Cannabinol: Difference between revisions
Hello - I have spent weeks working to expand on this page as part of a pharmacology course in my PhD training. I will publish my changes in multiple rounds to allow for easier review by Wikipedia editors. Round #1 includes a rewritten formation section (renamed formation and metabolism), a rewritten chemistry section (renamed chemical structure), and a rewritten introduction/summary at the top. I also added a diagram explaining CBN's unique biosynthesis. All original content is still present. Tags: nowiki added Visual edit |
Round #2 - replaced pharmacology section (including addition of 2 subsections, 1 timeline of CBN research progress and relevant legislation, and 1 diagram demonstrating the canonical mechanism of CB1 receptor signaling in the brain) |
||
| Line 77: | Line 77: | ||
Due to high lipophilicity and first-pass metabolism, there is low [[bioavailability]] of CBN and other cannabinoids following [[oral administration]]. CBN metabolism is mediated in part by [[Cytochrome P450|CYP450]] [[Protein isoform|isoforms]] 2C9 and 3A4. The metabolism of CBN may be [[Catalysis|catalyzed]] by UGTs ([[Glucuronosyltransferase|UDP-Glucuronosyltransferases]]), with a subset of UGT isoforms (1A7, 1A8, 1A9, 1A10, 2B7) identified as potential substrates associated with CBN [[glucuronidation]]. The [[bioavailability]] of CBN following administration via [[inhalation]] (e.g., smoking or vaporizing) is approximately 40% that of [[Intravenous therapy|intravenous administration]]. |
Due to high lipophilicity and first-pass metabolism, there is low [[bioavailability]] of CBN and other cannabinoids following [[oral administration]]. CBN metabolism is mediated in part by [[Cytochrome P450|CYP450]] [[Protein isoform|isoforms]] 2C9 and 3A4. The metabolism of CBN may be [[Catalysis|catalyzed]] by UGTs ([[Glucuronosyltransferase|UDP-Glucuronosyltransferases]]), with a subset of UGT isoforms (1A7, 1A8, 1A9, 1A10, 2B7) identified as potential substrates associated with CBN [[glucuronidation]]. The [[bioavailability]] of CBN following administration via [[inhalation]] (e.g., smoking or vaporizing) is approximately 40% that of [[Intravenous therapy|intravenous administration]]. |
||
== Pharmacology == |
== Pharmacology == |
||
[[File:Brief_History_of_CBN_(Emphasis_on_US_Legislation).png|thumb|620x620px|This timeline represents a simplified history of CBN with an emphasis on the complexity surrounding cannabis legislation in the US.]] |
|||
CBN acts as a partial [[agonist]] at the [[CB1 receptor|CB<sub>1</sub> receptors]], but has a higher affinity to [[CB2 receptor|CB<sub>2</sub>]] [[Receptor (biochemistry)|receptors]]; however, it has lower [[Affinity (pharmacology)|affinities]] relative to THC.<ref name="pmid11020293">{{cite journal | vauthors = Mahadevan A, Siegel C, Martin BR, Abood ME, Beletskaya I, Razdan RK | title = Novel cannabinol probes for CB1 and CB2 cannabinoid receptors | journal = Journal of Medicinal Chemistry | volume = 43 | issue = 20 | pages = 3778–3785 | date = October 2000 | pmid = 11020293 | doi = 10.1021/jm0001572 }}</ref><ref name="pmid9667767">{{cite journal | vauthors = Petitet F, Jeantaud B, Reibaud M, Imperato A, Dubroeucq MC | title = Complex pharmacology of natural cannabinoids: evidence for partial agonist activity of delta9-tetrahydrocannabinol and antagonist activity of cannabidiol on rat brain cannabinoid receptors | journal = Life Sciences | volume = 63 | issue = 1 | pages = PL1–PL6 | year = 1998 | pmid = 9667767 | doi = 10.1016/S0024-3205(98)00238-0 }}</ref><ref name="NCI_C84510">{{Cite web |title=Cannabinol (Code C84510) |url=https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=ncit&code=C84510 |work=NCI Thesaurus |publisher=National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services}}</ref> Both THC and CBN activate the CB<sub>1</sub> (K<sub>i</sub> = 211.2 nM) and CB<sub>2</sub> (K<sub>i</sub> = 126.4 nM) receptors.<ref name="pmid28826544">{{cite book | vauthors = Russo EB, Marcu J | title = Cannabinoid Pharmacology | chapter = Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads | series = Advances in Pharmacology | volume = 80 | pages = 67–134 | date = 2017 | pmid = 28826544 | doi = 10.1016/bs.apha.2017.03.004 | isbn = 978-0-12-811232-8 }}</ref> It is metabolised to [[11-OH-CBN]], which is a more potent CB<sub>1</sub> agonist than CBN but acts as a weak antagonist at CB<sub>2</sub>.<ref name="Rhee_1997">{{cite journal | vauthors = Rhee MH, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L, Breuer A, Mechoulam R | display-authors = 6 | title = Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase | journal = Journal of Medicinal Chemistry | volume = 40 | issue = 20 | pages = 3228–3233 | date = September 1997 | pmid = 9379442 | doi = 10.1021/jm970126f }}</ref> |
|||
