List of modafinil analogues and derivatives
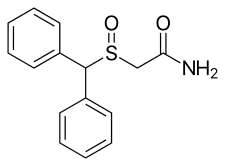
This page lists chemical compounds similar to modafinil, known as modafinil analogues and derivatives. These are structural analogues and derivatives of modafinil, a drug that affects dopamine levels in the brain in an unusual way (atypical dopamine reuptake inhibitor or DRI). Modafinil is a drug that helps keep people awake and alert (wakefulness-promoting agent or "eugeroic").[1][2]
Most of the listed modafinil analogues are drugs that specifically target dopamine reuptake (reabsorption of a neurotransmitter by a neurotransmitter transporter) with stronger effects (selective DRIs with improved potency) compared to modafinil.[3][2][4] The modafinil analogues are of interest in the potential treatment of a condition involving the misuse of stimulant drugs (psychostimulant use disorder or PSUD), as drugs that help increase motivation (pro-motivational agents) to treat motivational disorders,[4][5][6] and for treatment of neurodegenerative diseases such as Alzheimer's disease.[3][2][7][8]
Modafinil analogues acting as DRIs include both drugs similar to modafinil that affect dopamine without causing stimulant effects (atypical modafinil-like non-psychostimulant DRIs) such as flmodafinil and JJC8-016 and drugs that affect dopamine in a way similar to cocaine (classical or typical cocaine-like DRIs) such as JJC8-088. Besides their potential medical use, modafinil analogues, including adrafinil, flmodafinil, fladrafinil, and modafiendz, are also sold online as substances that are believed to improve cognitive functions such as memory and focus (nootropics or "cognitive enhancers").[1][9][10][11]
A limitation of some modafinil analogues such as JJC8-016 is blocking a specific protein (hERG) that can lead to heart problems (potent inhibition of the hERG antitarget and predicted cardiotoxicity).[8][2][12][13][14]
List of modafinil analogues and derivatives
[edit]- Adrafinil (Olmifon, CRL-40028, N-hydroxymodafinil) – prodrug of modafinil[1][9]
- CE-103 – DRI[15][16]
- CE-111 – DRI[6][15][17]
- CE-123 (or as (S)-CE-123) – DRI[4][15][18][19][20]
- CE-125 – DRI[6][15][21]
- CE-158 (or as (S,S)-CE-158) – DRI[4][22][23]
- Cinfenine – abandoned antidepressant and coronary vasodilator (similar in structure to JJC8-016)[24][25]
- CT-005094 (CT-0050904) – atypical DRI[26][27]
- CT-005404 (CT-5404) – atypical DRI[4][28][26][27]
- Fladrafinil (CRL-40941, fluorafinil, bisfluoroadrafinil) – modafinil-like agent, little-characterized (but possible prodrug of flmodafinil)[1][9]
- Flmodafinil (CRL-40940, NLS-4, JBG01-41, bisfluoromodafinil, lauflumide) – atypical DRI[1][9][29][30][31][32]
- (S)-(+)-Flmodafinil (JBG1-048) – atypical DRI[31][33]
- (R)-(–)-Flmodafinil (JBG1-049) – atypical DRI[31][33]
- GC03-04 – DRI[34]
- GC04-38 – DRI[34][35]
- JJC8-016 – poorly selective atypical DRI[1][8][2]
- JJC8-087 – DRI[34][35]
- JJC8-088 – classical/typical or cocaine-like DRI[8][2]
- JJC8-089 – DRI[8][2]
- JJC8-091 – atypical DRI[8][2]
- (S)-MK-26 – atypical DRI[4][36][37]
- Modafiendz (methylbisfluoromodafinil) – modafinil-like agent, little-characterized[1][10][11]
- Modafinil (Provigil, Alertec, Modavigil, CRL-40476) – atypical DRI, other actions[1][38]
- Armodafinil (Nuvigil, CRL-40982, CEP-10952, (R)-modafinil) – atypical DRI[1][39]
- Esmodafinil (CRL-40983, (S)-modafinil) – atypical DRI[1][38]
- Modafinil acid (modafinilic acid, CRL-40467) – inactive metabolite of modafinil[1][40]
- Modafinil sulfone (CRL-41056) – metabolite of modafinil with anticonvulsant effects but otherwise inactive[1][40]
- RDS03-94 (RDS3-094) – atypical DRI[3][8][41]
- RDS04-010 (RDS04-10, RDS4-010) – DRI[42][14]
In addition to the above, further modafinil analogues have also been described.[43][44][45][46][47][48][49][50][51][52][53][54][15][55]
References
[edit]- ^ a b c d e f g h i j k l Sousa A, Dinis-Oliveira RJ (2020). "Pharmacokinetic and pharmacodynamic of the cognitive enhancer modafinil: Relevant clinical and forensic aspects". Subst Abus. 41 (2): 155–173. doi:10.1080/08897077.2019.1700584. PMID 31951804.
