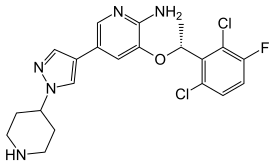
Crizotinib
 | |
| Clinical data | |
|---|---|
| Trade names | Xalkori, others |
| Other names | PF-02341066 1066 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a612018 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 43% |
| Protein binding | 91% |
| Metabolism | Liver (CYP3A4/CYP3A5-mediated) |
| Elimination half-life | 42 hours |
| Excretion | Faeces (63%), urine (22%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.166.440 |
| Chemical and physical data | |
| Formula | C21H22Cl2FN5O |
| Molar mass | 450.34 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Crizotinib, sold under the brand name Xalkori among others, is an anti-cancer medication used for the treatment of non-small cell lung carcinoma (NSCLC).[1][2][3][4] It acts as an ALK (anaplastic lymphoma kinase) and ROS1 (c-ros oncogene 1) inhibitor.[5][6][7]
Medical uses
Crizotinib is indicated for the treatment of metastatic non-small cell lung cancer (NSCLC) or relapsed or refractory, systemic anaplastic large cell lymphoma (ALCL) that is ALK-positive.[1][2]
It is also indicated for the treatment of unresectable, recurrent, or refractory inflammatory anaplastic lymphoma kinase (ALK)-positive myofibroblastic tumors (IMT).[1][8]
Mechanism of action

Crizotinib has an aminopyridine structure, and functions as a protein kinase inhibitor by competitive binding within the ATP-binding pocket of target kinases. About 4% of patients with non-small cell lung carcinoma have a chromosomal rearrangement that generates a fusion gene between EML4 ('echinoderm microtubule-associated protein-like 4') and ALK ('anaplastic lymphoma kinase'), which results in constitutive kinase activity that contributes to carcinogenesis and seems to drive the malignant phenotype.[10] The kinase activity of the fusion protein is inhibited by crizotinib.[10] Patients with this gene fusion are typically younger non-smokers who do not have mutations in either the epidermal growth factor receptor gene (EGFR) or in the K-Ras gene.[10][11] The number of new cases of ALK-fusion NSLC is about 9,000 per year in the U.S. and about 45,000 worldwide.[12][13]
ALK mutations are thought to be important in driving the malignant phenotype in about 15% of cases of neuroblastoma, a rare form of peripheral nervous system cancer that occurs almost exclusively in very young children.[14]
Crizotinib inhibits the c-Met/Hepatocyte growth factor receptor (HGFR) tyrosine kinase, which is involved in the oncogenesis of a number of other histological forms of malignant neoplasms.[15]
Crizotinib is thought to exert its effects through modulation of the growth, migration, and invasion of malignant cells.[15][16] Other studies suggest that crizotinib might also act via inhibition of angiogenesis in malignant tumors.[17]
Society and culture
Legal status
On August 24, 2011, the U.S. Food and Drug Administration approved crizotinib to treat certain late-stage (locally advanced or metastatic) non-small cell lung cancers that express the abnormal anaplastic lymphoma kinase (ALK) gene.[3] Approval required a companion molecular test for the EML4-ALK fusion. In March 2016, the U.S. Food and Drug Administration approved crizotinib in ROS1-positive non-small cell lung cancer.[18]
In October 2012, the European Medicines Agency (EMA) approved the use of crizotinib to treat non-small cell lung cancers that express the abnormal anaplastic lymphoma kinase (ALK) gene.[2][19]
Research
Lung cancer
Crizotinib caused tumors to shrink or stabilize in 90% of 82 patients carrying the ALK fusion gene.[11][12] Tumors shrank at least 30% in 57% of people treated.[12] [20] Most had adenocarcinoma, and had never smoked or were former smokers.[11] They had undergone treatment with an average of three other drugs prior to receiving crizotinib, and only 10% were expected to respond to standard therapy.[11][21] They were given 250 mg crizotinib twice daily for a median duration of six months.[11] Approximately 50% of these patients had at least one side effect, such as nausea, vomiting, or diarrhea.[21] Some responses to crizotinib have lasted up to 15 months.[21]
A Phase III trial, PROFILE 1007,[22] compares crizotinib to standard second line chemotherapy (pemetrexed or taxotere) in the treatment of ALK-positive NSCLC.[23][13][24] Additionally, a phase 2 trial, PROFILE 1005, studies patients meeting similar criteria who have received more than one line of prior chemotherapy.[13]
In February 2016, the J-ALEX phase III study comparing alectinib with crizotinib ALK-positive metastatic NSCLC was terminated early because an interim analysis showed that progression-free survival was longer with alectinib.[25] These results were confirmed in a 2017 analysis.[26]
Lymphomas
In people affected by relapsed or refractory ALK+ anaplastic large cell lymphoma, crizotinib produced objective response rates ranging from 65% to 90% and 3 year progression free survival rates of 60-75%. No relapse of the lymphoma was ever observed after the initial 100 days of treatment. Treatment must be continued indefinitely at present.[27][28][29]
Other cancers
Crizotinib is also being tested in clinical trials of advanced disseminated neuroblastoma.[30]
References
- ^ a b c d "Xalkori- crizotinib capsule". DailyMed. Archived from the original on 9 October 2021. Retrieved 18 April 2021.