CBN was the first cannabis compound to be isolated from [[cannabis]] extract in the late 1800s. Its structure and chemical synthesis were achieved by 1940, followed by some of the first preclinical research studies to determine the effects of individual cannabis-derived compounds in vivo.<ref name=":4">{{Cite journal |last=Pertwee |first=Roger G |date=2006 |title=Cannabinoid pharmacology: the first 66 years: Cannabinoid pharmacology |url=http://doi.wiley.com/10.1038/sj.bjp.0706406 |journal=British Journal of Pharmacology |language=en |volume=147 |issue=S1 |pages=S163–S171 |doi=10.1038/sj.bjp.0706406 |pmc=PMC1760722 |pmid=16402100}}</ref> |
|||
Both THC and CBN activate the CB1 (Ki = 211.2 nM) and CB2 (Ki = 126.4 nM) receptors.<ref name="Rhee_19972">{{cite journal |display-authors=6 |vauthors=Rhee MH, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L, Breuer A, Mechoulam R |date=September 1997 |title=Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase |journal=Journal of Medicinal Chemistry |volume=40 |issue=20 |pages=3228–3233 |doi=10.1021/jm970126f |pmid=9379442}}</ref> Each compound acts as a low affinity partial [[agonist]] at [[CB1 receptor|CB1 receptors]] with THC demonstrating 10-13x greater affinity to the CB1 receptor.<ref name="Rhee_19972" /><ref name=":6">{{Cite book |url=https://www.worldcat.org/oclc/65169431 |title=Cannabinoids |date=2005 |publisher=Springer |others=Mary Ellen Abood, R. G. Pertwee |isbn=3-540-22565-X |location=Berlin |oclc=65169431}}</ref><ref name=":5">{{Cite journal |last=Corroon |first=Jamie |date=2021-08-31 |title=Cannabinol and Sleep: Separating Fact from Fiction |url=https://www.liebertpub.com/doi/10.1089/can.2021.0006 |journal=Cannabis and Cannabinoid Research |language=en |pages=can.2021.0006 |doi=10.1089/can.2021.0006 |issn=2578-5125 |pmc=PMC8612407 |pmid=34468204}}</ref><ref name=":4" /><ref name=":2">{{cite journal |vauthors=Andre CM, Hausman JF, Guerriero G |date=2016-02-04 |title=Cannabis sativa: The Plant of the Thousand and One Molecules |journal=Frontiers in Plant Science |volume=7 |pages=19 |doi=10.3389/fpls.2016.00019 |pmc=4740396 |pmid=26870049 |doi-access=free}}</ref><ref name=":8">{{Cite journal |last=Aizpurua-Olaizola |first=Oier |last2=Elezgarai |first2=Izaskun |last3=Rico-Barrio |first3=Irantzu |last4=Zarandona |first4=Iratxe |last5=Etxebarria |first5=Nestor |last6=Usobiaga |first6=Aresatz |date=2017 |title=Targeting the endocannabinoid system: future therapeutic strategies |url=https://linkinghub.elsevier.com/retrieve/pii/S1359644616302926 |journal=Drug Discovery Today |language=en |volume=22 |issue=1 |pages=105–110 |doi=10.1016/j.drudis.2016.08.005}}</ref> Compared to THC, CBN has an equivalent or higher affinity to [[CB2 receptor|CB2]] [[Receptor (biochemistry)|receptors]]<ref name="Rhee_19972" /><ref name=":4" />, which are located throughout the central and [[peripheral nervous system]], but are primarily associated with [[Immune system|immune function]]. CB2 receptors are known to be located on immune cells throughout the body, including [[Macrophage|macrophages]], [[T cell|T cells]], and [[B cell|B cells]]. These immune cells have been shown to decrease production of immune-related chemical signals (e.g., [[Cytokine|cytokines]]) or undergo [[apoptosis]] as a consequence of CB2 agonism by CBN.<ref name="NCI_C845102">{{Cite web |title=Cannabinol (Code C84510) |url=https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=ncit&code=C84510 |work=NCI Thesaurus |publisher=National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services}}</ref> In cell culture, CBN demonstrates antimicrobial effects, particularly in instances of antibiotic-resistant bacteria.<ref>{{Cite journal |last=Pattnaik |first=Falguni |last2=Nanda |first2=Sonil |last3=Mohanty |first3=Shobhangam |last4=Dalai |first4=Ajay K. |last5=Kumar |first5=Vivek |last6=Ponnusamy |first6=Senthil Kumar |last7=Naik |first7=Satyanarayan |date=2022 |title=Cannabis: Chemistry, extraction and therapeutic applications |url=https://linkinghub.elsevier.com/retrieve/pii/S0045653521034846 |journal=Chemosphere |language=en |volume=289 |pages=133012 |doi=10.1016/j.chemosphere.2021.133012}}</ref> CBN has also been reported to act as an [[TRPA1|ANKTM1]] channel agonist at high concentrations (>20nM).<ref name=":6" /> While some [[Cannabinoid|phytocannabinoids]] have been shown to interact with [[Nociception|nociceptive]] and immune-related signaling via [[Transient receptor potential channel|transient receptor potential channels]] (e.g., TRPV1 and TRPM8), there is currently limited evidence to suggest that CBN acts in this way.<ref name=":6" /><ref name=":12">{{Cite journal |last=Muller |first=Chanté |last2=Morales |first2=Paula |last3=Reggio |first3=Patricia H. |date=2019-01-15 |title=Cannabinoid Ligands Targeting TRP Channels |url=https://www.frontiersin.org/article/10.3389/fnmol.2018.00487/full |journal=Frontiers in Molecular Neuroscience |volume=11 |pages=487 |doi=10.3389/fnmol.2018.00487 |issn=1662-5099 |pmc=PMC6340993 |pmid=30697147}}</ref> In preclinical rodent studies, CBN, [[anandamide]] and other CB1 agonists have demonstrated inhibitory effects on GI motility, reversible via CB1R blockade (i.e., antagonism).<ref name=":6" /> |
