Tetrodotoxin: Difference between revisions
m →Chemical synthesis: lower case 's'. |
|||
| Line 94: | Line 94: | ||
== Chemical synthesis == |
== Chemical synthesis == |
||
In 1964 a team of |
In 1964 a team of scientists led by [[Robert B. Woodward]] at Harvard University elucidated the structure of tetrodotoxin.<ref name="Woodward_1964">{{cite journal | vauthors = Woodward RB | author-link = Robert B. Woodward | title = The Structure of Tetrodotoxin | journal = Pure Appl. Chem. | volume = 9 | issue = 1 | pages = 49–75 | year = 1964 | pmid = | doi = 10.1351/pac196409010049 | url = http://pac.iupac.org/publications/pac/pdf/1964/pdf/0901x0049.pdf }}</ref> The structure was confirmed by X-ray crystallography in 1970.<ref>{{ cite journal | first1 = Akio | last1 = Furusaki | first2 = Yujiro | last2 = Tomiie | first3 = Isamu | last3 = Nitta | title = The Crystal and Molecular Structure of Tetrodotoxin Hydrobromide | journal = [[Bulletin of the Chemical Society of Japan]] | volume = 43 | year = 1970 | issue = 11 | pages = 3332–3341 | doi = 10.1246/bcsj.43.3332 }}</ref> [[Yoshito Kishi]] and coworkers at [[Nagoya University]], [[Nagoya]], [[Japan]], (now at Harvard University) reported the first [[total synthesis]] of D,L-tetrodotoxin in 1972.<ref name="Kishi_1972a">{{cite journal | vauthors = Kishi Y, Aratani M, Fukuyama T, Nakatsubo F, Goto T | title = Synthetic studies on tetrodotoxin and related compounds. 3. A stereospecific synthesis of an equivalent of acetylated tetrodamine | journal = Journal of the American Chemical Society | volume = 94 | issue = 26 | pages = 9217–9 | date = Dec 1972 | pmid = 4642370 | doi = 10.1021/ja00781a038 }}</ref><ref name="Kishi_1972b">{{cite journal | vauthors = Kishi Y, Fukuyama T, Aratani M, Nakatsubo F, Goto T | title = Synthetic studies on tetrodotoxin and related compounds. IV. Stereospecific total syntheses of DL-tetrodotoxin | journal = Journal of the American Chemical Society | volume = 94 | issue = 26 | pages = 9219–21 | date = Dec 1972 | pmid = 4642371 | doi = 10.1021/ja00781a039 }}</ref> M. Isobe and coworkers at Nagoya University, Japan<ref name="Taber_2005">{{cite web | url = http://www.organic-chemistry.org/Highlights/2005/02May.shtm | title = Synthesis of (-)-Tetrodotoxin | vauthors = Taber D | date = 2005-05-02 | work = Organic Chemistry Portal | publisher = organic-chemistry.org }}</ref><ref name="Isobe_2003">{{cite journal | vauthors = Ohyabu N, Nishikawa T, Isobe M | title = First asymmetric total synthesis of tetrodotoxin | journal = Journal of the American Chemical Society | volume = 125 | issue = 29 | pages = 8798–805 | date = Jul 2003 | pmid = 12862474 | doi = 10.1021/ja0342998 }}</ref><ref name="Isobe_2004">{{cite journal | vauthors = Nishikawa T, Urabe D, Isobe M | title = An efficient total synthesis of optically active tetrodotoxin | journal = Angewandte Chemie | volume = 43 | issue = 36 | pages = 4782–5 | date = Sep 2004 | pmid = 15366086 | doi = 10.1002/anie.200460293 }}</ref> and J. Du Bois ''et al.'' at [[Stanford University]], U.S., reported the [[asymmetric synthesis|asymmetric]] total synthesis of tetrodotoxin in 2003.<ref name="Dubois_2003">{{cite journal | vauthors = Hinman A, Du Bois J | title = A stereoselective synthesis of (-)-tetrodotoxin | journal = Journal of the American Chemical Society | volume = 125 | issue = 38 | pages = 11510–1 | date = Sep 2003 | pmid = 13129349 | doi = 10.1021/ja0368305 }}</ref> The two 2003 syntheses used very different strategies, with Isobe's route based on a [[Diels-Alder reaction|Diels-Alder approach]] and Du Bois's work using C-H bond activation. Since then, methods have rapidly advanced, with several new strategies for the synthesis of tetrodotoxin having been developed.<ref>{{cite journal | vauthors = Chau J, Ciufolini MA | title = The chemical synthesis of tetrodoxin: an ongoing quest | journal = Marine Drugs | volume = 9 | issue = 10 | pages = 2046–74 | date = 2011 | pmid = 22073009 | pmc = 3210618 | doi = 10.3390/md9102046 }}</ref><ref>{{cite journal | vauthors = Sato K, Akai S, Yoshimura J | title = Stereocontrolled total synthesis of tetrodotoxin from myo-inositol and D-glucose by three routes: aspects for constructing complex multi-functionalized cyclitols with branched-chain structures | journal = Natural Product Communications | volume = 8 | issue = 7 | pages = 987–98 | date = Jul 2013 | pmid = 23980434 }}</ref> |
||
== Poisoning == |
== Poisoning == |
||
Revision as of 17:00, 31 July 2016
This scientific article needs additional citations to secondary or tertiary sources. (February 2016) |
| |

| |
| Names | |
|---|---|
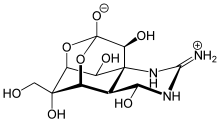
| IUPAC name
(4R,4aR,5R,6S,7S,8S,8aR,10S,12S)-2-azaniumylidene-4,6,8,12-tetrahydroxy-6-(hydroxymethyl)-2,3,4,4a,5,6,7,8-octahydro-1H-8a,10-methano-5,7-(epoxymethanooxy)quinazolin-10-olate
| |
| Other names
anhydrotetrodotoxin, 4-epitetrodotoxin, tetrodonic acid, TTX
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.022.236 |
| KEGG | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C11H17N3O8 | |
| Molar mass | 319.268 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Tetrodotoxin, frequently abbreviated as TTX, is a potent neurotoxin. Its name derives from Tetraodontiformes, an order that includes pufferfish, porcupinefish, ocean sunfish, and triggerfish; several species that carry the toxin. Although tetrodotoxin was discovered in these fish and found in several other aquatic animals (e.g., in blue-ringed octopuses, rough-skinned newts, and moon snails), it is actually produced by certain infecting or symbiotic bacteria like Pseudoalteromonas, Pseudomonas, and Vibrio as well as other species found in the animals.