- ^ a b c d "Xalkori EPAR". European Medicines Agency (EMA). Archived from the original on 19 April 2021. Retrieved 18 April 2021.
- ^ a b "Drug Approval Package: Xalkori Capsules (crizotinib) NDA #202570". U.S. Food and Drug Administration (FDA). 27 September 2011. Archived from the original on 7 April 2021. Retrieved 18 April 2021.
- ^ "Summary Review for Regulatory Action" (PDF). U.S. Food and Drug Administration (FDA). August 26, 2011. Archived (PDF) from the original on April 6, 2021. Retrieved April 19, 2021.
- ^ Forde PM, Rudin CM (June 2012). "Crizotinib in the treatment of non-small-cell lung cancer". Expert Opinion on Pharmacotherapy. 13 (8): 1195–201. doi:10.1517/14656566.2012.688029. PMID 22594847. S2CID 23715951.
- ^ Roberts PJ (2013). "Clinical use of crizotinib for the treatment of non-small cell lung cancer". Biologics: Targets and Therapy. 7: 91–101. doi:10.2147/BTT.S29026. PMC 3643289. PMID 23671386.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Sahu A, Prabhash K, Noronha V, Joshi A, Desai S (April 2013). "Crizotinib: A comprehensive review". South Asian Journal of Cancer. 2 (2): 91–7. doi:10.4103/2278-330X.110506. PMC 3876666. PMID 24455567.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "FDA approves crizotinib for ALK-positive inflammatory myofibroblastic tumor". U.S. Food and Drug Administration (FDA) (Press release). 14 July 2022. Archived from the original on 14 July 2022. Retrieved 14 July 2022.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ Cui JJ, Tran-Dubé M, Shen H, Nambu M, Kung PP, Pairish M, et al. (September 2011). "Structure based drug design of crizotinib (PF-02341066), a potent and selective dual inhibitor of mesenchymal-epithelial transition factor (c-MET) kinase and anaplastic lymphoma kinase (ALK)". Journal of Medicinal Chemistry. 54 (18): 6342–63. doi:10.1021/jm2007613. PMID 21812414.
- ^ a b c "Maintenance Therapy for Non-Small Cell Lung Cancer". MedscapeCME. 2010-05-12. Archived from the original on 2012-12-06. Retrieved 2010-06-07.
- ^ a b c d e "ALK inhibitor crizotinib has high response rate in patients with ALK-positive NSCLC". HemOncToday. 2010-06-05. Archived from the original on 2020-04-06. Retrieved 2010-06-07.
- ^ a b c Winslow R (2010-06-07). "Advances Come in War on Cancer". The Wall Street Journal. Archived from the original on 2021-10-09. Retrieved 2010-06-07.
- ^ a b c "Pfizer Oncology To Present New Clinical Data From Ten Molecules Across Multiple Tumor Types" (PDF) (Press release). Pfizer Oncology. 2010-05-20. Archived from the original (PDF) on 2010-06-12. Retrieved 2010-06-07.
- ^ Janoueix-Lerosey I, Schleiermacher G, Delattre O (March 2010). "Molecular pathogenesis of peripheral neuroblastic tumors". Oncogene. 29 (11): 1566–79. doi:10.1038/onc.2009.518. PMID 20101209.
- ^ a b Clinical trial number NCT00585195 for "A Study Of Oral PF-02341066, A c-Met/Hepatocyte Growth Factor Tyrosine Kinase Inhibitor, In Patients With Advanced Cancer" at ClinicalTrials.gov
- ^ Christensen JG, Zou HY, Arango ME, Li Q, Lee JH, McDonnell SR, et al. (December 2007). "Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma". Molecular Cancer Therapeutics. 6 (12 Pt 1): 3314–22. doi:10.1158/1535-7163.MCT-07-0365. PMID 18089725.
- ^ Zou HY, Li Q, Lee JH, Arango ME, McDonnell SR, Yamazaki S, et al. (May 2007). "An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms". Cancer Research. 67 (9): 4408–17. doi:10.1158/0008-5472.CAN-06-4443. PMID 17483355.
- ^ "NICE backs Pfizer's Xalkori after squeezing out a new discount - FiercePharma". 18 August 2016. Archived from the original on 8 August 2020. Retrieved 18 August 2016.