|||
In considering the efficacy of cannabis-based products, there remains controversy surrounding a concept termed “the entourage effect”. This concept describes a widely-observed but poorly-understood synergistic effect of cannabinoid activity when phytocannabinoids are coadministered with other naturally-occurring chemical compounds in the cannabis plant (e.g., flavonoids, terpenoids, alkaloids). This entourage effect is often cited to explain the superior efficacy observed in some studies of whole-plant-derived cannabis therapeutics as compared to isolated or synthesized individual cannabis constituents<ref name=":14">{{Cite journal |last=Legare |first=Christopher A. |last2=Raup-Konsavage |first2=Wesley M. |last3=Vrana |first3=Kent E. |date=2022 |title=Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals |url=https://www.karger.com/Article/FullText/521683 |journal=Pharmacology |language=en |volume=107 |issue=3-4 |pages=131–149 |doi=10.1159/000521683 |issn=0031-7012}}</ref>. |
|||
=== Common Cannabinoids - Putative Receptor Targets & Therapeutic Properties === |
|||
The below table highlights several common cannabinoids along with putative receptor targets and therapeutic properties. Exogenous (plant-derived) phytocannabinoids are identified with an asterisk while remaining chemicals represent well-known endocannabinoids (i.e., endogenously-produced cannabinoid receptor ligands). |
|||
{| class="wikitable" |
|||
|'''Full Name''' |
|||
|'''Known Receptor Targets''' |
|||
|'''Putative Therapeutic Properties''' |
|||
|- |
|||
|*Cannabichromene (CBC) |
|||
|· Agonist at CB2<ref name=":0">{{Cite journal |last=Sampson |first=Peter B. |date=2021-01-22 |title=Phytocannabinoid Pharmacology: Medicinal Properties of Cannabis sativa Constituents Aside from the "Big Two" |url=https://pubmed.ncbi.nlm.nih.gov/33356248 |journal=Journal of Natural Products |volume=84 |issue=1 |pages=142–160 |doi=10.1021/acs.jnatprod.0c00965 |issn=1520-6025 |pmid=33356248}}</ref>, TRPV3, and most potent phytocannabinoid at TRPA1<ref name=":0" /><ref name=":12" /> |
|||
· Very low efficacy at TRPV1 and TRPV4, but may reduce expression of TRPV4 in the presence of inflammation<ref name=":12" /> |
|||
· High affinity for CB1 but no observed functional activity<ref name=":0" /> |
|||
· Antagonist at TRPM8<ref name=":12" /> |
|||
|· Antimicrobial and anti-inflammatory<ref name=":0" /> |
|||
· Potential neuroprotective effects<ref name=":0" /> |
|||
· Potential efficacy in treatment of inflammatory pain<ref name=":0" /> |
|||
|- |
|||
|*Cannabidiol (CBD) |
|||
|· Very weak affinity for CB1 and CB2<ref name=":13">{{Cite journal |last=Cherkasova |first=Viktoriia |last2=Wang |first2=Bo |last3=Gerasymchuk |first3=Marta |last4=Fiselier |first4=Anna |last5=Kovalchuk |first5=Olga |last6=Kovalchuk |first6=Igor |date=2022-10-20 |title=Use of Cannabis and Cannabinoids for Treatment of Cancer |url=https://www.mdpi.com/2072-6694/14/20/5142 |journal=Cancers |language=en |volume=14 |issue=20 |pages=5142 |doi=10.3390/cancers14205142 |issn=2072-6694 |pmc=PMC9600568 |pmid=36291926}}</ref> |
|||
· Conflicting reports but generally described as negative allosteric modulator at CB1 & CB2, altering THC activity when THC & CBD are coadministered<ref name=":13" /> |
|||
· Agonist at TRPA1<ref name=":12" />, TRPV1 (high potency at this “capsaicin receptor” without ablative effects<ref name=":12" />), TRPV2, TRPV3, PPARγ, 5-HT1A, A2 and A1 adenosine receptors<ref name=":13" /> |
|||
· Highest potency at TRPV1<ref name=":12" /> |
|||
· Antagonist at GPR55, GPR18, 5-HT3A<ref name=":13" />, with highest potency as antagonist at TRPM8<ref name=":12" /> |
|||
· Inverse agonist at GPR3, GPR6, and GPR12<ref name=":13" /> |
|||
|· Anti-inflammatory<ref name=":10">{{cite journal |vauthors=Mead A |date=2019-06-14 |title=Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States |journal=Frontiers in Plant Science |volume=10 |pages=697 |doi=10.3389/fpls.2019.00697 |pmc=6590107 |pmid=31263468 |doi-access=free}}</ref><ref name=":12" /> |
|||
· Anti-convulsant<ref name=":10" /> |
|||
· Potential efficacy in treatment of inflammatory and chronic pain<ref name=":12" /> |
|||
|- |
|||
|*Cannabigerol (CBG) |
|||
|· Low affinity agonist and partial agonist at CB1 and CB2, respectively<ref name=":0" /> |
|||
· Agonist at α2adrenoceptor<ref name=":0" /> and TRP channels such as TRPA1, TRPV2, and TRPV3, with highest potency as agonist at TRPV1<ref name=":12" /> |