Tetrodotoxin inhibits the firing of action potentials in nerves by binding to the voltage-gated sodium channels in nerve cell membranes and blocking the passage of sodium ions (responsible for the rising phase of an action potential) into the nerve cell.[1]
Its mechanism of action, selective blocking of the sodium channel, was shown definitively in 1964 by Toshio Narahashi and John W. Moore at Duke University, using the sucrose gap voltage clamp technique.[2]
Sources in nature
Apart from their bacterial species of most likely ultimate biosynthetic origin (see below), tetrodotoxin has been isolated from widely differing animal species, including:[3]
- various pufferfish species,[1][3][4]
- certain angelfish,[5]
- several species of the blue-ringed octopus,[1][3][4] including Hapalochlaena maculosa (where it was called "maculotoxin"),[4]
- species of Niotha gastropods,[1][3][4]
- species of genus Naticidae (moon snails),[3][6]
- several starfish, including Astropecten species,[1][3][4]
- several species of xanthid crabs.[3][4]
- species of Chaetognatha (arrow worms),[1][3]
- species of Nemertea (ribbon worms),[1][3]
- a polyclad flatworm,[3]
- land planarians of the genus Bipalium,[7]
- toads of the genus Atelopus,[3] and
- western, rough-skinned newts of the genus Taricha (wherein it was originally termed "tarichatoxin"),[3]
Tarichatoxin was shown to be identical to TTX in 1964 by Mosher and coworkers,[8][9] and the identity of maculotoxin and TTX was reported in Science in 1978,[10] and the synonymity of these two toxins is supported in modern reports (e.g., at Pubchem[11] and in modern toxicology textbooks[12]) though historic monographs questioning this continue in reprint.[13]
The toxin is variously used by metazoans as a defensive biotoxin to ward off predation, or as both a defensive and predatory venom (e.g., in octopi, chaetognaths, and ribbon worms).[citation needed] Even though the toxin acts as a defense mechanism, some predators such as the common garter snake have developed insensitivity to TTX, which allows them to prey upon toxic newts.[14]
The association of TTX with consumed, infecting, or symbiotic bacterial populations within the metazoan species from which it is isolated is, as of 2016, relatively clear;[3] presence of TTX-producing bacteria within a metazoan's microbiome is determined by culture methods, the presence of the toxin by chemical analysis, and the association of the bacteria with TTX production by toxicity assay of media in which suspected bacteria are grown.[4] As Lago et al. note, "there is good evidence that uptake of bacteria producing TTX is an important element of TTX toxicity in marine metazoans that present this toxin."[4] TTX-producing bacteria include Actinomyces, Aeromonas, Alteromonas, Bacillus, Pseudomonas, and Vibrio species;[4] in the following animals, specific bacterial species have been implicated:[3]
- Vibrio species including Vibrio alginolyticus, from the puffer fish, Fugu vermicularis,[1][3][4][15]
- Vibrio alginolyticus, from the starfish species Astropecten polyanthus,[1][4]
- Aeromonas species from the puffer fish, Takifugu obscures,[1][4]
- both Vibrio, Pseudomonas, and Aeromonas species from gastropod Niotha clathrata,[1][3][4]
- Alteromonas, Bacillus, Pseudomonas, and Vibrio species from blue-ringed octopi, species Hapalochlaena macula,[1][3][4][16]
- Vibrio species, including Vibrio alginolyticus again, in arrow worms, phylum Chaetognatha,[3][17] and
- Vibrio species, again, in ribbon worms, phylum Nemertea.[3][18]
The association of bacterial species with the production of the toxin is unequivocal—Lago and coworkers state, "[e]ndocellular symbiotic bacteria have been proposed as a possible source of eukaryotic TTX by means of an exogenous pathway,"[4] and Chau and coworkers note that the "widespread occurrence of TTX in phylogenetically distinct organisms… strongly suggests that symbiotic bacteria play a role in TTX biosynthesis"[3]—although the correlation has been extended to most but not all metazoans in which the toxin has been identified.[1][3][4] To the contrary, there has been a failure in a single case, that of newts (Taricha granulosa), to detect TTX-producng bacteria in the tissues with highest toxin levels (skin, ovaries, muscle), using PCR methods, although technical concerns about the approach have been raised.[3] Critically for the general argument, Takifugu rubripes puffers captured and raised in laboratory on controlled, TTX-free diets "lose toxicity over time," while cultured, TTX-free Fugu niphobles puffers fed on TTX-containing diets saw TTX in the livers of the fishes increase to toxic levels.[3] Hence, as bacterial species that produce TTX are broadly present in aquatic sediments, a strong case is made for ingestion of TTX and/or TTX-producing bacteria, with accumulation and possible subsequent colonization and production.[3] Nevertheless, without clear biosynthetic pathways (not yet found in metazoans, but shown for bacteria),[19] it remains uncertain whether it is simply via bacteria that each metazoan accumulates TTX; whether the quantities can be sufficiently explained by ingestion, this plus colonization, or some other mechanism.[1][3][4]
Biochemistry
This section needs additional citations for verification. (April 2016) |
Tetrodotoxin binds to what is known as site 1 of the fast voltage-gated sodium channel.[20] Site 1 is located at the extracellular pore opening of the ion channel. The binding of any molecules to this site will temporarily disable the function of the ion channel. Saxitoxin, neosaxitoxin and several of the conotoxins also bind the same site.