- ^ a b c d e f g h Aggarwal S, Mortensen OV (2023). "Discovery and Development of Monoamine Transporter Ligands". Drug Development in Psychiatry. Advances in Neurobiology. Vol. 30. Cham: Springer. pp. 101–129. doi:10.1007/978-3-031-21054-9_4. ISBN 978-3-031-21053-2. PMC 10074400. PMID 36928847.
- ^ a b c Tanda G, Hersey M, Hempel B, Xi ZX, Newman AH (February 2021). "Modafinil and its structural analogues as atypical dopamine uptake inhibitors and potential medications for psychostimulant use disorder". Curr Opin Pharmacol. 56: 13–21. doi:10.1016/j.coph.2020.07.007. PMC 8247144. PMID 32927246.
- ^ a b c d e f Salamone JD, Correa M (January 2024). "The Neurobiology of Activational Aspects of Motivation: Exertion of Effort, Effort-Based Decision Making, and the Role of Dopamine". Annual Review of Psychology. 75 (1): 1–32. doi:10.1146/annurev-psych-020223-012208. hdl:10234/207207. PMID 37788571.
- ^ Treadway MT, Salamone JD (2022). "Vigor, Effort-Related Aspects of Motivation and Anhedonia". Curr Top Behav Neurosci. Current Topics in Behavioral Neurosciences. 58. Cham: 325–353. doi:10.1007/7854_2022_355. ISBN 978-3-031-09682-2. PMID 35505057.
- ^ a b c Shaikh A, Ahmad F, Teoh SL, Kumar J, Yahaya MF (2023). "Targeting dopamine transporter to ameliorate cognitive deficits in Alzheimer's disease". Front Cell Neurosci. 17: 1292858. doi:10.3389/fncel.2023.1292858. PMC 10679733. PMID 38026688.
- ^ Hersey M, Bacon AK, Bailey LG, Coggiano MA, Newman AH, Leggio L, et al. (2021). "Psychostimulant Use Disorder, an Unmet Therapeutic Goal: Can Modafinil Narrow the Gap?". Front Neurosci. 15: 656475. doi:10.3389/fnins.2021.656475. PMC 8187604. PMID 34121988.
- ^ a b c d e f g Newman AH, Ku T, Jordan CJ, Bonifazi A, Xi ZX (January 2021). "New Drugs, Old Targets: Tweaking the Dopamine System to Treat Psychostimulant Use Disorders". Annu Rev Pharmacol Toxicol. 61 (1): 609–628. doi:10.1146/annurev-pharmtox-030220-124205. PMC 9341034. PMID 33411583.
- ^ a b c d Schifano F, Catalani V, Sharif S, Napoletano F, Corkery JM, Arillotta D, et al. (April 2022). "Benefits and Harms of 'Smart Drugs' (Nootropics) in Healthy Individuals". Drugs. 82 (6): 633–647. doi:10.1007/s40265-022-01701-7. PMID 35366192.
[Modafinil] is widely available for online purchase [105] and it is of interest that a range of modafinil derivatives are actively being discussed on web fora, including: adrafinil, fladrafinil, flmodafinil, and N-methyl-4,4′-difluoro-modafinil [8]. Finally, the modafinil R-enantiomer armodafinil, which is being used to improve wakefulness in patients with excessive sleepiness [106], is currently the subject of an anecdotal debate relating to its properties as a [cognitive enhancer] [107].
- ^ a b Napoletano F, Schifano F, Corkery JM, Guirguis A, Arillotta D, Zangani C, et al. (2020). "The Psychonauts' World of Cognitive Enhancers". Frontiers in Psychiatry. 11: 546796. doi:10.3389/fpsyt.2020.546796. PMC 7516264. PMID 33024436.
2-{[bis(4-fluorophenyl)methyl]sulfinyl}-N-methylacetamide is the bis-fluoro-N-methyl analogue of the substance modafinil and is currently marketed by online sellers as a nootropic substance called 'modafiendz'.
- ^ a b Dowling G, Kavanagh PV, Talbot B, O'Brien J, Hessman G, McLaughlin G, et al. (March 2017). "Outsmarted by nootropics? An investigation into the thermal degradation of modafinil, modafinic acid, adrafinil, CRL-40,940 and CRL-40,941 in the GC injector: formation of 1,1,2,2-tetraphenylethane and its tetra fluoro analogue" (PDF). Drug Testing and Analysis. 9 (3): 518–528. doi:10.1002/dta.2142. PMID 27928893.