|||
· Readily desensitizes but low affinity for TRPV4<ref name=":12" /> |
|||
· Antagonist at 5-HT1A<ref name=":0" /> and TRPM8<ref name=":12" /> |
|||
|· Anti-microbial, anti-inflammatory, and anti-nociceptive effects<ref name=":0" /> |
|||
· Neuroprotective properties via mitigation of oxidative stress<ref name=":0" /> |
|||
· Potential anti-tumor agent<ref name=":0" /> |
|||
· Potential efficacy in treatment of chemotherapy-induced muscle atrophy and weight loss<ref name=":0" /> |
|||
|- |
|||
|*Cannabinol (CBN) |
|||
|· Agonist at CB1 and CB2, with some evidence of slightly higher affinity at CB2<ref name=":0" /> |
|||
· Low affinity agonist at TRPV1, TRPV2, TRPV3, TRPV4, and TRPA1<ref name=":12" />, but readily desensitizes TRPV4<ref name=":12" /> |
|||
· Antagonist at TRPM8<ref name=":12" /> |
|||
|· Antimicrobial and anti-inflammatory / immunosuppressive effects<ref name=":0" /> |
|||
· Potential efficacy in treatment of ocular disease and epidermolysis bullosa<ref name=":0" /> |
|||
· Reported neuroprotective effects (synergistic if coadministered with other cannabinoids)<ref name=":0" /> |
|||
· Relevance to pain, itch, and inflammation via TRP channel activity<ref name=":0" /> |
|||
|- |
|||
|*Tetrahydrocannabinol (THC) |
|||
or |
|||
Delta-9-Tetrahydrocannabinol (D9THC) |
|||
|· Agonist at CB1 and CB2, as well as GPR55, GPR18, PPARγ, and TRPA1<ref name=":12" /><ref name=":13" /> |
|||
· Antagonist at TRPM8<ref name=":12" /><ref name=":13" /> and 5-HT3A<ref name=":13" /> |
|||
· Differing activity across TRP channels: highest potency phytocannabinoid at TRPV2; modest activity at TRPV3, TRPV4, TRPA1, and TRPM8; no activity observed at TRPV1<ref name=":12" /> |
|||
· Importantly, 11-OH-THC, the active metabolite generated via first-pass-metabolism of THC, demonstrates different binding profile at TRP channels<ref name=":12" /> |
|||
|· Potential relevance to sleep induction (e.g., increased adenosine levels<ref name=":13" />) and increased quality of sleep<ref name=":12" /> |
|||
· Dose-dependent anxiolytic effects<ref name=":12" />, with anxiogenic effects at high doses |
|||
· Appetite stimulation<ref name=":12" /><ref name=":14" /> |
|||
· Anti-nausea<ref name=":12" /><ref name=":14" /> |
|||
· In combination with CBD, potential efficacy in treatment of spasticity, neuropathic pain and muscle spasticity (see Sativex: THC-containing therapeutic approved in Europe as treatment for Multiple Sclerosis) |
|||
|- |
|||
|*2-Arachidonoylglycerol (2-AG) |
|||
|· Partial agonist at CB1 (e.g., on lysosomal surface, increasing lysosomal integrity) and CB2<ref name=":13" /> |
|||
· Agonist at GPR55, GPR18, GPR119, PPAR, and robust activation at TRPV4<ref name=":12" /><ref name=":13" /> |
|||
|· Anti-oxidative properties<ref name=":13" /> |
|||
· Increased lysosomal stability & integrity<ref name=":13" /> |
|||
· Attenuation of mitochondrial damage during cell stress<ref name=":13" /> |
|||
|- |
|||
|Anandamide (AEA) |
|||
|· Agonist at GPR18, GPR119, and PPAR, with robust activation at TRPV4, and very high efficacy at TRPA1<ref name=":12" /><ref name=":13" /> |
|||
· Potent partial agonist at GPR55<ref name=":13" /><ref name=":14" /> |
|||
· Low-affinity full agonist at TRPV1<ref name=":12" /><ref name=":14" />, with similar but less potent affinity as compared to capsaicin<ref name=":12" /> |
|||
· Antagonist at TRPM8<ref name=":12" /> |
|||
|· Anti-oxidative properties<ref name=":13" /> |
|||
|} |
|||
=== Neurotransmitter Interactions === |
|||
[[File:DSI_DSE_Diagram_-_Mechanism_of_Action_of_eCB_ligands_at_CB1R_in_the_brain.jpg|thumb|650x650px|In the brain, the canonical mechanism of CB1 receptor activation is a form of short-term [[synaptic plasticity]] initiated via [[retrograde signaling]] of [[endogenous]] CB1 agonists such as [[2-Arachidonoylglycerol|2AG]] or [[Anandamide|AEA]] (two primary endocannabinoids).]] |
|||
In the brain, the canonical mechanism of CB1 receptor activation is a form of short-term [[synaptic plasticity]] initiated via [[retrograde signaling]] of [[endogenous]] CB1 agonists such as [[2-Arachidonoylglycerol|2AG]] or [[Anandamide|AEA]] (two primary endocannabinoids). This mechanism of action is called depolarization-induced suppression of inhibition (DSI) or depolarization-induced suppression of excitation (DSE)<ref name=":9">{{Cite journal |last=Diana |first=Marco A |last2=Marty |first2=Alain |date=2004 |title=Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE): DSI/DSE: two forms of CB1R-mediated plasticity |url=http://doi.wiley.com/10.1038/sj.bjp.0705726 |journal=British Journal of Pharmacology |language=en |volume=142 |issue=1 |pages=9–19 |doi=10.1038/sj.bjp.0705726 |pmc=PMC1574919 |pmid=15100161}}</ref>, depending on the classification of the [[Chemical synapse|presynaptic neuron]] acted upon by the retrograde messenger. In the case of CB1R agonism on the presynaptic membrane of a [[Γ-Aminobutyric acid|GABAergic interneuron]], activation leads to a net effect of increased activity, while the same activity on a [[Glutamate (neurotransmitter)|glutamatergic neuron]] leads to the opposite net effect. The release of other neurotransmitters is also modulated in this way, particularly [[dopamine]], [[dynorphin]], [[oxytocin]], and [[vasopressin]].<ref name=":9" /> |