The use of this toxin as a biochemical probe has elucidated two distinct types of voltage-gated sodium channels present in humans: the tetrodotoxin-sensitive voltage-gated sodium channel (TTX-s Na+ channel) and the tetrodotoxin-resistant voltage-gated sodium channel (TTX-r Na+ channel). Tetrodotoxin binds to TTX-s Na+ channels with a binding affinity of 5–15 nM, while the TTX-r Na+ channels bind TTX with low micromolar affinity.[21][failed verification] Nerve cells containing TTX-r Na+ channels are located primarily in cardiac tissue, while nerve cells containing TTX-s Na+ channels dominate the rest of the body.
TTX and its analogs have historically been important agents for use as chemical tool compounds, for use in channel characterization and in fundamental studies of channel function.[22][23] The prevalence of TTX-s Na+ channels in the central nervous system makes tetrodotoxin a valuable agent for the silencing of neural activity within a cell culture.
Chemical synthesis
In 1964 a team of scientists led by Robert B. Woodward at Harvard University elucidated the structure of tetrodotoxin.[24] The structure was confirmed by X-ray crystallography in 1970.[25] Yoshito Kishi and coworkers at Nagoya University, Nagoya, Japan, (now at Harvard University) reported the first total synthesis of D,L-tetrodotoxin in 1972.[26][27] M. Isobe and coworkers at Nagoya University, Japan[28][29][30] and J. Du Bois et al. at Stanford University, U.S., reported the asymmetric total synthesis of tetrodotoxin in 2003.[31] The two 2003 syntheses used very different strategies, with Isobe's route based on a Diels-Alder approach and Du Bois's work using C-H bond activation. Since then, methods have rapidly advanced, with several new strategies for the synthesis of tetrodotoxin having been developed.[32][33]
Poisoning
Toxicity
TTX is extremely toxic. The Material Safety Data Sheet for TTX lists the oral median lethal dose (LD50) for mice as 334 μg per kg.[34] For comparison, the oral LD50 of potassium cyanide for mice is 8.5 mg per kg,[35] demonstrating that even orally, TTX is more poisonous than cyanide. TTX is even more dangerous if injected; the amount needed to reach a lethal dose by injection is only 8 μg per kg in mice.[36]
The toxin can enter the body of a victim by ingestion, injection, or inhalation, or through abraded skin.[37]
Poisoning occurring as a consequence of consumption of fish from the order Tetraodontiformes is extremely serious. The organs (e.g. liver) of the pufferfish can contain levels of tetrodotoxin sufficient to produce the described paralysis of the diaphragm and corresponding death due to respiratory failure.[38] Toxicity varies between species and at different seasons and geographic localities, and the flesh of many pufferfish may not be dangerously toxic.[1]
The mechanism of toxicity is through the blockage of fast voltage-gated sodium channels, which are required for the normal transmission of signals between the body and brain.[39] As a result, TTX causes loss of sensation, and paralysis of voluntary muscles including the diaphragm and intercostal muscles, stopping breathing.[40]
History

The therapeutic uses of puffer fish (tetraodon) eggs were mentioned in the first Chinese pharmacopea (Pen-T’ so Ching, The Book of Herbs, allegedly 2838–2698 BC by Shénnóng Běn Cǎo Jīng; but a later date is more likely), where they were classified as having ‘medium’ toxicity, but could have a tonic effect when used at the correct dose. The principle use was “to arrest convulsive diseases”.[41] In the Pen-T’ so Kang Mu (Index Herbacea or The Great Herbal by Li Shih-Chen, 1596) some types of the fish Ho-Tun (the current Chinese name for tetraodon) were also recognized as both toxic and (at the right dose) could be used to prepare a tonic. Increased toxicity in Ho-Tun was noted in fish caught at sea (rather than river) after the month of March. It was recognized that the most poisonous parts were the liver and eggs, but that toxicity could be reduced by soaking the eggs,[41] noting that tetrodotoxin is slightly water-soluble, and soluble at 1 mg/mL in slightly acidic solutions.[42]
The German physician Engelbert Kaempfer, in his "A History of Japan" (translated and published in English in 1727), described how well known the toxic effects of the fish were, to the extent that it would be used for suicide and that the Emperor specifically decreed that soldiers were not permitted to eat it. There is also evidence from other sources that knowledge of such toxicity was widespread throughout southeast Asia and India.[41]
The first recorded cases of TTX poisoning affecting Westerners are from the logs of Captain James Cook from 7 September 1774.[38] On that date Cook recorded his crew eating some local tropic fish (pufferfish), then feeding the remains to the pigs kept on board. The crew experienced numbness and shortness of breath, while the pigs were all found dead the next morning. In hindsight, it is clear that the crew survived a mild dose of tetrodotoxin, while the pigs ate the pufferfish body parts that contain most of the toxin, thus being fatally poisoned.