2-[(Diphenylmethyl)sulfinyl]acetamide (modafinil) is commonly prescribed for the treatment of narcolepsy and increasing popularity and off-label use as a cognitive enhancer resulted in a reputation as an intelligence boosting 'wonder drug'. Common alternatives available from online shops and other retail outlets include 2-[(diphenylmethyl)sulfinyl]-N-hydroxyacetamide (adrafinil), 2-([bis(4-fluorophenyl)methyl]sulfinyl)acetamide (CRL-40,940), 2-([bis(4-fluorophenyl)methyl]sulfinyl)-N-hydroxyacetamide (CRL-40,941) and N-methyl-4,4-difluoro-modafinil (modafiendz), respectively. [...] CRL-40,941 and modafiendz are also wakefulness promoting agents and related to modafinil and adrafinil (Figure 1).
- ^ Rahimi O, Cao J, Lam J, Childers SR, Rais R, Porrino LJ, et al. (March 2023). "The Effects of the Dopamine Transporter Ligands JJC8-088 and JJC8-091 on Cocaine versus Food Choice in Rhesus Monkeys". J Pharmacol Exp Ther. 384 (3): 372–381. doi:10.1124/jpet.122.001363. PMC 9976790. PMID 36507847.
However, JJC8-016 failed cardiac safety tests by exhibiting relatively high affinity at hERG channels; thus, this analogue was abandoned from further development.
- ^ Lee KH, Fant AD, Guo J, Guan A, Jung J, Kudaibergenova M, et al. (September 2021). "Toward Reducing hERG Affinities for DAT Inhibitors with a Combined Machine Learning and Molecular Modeling Approach". J Chem Inf Model. 61 (9): 4266–4279. doi:10.1021/acs.jcim.1c00856. PMC 9593962. PMID 34420294.
From this validation set of DAT inhibitors, we noticed that a pair of analogues with similar chemical structures, JJC8-01646 and JJC8-08813 (Tanimoto similarity = 0.62, Figure S6), have opposite trends of affinities at DAT and hERG. JJC8-088 has ~90-fold higher affinity than JJC8-016 at DAT (Ki = 2.6 and 234.4 nM, respectively), but has ~2-fold lower affinity than JJC8-016 at hERG (IC50 = 0.13 and 0.06 μM, respectively).
- ^ a b Ku TC, Cao J, Won SJ, Guo J, Camacho-Hernandez GA, Okorom AV, et al. (February 2024). "Series of (([1,1'-Biphenyl]-2-yl)methyl)sulfinylalkyl Alicyclic Amines as Novel and High Affinity Atypical Dopamine Transporter Inhibitors with Reduced hERG Activity". ACS Pharmacol Transl Sci. 7 (2): 515–532. doi:10.1021/acsptsci.3c00322. PMC 10863442. PMID 38357284.
- ^ a b c d e Kalaba P, Ilić M, Aher NY, Dragačević V, Wieder M, Zehl M, et al. (January 2020). "Structure-Activity Relationships of Novel Thiazole-Based Modafinil Analogues Acting at Monoamine Transporters". J Med Chem. 63 (1): 391–417. doi:10.1021/acs.jmedchem.9b01938. PMID 31841637.
- ^ Sase A, Aher YD, Saroja SR, Ganesan MK, Sase S, Holy M, et al. (March 2016). "A heterocyclic compound CE-103 inhibits dopamine reuptake and modulates dopamine transporter and dopamine D1-D3 containing receptor complexes". Neuropharmacology. 102: 186–196. doi:10.1016/j.neuropharm.2015.07.039. PMID 26407764.
- ^ Saroja SR, Aher YD, Kalaba P, Aher NY, Zehl M, Korz V, et al. (October 2016). "A novel heterocyclic compound targeting the dopamine transporter improves performance in the radial arm maze and modulates dopamine receptors D1-D3". Behav Brain Res. 312: 127–137. doi:10.1016/j.bbr.2016.06.011. PMID 27288589.
- ^ Nikiforuk A, Kalaba P, Ilic M, Korz V, Dragačević V, Wackerlig J, et al. (2017). "A Novel Dopamine Transporter Inhibitor CE-123 Improves Cognitive Flexibility and Maintains Impulsivity in Healthy Male Rats". Front Behav Neurosci. 11: 222. doi:10.3389/fnbeh.2017.00222. PMC 5711856. PMID 29230168.