|||
==Legal status== |
==Legal status== |
||
Revision as of 22:03, 7 December 2022
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral, inhaled |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.216.772 |
| Chemical and physical data | |
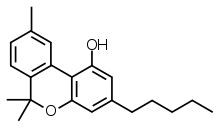
| Formula | C21H26O2 |
| Molar mass | 310.437 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 77 °C (171 °F) [1] |
| Solubility in water | Insoluble in water,[2] soluble in methanol[3] and ethanol[4] mg/mL (20 °C) |
| |
| |
| | |
Cannabinol (CBN) is a mildly psychoactive cannabinoid that acts as a low affinity partial agonist at both CB1 and CB2 receptors. This activity at CB1 and CB2 receptors constitutes interaction of CBN with the endocannabinoid system (ECS), which is responsible for regulating many important functions in the body. Through its mechanism of partial agonism at the CB1R, CBN is thought to interact with other kinds of neurotransmission (e.g., dopaminergic, serotonergic, cholinergic, and noradrenergic).
CBN was the first cannabis compound to be isolated from cannabis extract in the late 1800s. Its structure and chemical synthesis were achieved by 1940, followed by some of the first pre-clinical research studies to determine the effects of individual cannabis-derived compounds in vivo. Although CBN shares the same mechanism of action as other more well-known phytocannabinoids (e.g., delta-9 tetrahydrocannabinol or D9THC), it has a lower affinity for CB1 receptors, meaning that much higher doses of CBN are required in order to experience physiologic effects (e.g., mild sedation) associated with CB1R agonism. Although scientific reports are conflicting, the majority of findings suggest that CBN has a slightly higher affinity for CB2 as compared to CB1. Although CBN has been marketed as a sleep aid in recent years, there is a lack of scientific evidence to support these claims, warranting skepticism on the part of consumers.
Chemical Structure
Cannabinoid receptor agonists are categorized into four groups based on chemical structure. CBN, as one of the many phytocannabinoids derived from Cannabis Sativa L, is considered a classical cannabinoid. Other examples of compounds in this group include dibenzopyran derivatives such as D9THC, well-known for underlying the subjective “high” experienced by cannabis users, as well as D8THC, and their synthetic analogs. In contrast, endogenously produced cannabinoids (i.e., endocannabinoids), which also exert effects through CB agonism, are considered eicosanoids, distinguished by notable differences in chemical structure.
Compared to D9THC, one additional aromatic ring confers CBN with a slower and more limited metabolic profile - see CBN Formation & Metabolism, below. In contrast to THC, CBN has no double bond isomers nor stereoisomers. CBN can degrade into HU-345 from oxidation. In the case of oral administration of CBN, first-pass metabolism in the liver involves the addition of a hydroxyl group at C9 or C11, increasing the affinity and specificity of CBN for both CB1 and CB2 receptors (see 11-OH-CBN).
Formation & Metabolism
This diagram represents the biosynthetic and metabolic pathways by which phytocannabinoids (e.g., CBD, THC, CBN) are created in the cannabis plant. Starting with CBG-A, the acidic forms of certain phytocannabinoids are generated via enzymatic conversion. From there, decarboxylation (i.e., catalyzed by combustion or heat) yields the most well-known metabolites present in the cannabis plant. CBN is unique in that it does not arise from a pre-existing acidic form, but rather is generated through the oxidation of THC.
CBN is unique among phytocannabinoids in that its biosynthetic pathway involves conversion directly from D9THC, rather than from an acidic precursor form of CBN (e.g., D9THC arises through decarboxylation of THC-A). CBN can be found in trace amounts in the Cannabis plant, found mostly in cannabis that is aged and stored, allowing for CBN formation through the oxidation of the cannabis plant's main psychoactive and intoxicating chemical, tetrahydrocannabinol (THC). This process of oxidation occurs via exposure to heat, oxygen, and/or light. Although reports are limited, CBN-A has also been measured at very low levels in the cannabis plant, thought to have formed via hydrolyzation of THC-A (see Phytocannabinoid Biosynthesis diagram, below).