The toxin was first isolated and named in 1909 by Japanese scientist Dr. Yoshizumi Tahara.[38] It was one of the agents studied by Japan's Unit 731, which evaluated biological weapons on human subjects in the 1930s.[43]
Symptoms and treatment
The diagnosis of pufferfish poisoning is based on the observed symptomatology and recent dietary history.[44]
Symptoms typically develop within 30 minutes of ingestion, but may be delayed by up to four hours; however, if the dose is fatal, symptoms are usually present within 17 minutes of ingestion.[38] Paresthesia of the lips and tongue is followed by developing paresthesia in the extremities, hypersalivation, sweating, headache, weakness, lethargy, incoordination, tremor, paralysis, cyanosis, aphonia, dysphagia, and seizures. The gastrointestinal symptoms are often severe and include nausea, vomiting, diarrhea, and abdominal pain; death is usually secondary to respiratory failure.[40][44] There is increasing respiratory distress, speech is affected, and the victim usually exhibits dyspnea, cyanosis, mydriasis, and hypotension. Paralysis increases, and convulsions, mental impairment, and cardiac arrhythmia may occur. The victim, although completely paralyzed, may be conscious and in some cases completely lucid until shortly before death, which generally occurs within 4 to 6 hours (range ~20 minutes to ~8 hours). However, some victims enter a coma.[40][45]
If the patient survives 24 hours, recovery without any residual effects will usually occur over a few days.[44]
Therapy is supportive and based on symptoms, with aggressive early airway management.[38] If ingested, treatment can consist of emptying the stomach, feeding the victim activated charcoal to bind the toxin, and taking standard life-support measures to keep the victim alive until the effect of the poison has worn off.[38] Alpha adrenergic agonists are recommended in addition to intravenous fluids to combat hypotension; anticholinesterase agents "have been proposed as a treatment option but have not been tested adequately".[45]
No antidote has been developed and approved for human use, but a primary research report (preliminary result) indicates that a monoclonal antibody specific to tetrodotoxin is in development by USAMRIID that was effective, in the one study, for reducing toxin lethality in tests on mice.[46]
Geographic frequency of toxicity
Poisonings from tetrodotoxin have been almost exclusively associated with the consumption of pufferfish from waters of the Indo-Pacific ocean regions, but pufferfishes from other regions are much less commonly eaten. Several reported cases of poisonings, including fatalities, involved pufferfish from the Atlantic Ocean, Gulf of Mexico, and Gulf of California. There have been no confirmed cases of tetrodotoxicity from the Atlantic pufferfish, Sphoeroides maculatus, but in three studies, extracts from fish of this species were highly toxic in mice. Several recent intoxications from these fishes in Florida were due to saxitoxin, which causes paralytic shellfish poisoning with very similar symptoms and signs. The trumpet shell Charonia sauliae has been implicated in food poisonings, and evidence suggests it contains a tetrodotoxin derivative. There have been several reported poisonings from mislabelled pufferfish, and at least one report of a fatal episode in Oregon when an individual swallowed a rough-skinned newt Taricha granulosa.[47]
In 2009, a major scare in the Auckland Region of New Zealand was sparked after several dogs died eating Pleurobranchaea maculata (grey side-gilled seaslug) on beaches.[48] Children and pet owners were asked to avoid beaches, and recreational fishing was also interrupted for a time. After exhaustive analysis, it was found that the sea slugs must have ingested tetrodotoxin.[49]
- Statistical factors
Statistics from the Tokyo Bureau of Social Welfare and Public Health indicate 20–44 incidents of fugu poisoning per year between 1996 and 2006 in the entire country, leading to 34–64 hospitalizations and 0–6 deaths per year, for an average fatality rate of 6.8%.[50] Of the 23 incidents recorded within Tokyo between 1993 and 2006, only one took place in a restaurant, while the others all involved fishermen eating their catch.[50] From 2006 through 2009 in Japan there were 119 incidents involving 183 people but only 7 people died.[51]
Only a few cases have been reported in the United States, and outbreaks in countries outside the Indo-Pacific area are rare.[citation needed] In Haiti, tetrodotoxin is thought to have been used in voodoo preparations, in so-called zombie poisons, where subsequent careful analysis has repeatedly called early studies into question on technical grounds, and have failed to identify the toxin in any preparation,[52][53][54] such that discussion of the matter has all but disappeared from the primary literature since the early 1990s. Kao and Yasumoto concluded in the first of their papers in 1986 that "the widely circulated claim in the lay press to the effect that tetrodotoxin is the causal agent in the initial zombification process is without factual foundation.”[52]: 748
Genetic background is not a factor in susceptibility to tetrodotoxin poisoning. This toxicosis may be avoided by not consuming animal species known to contain tetrodotoxin, principally pufferfish; other tetrodotoxic species are not usually consumed by humans.
- Fugu as a food
Poisoning from tetrodotoxin is of particular public health concern in Japan, where pufferfish "fugu" is a traditional delicacy. It is prepared and sold in special restaurants where trained and licensed chefs carefully remove the viscera to reduce the danger of poisoning.[55] There is potential for misidentification and mislabelling, particularly of prepared, frozen fish products.
Food analysis
The mouse bioassay developed for paralytic shellfish poisoning (PSP) can be used to monitor tetrodotoxin in pufferfish and is the current method of choice. An HPLC method with post-column reaction with alkali and fluorescence has been developed to determine tetrodotoxin and its associated toxins. The alkali degradation products can be confirmed as their trimethylsilyl derivatives by gas chromatography/mass spectrometry.
Detection in body fluids
Tetrodotoxin may be quantified in serum, whole blood or urine to confirm a diagnosis of poisoning in hospitalized patients or to assist in the forensic investigation of a case of fatal overdosage. Most analytical techniques involve mass spectrometric detection following gas or liquid chromatographic separation.[56]
Modern therapeutic research
Tetrodotoxin has been investigated as a possible treatment for cancer-associated pain. Early clinical trials demonstrate significant pain relief in some patients.[57][58]
In addition to the cancer pain application mentioned, mutations in one particular TTX-sensitive Na+ channel are associated with some migraine headaches,[59] although it is unclear as to whether this has any therapeutic relevance for most people with migraine.[60]
Tetrodotoxin has been used clinically to relieve the headache associated with heroin withdrawal.[61]
Regulation
In the U.S., tetrodotoxin appears on the select agents list of the Department of Health and Human Services,[62] and scientists must register with HHS to use tetrodotoxin in their research. However, investigators possessing less than 100 mg are exempt from regulation.[63]
Popular culture
Tetrodotoxin serves as a plot device for characters to fake death, as in the films Miami Vice (1985),[64] Hello Again (1987), The A-Team (2010) and Captain America: The Winter Soldier (2014), and in episodes of Nikita, CSI: NY (Season 4, episode 9 "Boo") and Chuck. In Law Abiding Citizen (2009) its paralysis is presented as a method of assisting torture. The toxin is used as a weapon in Covert Affairs.[65][66]
In the sci-fi series Orphan Black, a half organic, half mechanical "maggot bot" engineered by Evie Cho as a vector for gene therapy delivery to patients, makes use of tetrodotoxin as a defence mechanism to protect the device against tampering.
