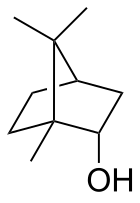
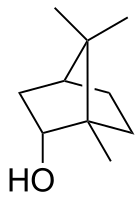
Borneol
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
rel-(1R,2S,4R)-1,7,7-Trimethylbicyclo[2.2.1]heptan-2-ol
| |||
| Other names
1,7,7-Trimethylbicyclo[2.2.1]heptan-2-endo-ol
endo-2-Bornanol | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.006.685 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID
|
|||
| UNII | |||
| UN number | 1312 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C10H18O | |||
| Molar mass | 154.253 g·mol−1 | ||
| Appearance | colorless to white lumps | ||
| Odor | pungent, camphor-like | ||
| Density | 1.011 g/cm3 (20 °C)[1] | ||
| Melting point | 208 °C (406 °F; 481 K) | ||
| Boiling point | 213 °C (415 °F; 486 K) | ||
| slightly soluble (D-form) | |||
| Solubility | soluble in chloroform, ethanol, acetone, ether, benzene, toluene, decalin, tetralin | ||
| −1.26×10−4 cm3/mol | |||
| Hazards | |||
| GHS labelling: | |||

| |||
| Warning | |||
| H228 | |||
| P210, P240, P241, P280, P370+P378 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 65 °C (149 °F; 338 K) | ||
| Safety data sheet (SDS) | External MSDS | ||
| Related compounds | |||
Related compounds
|
Bornane (hydrocarbon) | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Borneol is a bicyclic organic compound and a terpene derivative. The hydroxyl group in this compound is placed in an endo position. Being chiral, borneol exists as two enantiomers. Both (+)-borneol (older name d-borneol) and (−)-borneol (l-borneol) are found in nature.
Reactions
Borneol is easily oxidized to the ketone (camphor). One historical name for borneol is Borneo camphor which explains the name.
Occurrence
The compound was named in 1842 by the French chemist Charles Frédéric Gerhardt.[2] Borneol can be found in several species of Heterotheca,[3] Artemisia, Callicarpa,[4] Dipterocarpaceae, Blumea balsamifera and Kaempferia galanga.[5]
It is one of the chemical compounds found in castoreum. This compound is gathered from the beaver's plant food.[6]
Synthesis
Borneol can be synthesized by reduction of camphor by the Meerwein–Ponndorf–Verley reduction (a reversible process). Reduction of camphor with sodium borohydride (fast and irreversible) gives instead the isomer isoborneol as the kinetically controlled reaction product.
Uses
Whereas d-borneol was the enantiomer that used to be the most readily available commercially, the more commercially available enantiomer now is l-borneol, which also occurs in nature.
Borneol from Dipterocarpus spp. is used in traditional Chinese medicine. An early description is found in the Bencao Gangmu.
Borneol is a component of many essential oils[7] and it is a natural insect repellent.[8] It also generates a TRPM8-mediated cooling sensation similar to menthol.[9]
Laevo-borneol is used as an aroma chemical in perfumery. It has a balsamic odour type with pine, woody and camphoraceous facets.
Use in organic chemistry
Derivatives of isoborneol are used as ligands in asymmetric synthesis:
- (2S)-(−)-3-exo-(morpholino)isoborneol or MIB[10] with a morpholine substituent in the α-hydroxyl position.
- (2S)-(−)-3-exo-(dimethylamino)isoborneol or DAIB[11] with a dimethylamino substituent in the α-hydroxyl position
Toxicology
Borneol may cause eye, skin, and respiratory irritation; it is harmful if swallowed.[12]
Skin Irritation
Borneol has been shown to have little to no irritation effect when applied to the human skin at a small dose.[13] However, a long term exposure to borneol may cause mild irritation.[14]
Phototoxicity and photoallergy
Since Borneol does not absorb UV light at 290 nm to 400 nm, it doesn't have any phototoxic or photoallergic potential.[13]
Derivatives
The bornyl group is a univalent radical C10H17 derived from borneol by removal of hydroxyl and is also known as 2-bornyl.[15] Isobornyl is the univalent radical C10H17 that is derived from isoborneol.[16] The structural isomer fenchol is also a widely used compound derived from certain essential oils.
Bornyl acetate is the acetate ester of borneol.
Notes and references
- ^ Lide, D. R., ed. (2005). CRC Handbook of Chemistry and Physics (86th ed.). Boca Raton (FL): CRC Press. p. 3.56. ISBN 0-8493-0486-5.
- ^ C. Gerhardt (1842) "Sur la transformation de l'essence de valériane en camphre de Bornéo et en camphre des laurinées" (On the transformation of the essence of valerian into Borneo camphor and into laurel camphor), Comptes rendus, 14 : 832-835. From p. 834: "Je donne, par cette raison, à l'hydrogène carboné de l'essence de valériane, le nom de bornéène, et, au camphre lui-même, celui de bornéol." (I give, for this reason [namely, that the compound that Gerhardt had obtained from valerian oil was identical to that obtained by Pelouze from camphor from Borneo], to the hydrocarbon from valerian essence, the name bornéène, and, to camphor itself, that of borneol.)
- ^ Lincoln, D.E., B.M. Lawrence. 1984. "The volatile constituents of camphorweed, Heterotheca subaxillaris". Phytochemistry 23(4): 933-934
- ^ "Species Information". sun.ars-grin.gov. Retrieved 2008-03-02.
- ^ Wong, K. C.; Ong, K. S.; Lim, C. L. (2006). "Composition of the essential oil of rhizomes of Kaempferia Galanga L.". Flavour and Fragrance Journal. 7 (5): 263–266. doi:10.1002/ffj.2730070506.
- ^ The Beaver: Its Life and Impact. Dietland Muller-Schwarze, 2003, page 43 (book at google books)
- ^ Plants containing borneol Archived 2015-09-23 at the Wayback Machine (Dr. Duke's Phytochemical and Ethnobotanical Databases)]
- ^ "Chemical Information". sun.ars-grin.gov. Archived from the original on 2004-11-07. Retrieved 2008-03-02.
- ^ Chen, GL; Lei, M; Zhou, LP; Zeng, B; Zou, F (2016). "Borneol Is a TRPM8 Agonist that Increases Ocular Surface Wetness". PLOS ONE. 11 (7): e0158868. Bibcode:2016PLoSO..1158868C. doi:10.1371/journal.pone.0158868. PMC 4957794. PMID 27448228.
- ^ "(2S)-(−)-3-exo-(MORPHOLINO)ISOBORNEOL [(−)-MIB]". Organic Syntheses. 82: 87. 2005. doi:10.15227/orgsyn.082.0087.
- ^ "(2S)-(−)-3-exo-(DIMETHYLAMINO)ISOBORNEOL [(2S)-(−)-DAIB]". Organic Syntheses. 79: 130. 2002. doi:10.15227/orgsyn.079.0130.
- ^ Material Safety Data Sheet, Fisher Scientific
- ^ a b Bhatia, S.P.; Letizia, C.S.; Api, A.M. (November 2008). "Fragrance material review on borneol". Food and Chemical Toxicology. 46 (11): S77–S80. doi:10.1016/j.fct.2008.06.031. PMID 18640181.
- ^ HAZARDOUS SUBSTANCE FACT SHEET
- ^ "Definition of BORNYL". www.merriam-webster.com.
- ^ "Definition of ISOBORNYL". www.merriam-webster.com.