When administered orally, CBN demonstrates a similar metabolism to D9THC, with the primary active metabolite produced through the hydrolyzation of C9 as part of first-pass metabolism in the liver. The active metabolite generated via this process is called 11-OH-CBN, which is 2x as potent as CBN, and has demonstrated activity as a weak CB2 antagonist. This metabolism starkly contrasts that of D9THC in terms of potency, given that 11-OH-THC has been reported to have 10x the potency of D9THC.
Due to high lipophilicity and first-pass metabolism, there is low bioavailability of CBN and other cannabinoids following oral administration. CBN metabolism is mediated in part by CYP450 isoforms 2C9 and 3A4. The metabolism of CBN may be catalyzed by UGTs (UDP-Glucuronosyltransferases), with a subset of UGT isoforms (1A7, 1A8, 1A9, 1A10, 2B7) identified as potential substrates associated with CBN glucuronidation. The bioavailability of CBN following administration via inhalation (e.g., smoking or vaporizing) is approximately 40% that of intravenous administration.
Pharmacology

CBN was the first cannabis compound to be isolated from cannabis extract in the late 1800s. Its structure and chemical synthesis were achieved by 1940, followed by some of the first preclinical research studies to determine the effects of individual cannabis-derived compounds in vivo.[5]
Both THC and CBN activate the CB1 (Ki = 211.2 nM) and CB2 (Ki = 126.4 nM) receptors.[6] Each compound acts as a low affinity partial agonist at CB1 receptors with THC demonstrating 10-13x greater affinity to the CB1 receptor.[6][7][8][5][9][10] Compared to THC, CBN has an equivalent or higher affinity to CB2 receptors[6][5], which are located throughout the central and peripheral nervous system, but are primarily associated with immune function. CB2 receptors are known to be located on immune cells throughout the body, including macrophages, T cells, and B cells. These immune cells have been shown to decrease production of immune-related chemical signals (e.g., cytokines) or undergo apoptosis as a consequence of CB2 agonism by CBN.[11] In cell culture, CBN demonstrates antimicrobial effects, particularly in instances of antibiotic-resistant bacteria.[12] CBN has also been reported to act as an ANKTM1 channel agonist at high concentrations (>20nM).[7] While some phytocannabinoids have been shown to interact with nociceptive and immune-related signaling via transient receptor potential channels (e.g., TRPV1 and TRPM8), there is currently limited evidence to suggest that CBN acts in this way.[7][13] In preclinical rodent studies, CBN, anandamide and other CB1 agonists have demonstrated inhibitory effects on GI motility, reversible via CB1R blockade (i.e., antagonism).[7]
In considering the efficacy of cannabis-based products, there remains controversy surrounding a concept termed “the entourage effect”. This concept describes a widely-observed but poorly-understood synergistic effect of cannabinoid activity when phytocannabinoids are coadministered with other naturally-occurring chemical compounds in the cannabis plant (e.g., flavonoids, terpenoids, alkaloids). This entourage effect is often cited to explain the superior efficacy observed in some studies of whole-plant-derived cannabis therapeutics as compared to isolated or synthesized individual cannabis constituents[14].
Common Cannabinoids - Putative Receptor Targets & Therapeutic Properties
The below table highlights several common cannabinoids along with putative receptor targets and therapeutic properties. Exogenous (plant-derived) phytocannabinoids are identified with an asterisk while remaining chemicals represent well-known endocannabinoids (i.e., endogenously-produced cannabinoid receptor ligands).
| Full Name | Known Receptor Targets | Putative Therapeutic Properties |
| *Cannabichromene (CBC) | · Agonist at CB2[15], TRPV3, and most potent phytocannabinoid at TRPA1[15][13]
· Very low efficacy at TRPV1 and TRPV4, but may reduce expression of TRPV4 in the presence of inflammation[13] · High affinity for CB1 but no observed functional activity[15] · Antagonist at TRPM8[13] |
· Antimicrobial and anti-inflammatory[15]
· Potential neuroprotective effects[15] · Potential efficacy in treatment of inflammatory pain[15] |
| *Cannabidiol (CBD) | · Very weak affinity for CB1 and CB2[16]
· Conflicting reports but generally described as negative allosteric modulator at CB1 & CB2, altering THC activity when THC & CBD are coadministered[16] · Agonist at TRPA1[13], TRPV1 (high potency at this “capsaicin receptor” without ablative effects[13]), TRPV2, TRPV3, PPARγ, 5-HT1A, A2 and A1 adenosine receptors[16] · Highest potency at TRPV1[13] · Antagonist at GPR55, GPR18, 5-HT3A[16], with highest potency as antagonist at TRPM8[13] · Inverse agonist at GPR3, GPR6, and GPR12[16] |
· Anti-inflammatory[17][13]
· Anti-convulsant[17] · Potential efficacy in treatment of inflammatory and chronic pain[13] |
| *Cannabigerol (CBG) | · Low affinity agonist and partial agonist at CB1 and CB2, respectively[15]