- ^ "Xalkori - EMEA/H/C/002489 - T/0059" (PDF). European Medicines Agency. 2012. Archived (PDF) from the original on 2018-10-04. Retrieved 2018-10-22.
- ^ Helwick (2010). "Novel Agent Demonstrates Striking Activity in ALK-positive NSCLC". Archived from the original on 2011-01-28. NB Fig 1.
- ^ a b c "Gene-based lung cancer drug shows promise". NBC News. 2010-05-07. Retrieved 2010-06-07.
- ^ "Crizotinib Clinical Trials – Currently Ongoing and/or Enrolling" (PDF). Fact Sheet. Pfizer. Archived (PDF) from the original on 2016-03-03. Retrieved 2014-08-16.
- ^ Clinical trial number NCT00932451 for "An Investigational Drug, PF-02341066, Is Being Studied In Patients With Advanced Non-Small Cell Lung Cancer With A Specific Gene Profile Involving The Anaplastic Lymphoma Kinase (ALK) Gene" at ClinicalTrials.gov
- ^ Clinical trial number NCT00932893 for "An Investigational Drug, PF-02341066 Is Being Studied Versus Standard Of Care In Patients With Advanced Non-Small Cell Lung Cancer With A Specific Gene Profile Involving The Anaplastic Lymphoma Kinase (ALK) Gene" at ClinicalTrials.gov
- ^ "Chugai's ALK Inhibitor "Alecensa" Trial Stopped Early for Benefit" (PDF). Roche. February 2016. Archived (PDF) from the original on 2016-04-18. Retrieved 2017-12-08.
- ^ "FDA approves Alecensa for ALK-positive metastatic non-small cell lung cancer". Healio. November 2017. Archived from the original on 2017-12-09. Retrieved 2017-12-08.
- ^ Gambacorti-Passerini C, et al. (2010). "Clinical Activity of Crizotinib In Advanced, Chemoresistant ALK+ Lymphoma Patients". Annual Meeting of the American Society of Hematology. Orlando, Florida.
- ^ Gambacorti-Passerini C, Messa C, Pogliani EM (February 2011). "Crizotinib in anaplastic large-cell lymphoma". The New England Journal of Medicine. 364 (8): 775–6. doi:10.1056/NEJMc1013224. PMID 21345110.
- ^ Gambacorti Passerini C, Farina F, Stasia A, Redaelli S, Ceccon M, Mologni L, et al. (February 2014). "Crizotinib in advanced, chemoresistant anaplastic lymphoma kinase-positive lymphoma patients". Journal of the National Cancer Institute. 106 (2): djt378. doi:10.1093/jnci/djt378. PMID 24491302.
- ^ Wood AC, Laudenslager M, Haglund EA, Attiyeh EF, Pawel B, Courtright J, Plegaria J, Christensen JG, Maris JM, Mosse YP (2009). "Inhibition of ALK mutated neuroblastomas by the selective inhibitor PF-02341066". J Clin Oncol. 27 (15s. suppl, abstr 10008b): 10008b. doi:10.1200/jco.2009.27.15_suppl.10008b. Archived from the original on 2014-08-16.
External links
- "Crizotinib". Drug Information Portal. U.S. National Library of Medicine.
- "Crizotinib". NCI Drug Dictionary. National Cancer Institute.
- "Crizotinib". National Cancer Institute. 11 October 2011.
- Clinical trial number NCT00585195 for "A Study Of Oral PF-02341066, A C-Met/Hepatocyte Growth Factor Tyrosine Kinase Inhibitor, In Patients With Advanced Cancer (PROFILE 1001)" at ClinicalTrials.gov
- Clinical trial number NCT00932893 for "An Investigational Drug, PF-02341066 Is Being Studied Versus Standard Of Care In Patients With Advanced Non-Small Cell Lung Cancer With A Specific Gene Profile Involving The Anaplastic Lymphoma Kinase (ALK) Gene" at ClinicalTrials.gov
- Clinical trial number NCT00939770 for "Crizotinib in Treating Younger Patients With Relapsed or Refractory Solid Tumors or Anaplastic Large Cell Lymphoma" at ClinicalTrials.gov
- Clinical trial number NCT01154140 for "A Clinical Trial Testing The Efficacy Of Crizotinib Versus Standard Chemotherapy Pemetrexed Plus Cisplatin Or Carboplatin In Patients With ALK Positive Non Squamous Cancer Of The Lung (PROFILE 1014)" at ClinicalTrials.gov
- Clinical trial number NCT01979536 for "Brentuximab Vedotin or Crizotinib and Combination Chemotherapy in Treating Patients With Newly Diagnosed Stage II-IV Anaplastic Large Cell Lymphoma" at ClinicalTrials.gov