Based on the presumption that tetrodotoxin is not always fatal, but at near-lethal doses can leave a person extremely unwell with the person remaining conscious,[44] tetrodotoxin has been alleged to result in zombieism, and has been suggested as an ingredient in Haitian Vodou preparations.[67] This idea appeared earlier, in the 1938 non-fiction book Tell My Horse by Zora Neale Hurston—reporting multiple accounts of purported tetrodotoxin poisoning in Haiti, by a voodoo sorcerer called the Bokor[68]—and popularized by Harvard-trained ethnobotanist Wade Davis,[67] but has been dismissed by the scientific community since the 1990s based on analytical chemistry-based tests of multiple preparations and review of earlier reports (see above).[52][53][54]
See also
- Clairvius Narcisse, a Haitian alleged to have been buried alive under the effect of the drug
- 4-Aminopyridine
- Conotoxin
- Neurotoxin
- Neosaxitoxin
- Saxitoxin
- Tectin
References
- ^ a b c d e f g h i j k l m n o Bane V, Lehane M, Dikshit M, O'Riordan A, Furey A (Feb 2014). "Tetrodotoxin: Chemistry, Toxicity, Source, Distribution and Detection". Toxins. 6 (2): 693–755. doi:10.3390/toxins6020693. PMC 3942760. PMID 24566728.
{{cite journal}}: CS1 maint: unflagged free DOI (link) CS1 maint: year (link) - ^ Narahashi T, Moore JW, Scott WR (May 1964). "Tetrodotoxin blockage of sodium conductance increase in lobster giant axons". The Journal of General Physiology. 47 (5): 965–74. doi:10.1085/jgp.47.5.965. PMC 2195365. PMID 14155438.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y For a more comprehensive list of TTX-producing bacterial species associated with metazoans from which the toxin has been isolated or toxicity observed, and for a thorough discussion of the research literature regarding bacterial origins (and the remaining contrary perspectives, e.g., in newts), as well as for a thorough speculative discussion regarding biosynthesis, see Chau R, Kalaitzis JA, Neilan BA (Jul 2011). "On the origins and biosynthesis of tetrodotoxin" (print, online review). Aquatic Toxicology. 104 (1–2): 61–72. doi:10.1016/j.aquatox.2011.04.001. PMID 21543051.
- ^ a b c d e f g h i j k l m n o p q Lago J, Rodríguez LP, Blanco L, Vieites JM, Cabado AG (2015). "Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses". Marine Drugs. 13 (10): 6384–406. doi:10.3390/md13106384. PMC 4626696. PMID 26492253.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sigma-Aldrich Tetrodotoxin (T8024) – Product Information Sheet.
- ^ Hwang DF, Tai KP, Chueh CH, Lin LC, Jeng SS (1991). "Tetrodotoxin and derivatives in several species of the gastropod Naticidae". Toxicon. 29 (8): 1019–24. doi:10.1016/0041-0101(91)90084-5. PMID 1949060.
- ^ Stokes AN, Ducey PK, Neuman-Lee L, Hanifin CT, French SS, Pfrender ME, Brodie ED, Brodie ED (2014). "Confirmation and distribution of tetrodotoxin for the first time in terrestrial invertebrates: two terrestrial flatworm species (Bipalium adventitium and Bipalium kewense)". PLOS One. 9 (6): e100718. doi:10.1371/journal.pone.0100718. PMC 4070999. PMID 24963791.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Scheuer PJ (1970). "Toxins from fish and other marine organisms". Advances in Food Research. 18: 141–61. PMID 4929140.
- ^ Mosher HS, Fuhrman FA, Buchwald HD, Fischer HG (May 1964). "Tarichatoxin—tetrodotoxin: a potent neurotoxin". Science. 144 (3622): 1100–10. doi:10.1126/science.144.3622.1100. PMID 14148429.
- ^ Sheumack DD, Howden ME, Spence I, Quinn RJ (Jan 1978). "Maculotoxin: a neurotoxin from the venom glands of the octopus Hapalochlaena maculosa identified as tetrodotoxin". Science. 199 (4325): 188–9. doi:10.1126/science.619451. PMID 619451.
Maculotoxin, a potent neurotoxin isolated from the posterior salivary glands of the blue-ringed octopus. Hapalochlaena maculosa, has now been identified as tetrodotoxin. This is the first reported case in which tetrodotoxin has been found to occur in a venom.
- ^ "Tetrodotoxin". PubChem. National Center for Biotechnology Information (NCBI).
- ^ Stine KE, Brown TM (2015). Principles of Toxicology (3rd ed.). Boca Raton, FL, USA: CRC Press. pp. 196, 390. ISBN 1466503432.