· Agonist at α2adrenoceptor[15] and TRP channels such as TRPA1, TRPV2, and TRPV3, with highest potency as agonist at TRPV1[13] · Readily desensitizes but low affinity for TRPV4[13] |
· Anti-microbial, anti-inflammatory, and anti-nociceptive effects[15]
· Neuroprotective properties via mitigation of oxidative stress[15] · Potential anti-tumor agent[15] · Potential efficacy in treatment of chemotherapy-induced muscle atrophy and weight loss[15] |
| *Cannabinol (CBN) | · Agonist at CB1 and CB2, with some evidence of slightly higher affinity at CB2[15]
· Low affinity agonist at TRPV1, TRPV2, TRPV3, TRPV4, and TRPA1[13], but readily desensitizes TRPV4[13] · Antagonist at TRPM8[13] |
· Antimicrobial and anti-inflammatory / immunosuppressive effects[15]
· Potential efficacy in treatment of ocular disease and epidermolysis bullosa[15] · Reported neuroprotective effects (synergistic if coadministered with other cannabinoids)[15] · Relevance to pain, itch, and inflammation via TRP channel activity[15] |
| *Tetrahydrocannabinol (THC)
or Delta-9-Tetrahydrocannabinol (D9THC) |
· Agonist at CB1 and CB2, as well as GPR55, GPR18, PPARγ, and TRPA1[13][16]
· Antagonist at TRPM8[13][16] and 5-HT3A[16] · Differing activity across TRP channels: highest potency phytocannabinoid at TRPV2; modest activity at TRPV3, TRPV4, TRPA1, and TRPM8; no activity observed at TRPV1[13] · Importantly, 11-OH-THC, the active metabolite generated via first-pass-metabolism of THC, demonstrates different binding profile at TRP channels[13] |
· Potential relevance to sleep induction (e.g., increased adenosine levels[16]) and increased quality of sleep[13]
· Dose-dependent anxiolytic effects[13], with anxiogenic effects at high doses · Appetite stimulation[13][14] · In combination with CBD, potential efficacy in treatment of spasticity, neuropathic pain and muscle spasticity (see Sativex: THC-containing therapeutic approved in Europe as treatment for Multiple Sclerosis) |
| *2-Arachidonoylglycerol (2-AG) | · Partial agonist at CB1 (e.g., on lysosomal surface, increasing lysosomal integrity) and CB2[16]
· Agonist at GPR55, GPR18, GPR119, PPAR, and robust activation at TRPV4[13][16] |
· Anti-oxidative properties[16]
· Increased lysosomal stability & integrity[16] · Attenuation of mitochondrial damage during cell stress[16] |
| Anandamide (AEA) | · Agonist at GPR18, GPR119, and PPAR, with robust activation at TRPV4, and very high efficacy at TRPA1[13][16]
· Potent partial agonist at GPR55[16][14] · Low-affinity full agonist at TRPV1[13][14], with similar but less potent affinity as compared to capsaicin[13] · Antagonist at TRPM8[13] |
· Anti-oxidative properties[16] |
Neurotransmitter Interactions

In the brain, the canonical mechanism of CB1 receptor activation is a form of short-term synaptic plasticity initiated via retrograde signaling of endogenous CB1 agonists such as 2AG or AEA (two primary endocannabinoids). This mechanism of action is called depolarization-induced suppression of inhibition (DSI) or depolarization-induced suppression of excitation (DSE)[18], depending on the classification of the presynaptic neuron acted upon by the retrograde messenger. In the case of CB1R agonism on the presynaptic membrane of a GABAergic interneuron, activation leads to a net effect of increased activity, while the same activity on a glutamatergic neuron leads to the opposite net effect. The release of other neurotransmitters is also modulated in this way, particularly dopamine, dynorphin, oxytocin, and vasopressin.[18]
Legal status
CBN is not listed in the schedules set out by the United Nations' Single Convention on Narcotic Drugs from 1961 nor their Convention on Psychotropic Substances from 1971,[19] so the signatory countries to these international drug control treaties are not required by these treaties to control CBN.
United States
According to the 2018 Farm Bill,[20] extracts from the Cannabis sativa L. plant, including CBN, are legal under US federal law as long as they have a delta-9 Tetrahydrocannabinol (THC) concentration of 0.3 percent or less. However, as of 2022 in the United States, CBN and other cannabis extracts remain illegal under federal law to prescribe for medical use or to use as an ingredient in dietary supplements or other foods,[21][22][23] and sales or possession of CBN could potentially be prosecuted under the Federal Analogue Act.[24] In December 2016, the Drug Enforcement Administration added marijuana extracts, which are defined as any "extract containing one or more cannabinoids that has been derived from any plant of the genus Cannabis, other than the separated resin", to Schedule I.[25]
References
- ^ Template:ChemID.
- ^ Lide DR (2012). CRC Handbook of Chemistry and Physics. CRC Press. pp. 3–90. ISBN 978-1-43988049-4.
- ^ "Cannabinol solution, analytical standard, for drug analysis". Sigma-Aldrich. c046.
- ^ "Cannabinol" (PDF). Biotrend. Archived from the original (PDF) on May 22, 2016.
- ^ a b c Pertwee, Roger G (2006). "Cannabinoid pharmacology: the first 66 years: Cannabinoid pharmacology". British Journal of Pharmacology. 147 (S1): S163–S171. doi:10.1038/sj.bjp.0706406. PMC 1760722. PMID 16402100.
