem Compound. National Center for Biotechnology Information. Retrieved 2 January 2014.
{{cite web}}:|section=ignored (help) - ^ Cruickshank CC, Dyer KR (July 2009). "A review of the clinical pharmacology of methamphetamine". Addiction. 104 (7): 1085–1099. doi:10.1111/j.1360-0443.2009.02564.x. PMID 19426289.
- ^ Flavahan NA (April 2005). "Phenylpropanolamine constricts mouse and human blood vessels by preferentially activating alpha2-adrenoceptors". Journal of Pharmacology and Experimentical Therapeutics. 313 (1): 432–9. doi:10.1124/jpet.104.076653. PMID 15608085.
- ^ "Advisories, Warnings and Recalls – 2001". Health Canada. 7 January 2009. Archived from the original on 3 May 2010. Retrieved 10 January 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Drugs Banned in India". Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India. Central Drugs Standard Control Organization. Retrieved 7 January 2014.
- ^ "Benzedrex Inhaler Nasal Decongestant Inhaler". B.F. Ascher & Co., Inc. Retrieved 19 December 2013.
- ^ "Proposed Rules". Federal Register. 50 (10): 2226–2227.
- ^ Prince v. Ascher, 90 P.3d 1020 (2004).
- ^ Fornazzari L, Carlen PL, Kapur BM (November 1986). "Intravenous abuse of propylhexedrine (Benzedrex) and the risk of brainstem dysfunction in young adults". Canadian Journal of Neurological Science. 13 (4): 337–9. PMID 2877725.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hunter Gillies, Wayne E. Derman, Timothy D. Noakes, Peter Smith, Alicia Evans, and Gary Gabriels (1 December 1996). "Pseudoephedrine is without ergogenic effects during prolonged exercise". Journal of Applied Physiology. 81 (6): 2611–2617. PMID 9018513.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hodges, K; Hancock S; Currel K; Hamilton B; Jeukendrup AE (February 2006). "Pseudoephedrine enhances performance in 1500-m runners". US National Library of Medicine National Institutes of Health. 38 (2): 329–33. doi:10.1249/01.mss.0000183201.79330.9c. PMID 16531903.
- ^ Dickens, Charles (1856) [Digitized 19 February 2010]. "The Orsons of East Africa". Household Words: A Weekly Journal, Volume 14. Bradbury & Evans. p. 176. Retrieved 7 January 2014.
 (Free eBook)
(Free eBook)
- ^ a b Al-Mugahed, Leen (October 2008). "Khat chewing in Yemen: turning over a new leaf – Khat chewing is on the rise in Yemen, raising concerns about the health and social consequences". World Health Organization. Retrieved 8 January 2014.
- ^ Nutt D, King LA, Blakemore C (March 2007). "Development of a rational scale to assess the harm of drugs of potential misuse". Lancet. 369 (9566): 1047–53. doi:10.1016/S0140-6736(07)60464-4. PMID 17382831.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Haight-Ashbury Free Medical Clinic, Journal of psychoactive drugs, Volume 41, (Haight-Ashbury Publications: 2009), p.3.
- ^ AJ Giannini, WC Price (1986). "Contemporary drugs of abuse". American Family Physician. 33: 207–213.
- ^ Dackis CA, Gold MS (1990). "Addictiveness of central stimulants". Advances in Alcohol & Substance Abuse. 9 (1–2): 9–26. doi:10.1300/J251v09n01_02. PMID 1974121.
- ^ AJ Giannini. Drug Abuse. Los Angeles, Health Information Press, 1999, pp.203–208
External links
- Archived 2008-06-05 at the Wayback Machine
- Archived 2006-09-22 at the Wayback Machine
- Asia & Pacific Amphetamine-Type Stimulants Information Centre (APAIC)