- ^ Gage, Peter W.; Dulhunty, Angela F. (2012) [1973]. "Effects of Toxin from the Blue-Ringed Octopus (Hapalochlaena maculosa) [Chapter III]". In Martin, D.F.; Padilla, G.M. (eds.). Marine Pharmacognosy: Action of Marine Biotoxins at the Cellular Level. Philadelphia, PA [New York, NY], USA: Elsevier [Academic Press]. pp. 85–106. ISBN 032315560X.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Brodie, Edmund D. III; Brodie, Edmund D. Jr (May 1990). "Tetrodotoxin Resistance in Garter Snakes: An Evolutionary Response of Predators to Dangerous Prey". Evolution. 44 (3): 651. doi:10.2307/2409442.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Noguchi, T.; Hwang, D.F.; Arakawa, O.; Sugita, H.; Deguchi, Y.; Shida, Y.; Hashimoto, K. (1987). "Vibrio alginolyticus, a tetrodotoxin-producing bacterium, in the intestines of the fish Fugu vermicularis vermicularis" (PDF). Marine Biology. 94 (4): 625–630. doi:10.1007/BF00431409.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Hwang DF, Arakawa O, Saito T, Noguchi T, Simidu U, Tsukamoto K, Shida Y, Hashimoto K (1988). "Tetrodotoxin-producing bacteria from the blue-ringed octopus Octopus maculosus [sic.]". Marine Biology. 100 (3): 327–332. doi:10.1007/BF00391147.
- ^ Thuesen EV, Kogure K (1989). "Bacterial production of tetrodotoxin in four species of Chaetognatha" (PDF). Biological Bulletin. 176 (2): 191–194. doi:10.2307/1541587. JSTOR 1541587.
- ^ Carroll, S.; McEvoy, E.G.; Gibson, R. (2003). "The production of tetrodotoxin-like substances by nemertean worms in conjunction with bacteria". Journal of Experimental Marine Biology and Ecology. 288 (1): 51–63. doi:10.1016/S0022-0981(02)00595-6.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ As Chau et al., op. cit., note, "Despite its long history and a thorough knowledge of its toxicity and pharmacology, neither the pathway to TTX nor even the biogenic origin of TTX is known. The debate into whether TTX is derived from bacteria or is endogenous to the host animals is on-going and the only published study into the substrates of TTX biosynthesis proved inconclusive."
- ^ Moczydlowski EG (Mar 2013). "The molecular mystique of tetrodotoxin". Toxicon. 63: 165–83. doi:10.1016/j.toxicon.2012.11.026. PMID 23261990.
- ^ "Tetrodotoxin". Guide to Pharmacology. IUPHAR/BPS.
- ^ Kao, C.Y. (1966). "Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomenon". Pharm Rev. 18 (2): 997–1049. PMID 5328391.
{{cite journal}}:|format=requires|url=(help) - ^ Blankenship, J.E. (1976). "Tetrodotoxin: From Poison to Powerful Tool". Perspectives in Biology and Medicine. 19 (4, Summer): 509–526. doi:10.1353/pbm.1976.0071.
{{cite journal}}:|format=requires|url=(help) - ^ Woodward RB (1964). "The Structure of Tetrodotoxin" (PDF). Pure Appl. Chem. 9 (1): 49–75. doi:10.1351/pac196409010049.
- ^ Furusaki, Akio; Tomiie, Yujiro; Nitta, Isamu (1970). "The Crystal and Molecular Structure of Tetrodotoxin Hydrobromide". Bulletin of the Chemical Society of Japan. 43 (11): 3332–3341. doi:10.1246/bcsj.43.3332.
- ^ Kishi Y, Aratani M, Fukuyama T, Nakatsubo F, Goto T (Dec 1972). "Synthetic studies on tetrodotoxin and related compounds. 3. A stereospecific synthesis of an equivalent of acetylated tetrodamine". Journal of the American Chemical Society. 94 (26): 9217–9. doi:10.1021/ja00781a038. PMID 4642370.
- ^ Kishi Y, Fukuyama T, Aratani M, Nakatsubo F, Goto T (Dec 1972). "Synthetic studies on tetrodotoxin and related compounds. IV. Stereospecific total syntheses of DL-tetrodotoxin". Journal of the American Chemical Society. 94 (26): 9219–21. doi:10.1021/ja00781a039. PMID 4642371.
- ^ Taber D (2005-05-02). "Synthesis of (-)-Tetrodotoxin". Organic Chemistry Portal. organic-chemistry.org.
- ^ Ohyabu N, Nishikawa T, Isobe M (Jul 2003). "First asymmetric total synthesis of tetrodotoxin". Journal of the American Chemical Society. 125 (29): 8798–805. doi:10.1021/ja0342998. PMID 12862474.
- ^ Nishikawa T, Urabe D, Isobe M (Sep 2004). "An efficient total synthesis of optically active tetrodotoxin". Angewandte Chemie. 43 (36): 4782–5. doi:10.1002/anie.200460293. PMID 15366086.
- ^ Hinman A, Du Bois J (Sep 2003). "A stereoselective synthesis of (-)-tetrodotoxin". Journal of the American Chemical Society. 125 (38): 11510–1. doi:10.1021/ja0368305. PMID 13129349.
- ^ Chau J, Ciufolini MA (2011). "The chemical synthesis of tetrodoxin: an ongoing quest". Marine Drugs. 9 (10): 2046–74. doi:10.3390/md9102046. PMC 3210618. PMID 22073009.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sato K, Akai S, Yoshimura J (Jul 2013). "Stereocontrolled total synthesis of tetrodotoxin from myo-inositol and D-glucose by three routes: aspects for constructing complex multi-functionalized cyclitols with branched-chain structures". Natural Product Communications. 8 (7): 987–98. PMID 23980434.
- ^ "Material Safety Data Sheet Tetrodotoxin ACC# 01139". Acros Organics N.V.
- ^ "Cyanides (as CN)". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Gilman, Alfred Goodman; Goodman, Louis Sanford; Gilman, Alfred Zack (1980). Goodman & Gilman's The pharmacological Basis of Therapeutics. New York: McGraw-Hill. p. 310. ISBN 0-07-146891-9.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Patockaa, Jiri; Stredab, Ladislav (April 23, 2002). Price, Richard (ed.). "Brief Review of Natural Nonprotein Neurotoxins". ASA Newsletter. 02–2 (89). Applied Science and Analysis inc.: 16–23. ISSN 1057-9419. Retrieved 26 May 2012.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c d e f Clark RF, Williams SR, Nordt SP, Manoguerra AS (1999). "A review of selected seafood poisonings". Undersea & Hyperbaric Medicine. 26 (3): 175–84. PMID 10485519.