{{cite journal}}: CS1 maint: PMC format (link) - ^ a b c Rhee MH, Vogel Z, Barg J, Bayewitch M, Levy R, Hanus L, et al. (September 1997). "Cannabinol derivatives: binding to cannabinoid receptors and inhibition of adenylylcyclase". Journal of Medicinal Chemistry. 40 (20): 3228–3233. doi:10.1021/jm970126f. PMID 9379442.
- ^ a b c d Cannabinoids. Mary Ellen Abood, R. G. Pertwee. Berlin: Springer. 2005. ISBN 3-540-22565-X. OCLC 65169431.
{{cite book}}: CS1 maint: others (link) - ^ Corroon, Jamie (August 31, 2021). "Cannabinol and Sleep: Separating Fact from Fiction". Cannabis and Cannabinoid Research: can.2021.0006. doi:10.1089/can.2021.0006. ISSN 2578-5125. PMC 8612407. PMID 34468204.
{{cite journal}}: CS1 maint: PMC format (link) - ^ Andre CM, Hausman JF, Guerriero G (February 4, 2016). "Cannabis sativa: The Plant of the Thousand and One Molecules". Frontiers in Plant Science. 7: 19. doi:10.3389/fpls.2016.00019. PMC 4740396. PMID 26870049.
- ^ Aizpurua-Olaizola, Oier; Elezgarai, Izaskun; Rico-Barrio, Irantzu; Zarandona, Iratxe; Etxebarria, Nestor; Usobiaga, Aresatz (2017). "Targeting the endocannabinoid system: future therapeutic strategies". Drug Discovery Today. 22 (1): 105–110. doi:10.1016/j.drudis.2016.08.005.
- ^ "Cannabinol (Code C84510)". NCI Thesaurus. National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services.
- ^ Pattnaik, Falguni; Nanda, Sonil; Mohanty, Shobhangam; Dalai, Ajay K.; Kumar, Vivek; Ponnusamy, Senthil Kumar; Naik, Satyanarayan (2022). "Cannabis: Chemistry, extraction and therapeutic applications". Chemosphere. 289: 133012. doi:10.1016/j.chemosphere.2021.133012.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac Muller, Chanté; Morales, Paula; Reggio, Patricia H. (January 15, 2019). "Cannabinoid Ligands Targeting TRP Channels". Frontiers in Molecular Neuroscience. 11: 487. doi:10.3389/fnmol.2018.00487. ISSN 1662-5099. PMC 6340993. PMID 30697147.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ a b c d e Legare, Christopher A.; Raup-Konsavage, Wesley M.; Vrana, Kent E. (2022). "Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals". Pharmacology. 107 (3–4): 131–149. doi:10.1159/000521683. ISSN 0031-7012.
- ^ a b c d e f g h i j k l m n o p q r Sampson, Peter B. (January 22, 2021). "Phytocannabinoid Pharmacology: Medicinal Properties of Cannabis sativa Constituents Aside from the "Big Two"". Journal of Natural Products. 84 (1): 142–160. doi:10.1021/acs.jnatprod.0c00965. ISSN 1520-6025. PMID 33356248.
- ^ a b c d e f g h i j k l m n o p q Cherkasova, Viktoriia; Wang, Bo; Gerasymchuk, Marta; Fiselier, Anna; Kovalchuk, Olga; Kovalchuk, Igor (October 20, 2022). "Use of Cannabis and Cannabinoids for Treatment of Cancer". Cancers. 14 (20): 5142. doi:10.3390/cancers14205142. ISSN 2072-6694. PMC 9600568. PMID 36291926.
{{cite journal}}: CS1 maint: PMC format (link) CS1 maint: unflagged free DOI (link) - ^ a b Mead A (June 14, 2019). "Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States". Frontiers in Plant Science. 10: 697. doi:10.3389/fpls.2019.00697. PMC 6590107. PMID 31263468.
- ^ a b Diana, Marco A; Marty, Alain (2004). "Endocannabinoid-mediated short-term synaptic plasticity: depolarization-induced suppression of inhibition (DSI) and depolarization-induced suppression of excitation (DSE): DSI/DSE: two forms of CB1R-mediated plasticity". British Journal of Pharmacology. 142 (1): 9–19. doi:10.1038/sj.bjp.0705726. PMC 1574919. PMID 15100161.
{{cite journal}}: CS1 maint: PMC format (link) - ^ "UN International Drug Control Conventions". United Nations Office on Drugs and Crime. United Nations Commission on Narcotic Drugs.
- ^ Commissioner, Office of the (October 18, 2021). "FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD)". FDA.
- ^ Mead A (June 14, 2019). "Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States". Frontiers in Plant Science. 10: 697. doi:10.3389/fpls.2019.00697. PMC 6590107. PMID 31263468.
- ^ Mead A (May 2017). "The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law". Epilepsy & Behavior. 70 (Pt B): 288–291. doi:10.1016/j.yebeh.2016.11.021. PMID 28169144.
- ^ "Section 1308.11 Schedule I". Code of Federal Regulations. Office of Diversion Control, Drug Enforcement Administration, U.S. Department of Justice. Archived from the original on February 9, 2012.
- ^ "Federal Controlled Substance Analogue Act Summary". Erowid Analog Law Vault. January 2001.
- ^ "Establishment of a New Drug Code for Marihuana Extract" (PDF). Federal Register. 81 (240). December 14, 2016.
External links
- Erowid Compounds found in Cannabis sativa