- ^ Rang, Humphrey; Ritter, James; Flower, Rod; Henderson, Graeme (2015). Rang & Dale's Pharmacology (8th ed.). Churchill Livingstone. ISBN 9780702053627.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b c "CDC – The Emergency Response Safety and Health Database: Biotoxin: TETRODOTOXIN – NIOSH". www.cdc.gov. Retrieved 2016-01-03.
- ^ a b c Kao CY (Jun 1966). "Tetrodotoxin, saxitoxin and their significance in the study of excitation phenomena". Pharmacological Reviews. 18 (2): 997–1049. PMID 5328391.
- ^ "T8024 Sigma Tetrodotoxin". Catalogue. Sigma-Aldrich. Retrieved 23 August 2015.
- ^ Eric Croddy; James J. Wirtz, eds. (2005). Weapons of Mass Destruction: Chemical and biological weapons. ABC-CLIO. ISBN 9781851094905.
- ^ a b c d Butterton, J.R.; Calderwell, S.B. (1998). "Acute infectious diarrhoea disease and bacterial food poisoning". In Fauci, Anthony S.; Braunwald, Eugene; Isselbacher, Kurt J.; Wilson, Jean D.; Martin, Joseph B.; Kasper, Dennis L.; Hauser, Stephen L.; Longo, Dan L. (eds.). Harrison's principles of internal medicine (14th ed.). New York: McGraw-Hill, Health Professions Division. pp. 796–601. ISBN 0070202915.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b Benzer, Theodore. "Tetrodotoxin Toxicity". Medscape. Retrieved 23 August 2015.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Rivera VR, Poli MA, Bignami GS (Sep 1995). "Prophylaxis and treatment with a monoclonal antibody of tetrodotoxin poisoning in mice". Toxicon. 33 (9): 1231–7. doi:10.1016/0041-0101(95)00060-Y. PMID 8585093.

- ^ Bradley SG, Klika LJ (Jul 1981). "A fatal poisoning from the Oregon rough-skinned newt (Taricha granulosa)". JAMA. 246 (3): 247. doi:10.1001/jama.1981.03320030039026. PMID 7241765.

- ^ McNabb P, Mackenzie L, Selwood A, Rhodes L, Taylor D, Cornelison C (2009). "Review of tetrodotoxins in the sea slug Pleurobranchaea maculata and coincidence of dog deaths along Auckland Beaches" (PDF). Auckland Regional Council Technical Report 2009/108. Cawthron Institute for the Auckland Regional Council.
- ^ Gibson, Eloise (15 August 2009). "Puffer fish toxin blamed for deaths of two dogs". The New Zealand Herald. Retrieved 19 November 2011.
{{cite news}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ a b 危険がいっぱい ふぐの素人料理 [Danger in fugu amateur cuisine] (in Japanese). Tokyo Bureau of Social Welfare and Public Health. Archived from the original on 28 January 2010.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ 自然毒のリスクプロファイル:魚類:フグ毒 [Fish: fugu poison risk profile of natural poison] (in Japanese). 厚生労働省 (Ministry of Health Labour and Welfare (Japan)). Archived from the original on 27 September 2011.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c Yasumoto T, Kao CY (1986). "Tetrodotoxin and the Haitian zombie". Toxicon. 24 (8): 747–9. doi:10.1016/0041-0101(86)90098-x. PMID 3775790.
- ^ a b Kao CY, Yasumoto T (1990). "Tetrodotoxin in "zombie powder"". Toxicon. 28 (2): 129–32. doi:10.1016/0041-0101(90)90330-a. PMID 2339427.
- ^ a b Hines, Terence (May–June 2008). "Zombies and Tetrodotoxin". Skeptical Inquirer. 32 (3): 60–62.
{{cite journal}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Warin, Rosemary H.; Steventon, Glyn B.; Mitchell, Steve C. (2007). Molecules of death. Imperial College Press. p. 390. ISBN 1-86094-814-6.
- ^ Baselt, Randall C. (2008). Disposition of toxic drugs and chemicals in man (8th ed.). Foster City, California: Biomedical Publications. pp. 1521–22. ISBN 978-0-9626523-7-0.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Hagen NA, Lapointe B, Ong-Lam M, Dubuc B, Walde D, Gagnon B, Love R, Goel R, Hawley P, Ngoc AH, du Souich P (Jun 2011). "A multicentre open-label safety and efficacy study of tetrodotoxin for cancer pain". Current Oncology. 18 (3): e109–16. doi:10.3747/co.v18i3.732. PMC 3108870. PMID 21655148.
- ^ Hagen NA, du Souich P, Lapointe B, Ong-Lam M, Dubuc B, Walde D, Love R, Ngoc AH (Apr 2008). "Tetrodotoxin for moderate to severe cancer pain: a randomized, double blind, parallel design multicenter study". Journal of Pain and Symptom Management. 35 (4): 420–9. doi:10.1016/j.jpainsymman.2007.05.011. PMID 18243639.
- ^ Nieto FR, Cobos EJ, Tejada MÁ, Sánchez-Fernández C, González-Cano R, Cendán CM (Feb 2012). "Tetrodotoxin (TTX) as a therapeutic agent for pain". Marine Drugs. 10 (2): 281–305. doi:10.3390/md10020281. PMC 3296997. PMID 22412801.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Stimmel, Barry (2002). "12: Heroin Addiction". Alcoholism, drug addiction, and the road to recovery: life on the edge. New York: Haworth Medical Press. ISBN 0-7890-0553-0.
Tetrodotoxin blocks the sodium currents and is believed to have potential as a potent analgesic and as an effective agent in detoxoification from heroin addiction without withdrawal symptoms and without producing physical dependence
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Song H, Li J, Lu CL, Kang L, Xie L, Zhang YY, Zhou XB, Zhong S (Aug 2011). "Tetrodotoxin alleviates acute heroin withdrawal syndrome: a multicentre, randomized, double-blind, placebo-controlled study". Clinical and Experimental Pharmacology & Physiology. 38 (8): 510–4. doi:10.1111/j.1440-1681.2011.05539.x. PMID 21575032.
- ^ "HHS and USDA Select Agents and Toxins 7 CFR Part 331, 9 CFR Part 121, and 42 CFR Part 73" (PDF). Archived from the original (PDF) on 17 January 2009. Retrieved 17 March 2013.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ^ "Permissible Toxin Amounts". Federal Select Agent Program. United States Centers for Disease Control and Prevention. Retrieved 17 March 2013.
- ^ Miami Vice (1984–1990) Tale of the Goat. IMDb
- ^ Miranda, Kitin (26 November 2014). "Covert Affairs Recap: Starlings of the Slipstream". Movie News Guide. Retrieved 25 July 2015.
{{cite web}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ "Covert Affairs: Starlings of the Slipstream (season 5, episode 12 , original air date 13 November 2014)". USA Networks. 2015. Retrieved 25 July 2015.
- ^ a b Davis, Wade (1985). 'The Serpent and the Rainbow (1st Touchstone ed.). New York: Simon and Schuster. ISBN 978-0671502478.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help) - ^ Hurston, Zora Neale (2009). Reed, Ishmael; Louis, Henry (eds.). Tell my horse: Voodoo and life in Haiti and Jamaica (1st Harper Perennial Modern Classics ed.). New York: Harper Perennial. p. 336. ISBN 978-0061695131.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)
Further reading
- Bane V, Lehane M, Dikshit M, O'Riordan A, Furey A (2014). "Tetrodotoxin: chemistry, toxicity, source, distribution and detection". Toxins. 6 (2): 693–755. doi:10.3390/toxins6020693. PMC 3942760. PMID 24566728.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Lago J, Rodríguez LP, Blanco L, Vieites JM, Cabado AG (2015). "Tetrodotoxin, an Extremely Potent Marine Neurotoxin: Distribution, Toxicity, Origin and Therapeutical Uses". Marine Drugs. 13 (10): 6384–406. doi:10.3390/md13106384. PMC 4626696. PMID 26492253.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - Moczydlowski EG (2013). "The molecular mystique of tetrodotoxin". Toxicon. 63: 165–83. doi:10.1016/j.toxicon.2012.11.026. PMID 23261990.
- Lange WR (1990). "Puffer fish poisoning". American Family Physician. 42 (4): 1029–33. PMID 2220511.
- Nagashima Y, Matsumoto T, Kadoyama K, Ishizaki S, Taniyama S, Takatani T, Arakawa O, Terayama M (2012). "Tetrodotoxin poisoning due to smooth-backed blowfish, Lagocephalus inermis and the toxicity of L. inermis caught off the Kyushu coast, Japan". Shokuhin Eiseigaku Zasshi. Journal of the Food Hygienic Society of Japan. 53 (2): 85–90. doi:10.3358/shokueishi.53.85. PMID 22688023.
- Padera RF, Tse JY, Bellas E, Kohane DS (2006). "Tetrodotoxin for prolonged local anesthesia with minimal myotoxicity". Muscle & Nerve. 34 (6): 747–53. doi:10.1002/mus.20618. PMID 16897761.
- Centers for Disease Control Prevention (CDC) (1996). "Tetrodotoxin poisoning associated with eating puffer fish transported from Japan—California, 1996". Morbidity and Mortality Weekly Report. 45 (19): 389–91. PMID 8609880.
- Cole JB, Heegaard WG, Deeds JR, McGrath SC, Handy SM (2015). "Tetrodotoxin poisoning outbreak from imported dried puffer fish—Minneapolis, Minnesota, 2014". Morbidity and Mortality Weekly Report. 63 (51): 1222–5. PMID 25551594.
- Liu SH, Tseng CY, Lin CC (2015). "Is neostigmine effective in severe pufferfish-associated tetrodotoxin poisoning?". Clinical Toxicology. 53 (1): 13–21. doi:10.3109/15563650.2014.980581. PMID 25410493.
- Rivera VR, Poli MA, Bignami GS (1995). "Prophylaxis and treatment with a monoclonal antibody of tetrodotoxin poisoning in mice". Toxicon. 33 (9): 1231–7. doi:10.1016/0041-0101(95)00060-y. PMID 8585093.
- Chang FC, Spriggs DL, Benton BJ, Keller SA, Capacio BR (1997). "4-Aminopyridine reverses saxitoxin (STX)- and tetrodotoxin (TTX)-induced cardiorespiratory depression in chronically instrumented guinea pigs". Fundamental and Applied Toxicology. 38 (1): 75–88. doi:10.1006/faat.1997.2328. PMID 9268607.
- Ahasan HA, Mamun AA, Karim SR, Bakar MA, Gazi EA, Bala CS (2004). "Paralytic complications of puffer fish (tetrodotoxin) poisoning". Singapore Medical Journal. 45 (2): 73–4. PMID 14985845.
- How CK, Chern CH, Huang YC, Wang LM, Lee CH (2003). "Tetrodotoxin poisoning". The American Journal of Emergency Medicine. 21 (1): 51–4. doi:10.1053/ajem.2003.50008. PMID 12563582.
External links
- Tetrodotoxin at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- Tetrodotoxin: essential data (1999)
- Tetrodotoxin from the Bad Bug Book at the U.S. Food and Drug Administration website
- New York Times, "Whatever Doesn't Kill Some Animals Can Make Them Deadly"
- U.S. National Library of Medicine: Hazardous Substances Databank – Tetrodotoxin