- ^ Kristofova M, Aher YD, Ilic M, Radoman B, Kalaba P, Dragacevic V, et al. (May 2018). "A daily single dose of a novel modafinil analogue CE-123 improves memory acquisition and memory retrieval". Behav Brain Res. 343: 83–94. doi:10.1016/j.bbr.2018.01.032. PMID 29410048.
- ^ Rotolo RA, Dragacevic V, Kalaba P, Urban E, Zehl M, Roller A, et al. (2019). "The Novel Atypical Dopamine Uptake Inhibitor (S)-CE-123 Partially Reverses the Effort-Related Effects of the Dopamine Depleting Agent Tetrabenazine and Increases Progressive Ratio Responding". Front Pharmacol. 10: 682. doi:10.3389/fphar.2019.00682. PMC 6611521. PMID 31316379.
- ^ Hussein AM, Aher YD, Kalaba P, Aher NY, Dragačević V, Radoman B, et al. (August 2017). "A novel heterocyclic compound improves working memory in the radial arm maze and modulates the dopamine receptor D1R in frontal cortex of the Sprague-Dawley rat". Behav Brain Res. 332: 308–315. doi:10.1016/j.bbr.2017.06.023. PMID 28629964.
- ^ Rotolo RA, Kalaba P, Dragacevic V, Presby RE, Neri J, Robertson E, et al. (November 2020). "Behavioral and dopamine transporter binding properties of the modafinil analogue (S, S)-CE-158: reversal of the motivational effects of tetrabenazine and enhancement of progressive ratio responding". Psychopharmacology (Berl). 237 (11): 3459–3470. doi:10.1007/s00213-020-05625-6. PMC 7572767. PMID 32770257.
- ^ Ebner K, Sartori SB, Murau R, Kopel F, Kalaba P, Dragačević V, et al. (March 2022). "The Novel Analogue of Modafinil CE-158 Protects Social Memory against Interference and Triggers the Release of Dopamine in the Nucleus Accumbens of Mice". Biomolecules. 12 (4): 506. doi:10.3390/biom12040506. PMC 9033101. PMID 35454095.
- ^ Elks J (2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer US. p. 278. ISBN 978-1-4757-2085-3. Retrieved October 20, 2024.
- ^ "Cinfenine". PubChem. Retrieved October 20, 2024.
- ^ a b Salamone JD, Rotolo RA, Murray F, McNamara B, Presby RE, Yang JH, et al. (November 5, 2018). 323.04 / AAA17 - The novel atypical dopamine transport inhibitors CT-005094 and CT-005404 reverse the effort-related motivational effects of the dopamine depleting agent tetrabenazine. Neuroscience 2018. Society for Neuroscience.
The present studies focused on recently synthesized atypical DAT inhibitors, CT-005094 and CT-005404. These compounds bind to DAT with high selectivity relative to the serotonin and norepinephrine transporters, and can elevate extracellular levels of DA as measured by microdialysis without stimulating DA release. In the present studies, CT-005094 and CT-005404 were assessed for their ability to reverse the effort-related motivational effects of tetrabenazine. Rats were tested using the fixed ratio 5/chow feeding choice test. Tetrabenazine (1.0 mg/kg) shifted choice behaviour, decreasing lever pressing and increasing chow intake. CT-005094 was co-administered at doses ranging from 2.0-16.0 mg/kg IP, and the 8.0 mg/kg dose partially but significantly reversed the effects of tetrabenazine. CT-005404 was orally active, and reversed the effects of tetrabenazine in the dose range of 15.0-30.0 mg/kg PO. Atypical DAT inhibitors such as CT-005094 and CT-005404 offer potential as a new avenue for drug treatment of motivational dysfunctions in humans.
- ^ a b Moscoso M, Sanchez S (2019). "Society for Neuroscience – 48th Annual Meeting. San Diego, California, USA – November 3–7, 2018". Drugs of the Future. 44 (1): 93. doi:10.1358/dof.2019.44.1.2954217.
The assessment of two other modafinil-based atypical DAT inhibitors, CT-005404 and CT-0050904, in the reversal of the [...]
- ^ Rotolo RA, Presby RE, Tracy O, Asar S, Yang JH, Correa M, et al. (February 2021). "The novel atypical dopamine transport inhibitor CT-005404 has pro-motivational effects in neurochemical and inflammatory models of effort-based dysfunctions related to psychopathology". Neuropharmacology. 183: 108325. doi:10.1016/j.neuropharm.2020.108325. PMID 32956676.
- ^ Konofal E (August 2024). "From past to future: 50 years of pharmacological interventions to treat narcolepsy". Pharmacol Biochem Behav. 241: 173804. doi:10.1016/j.pbb.2024.173804. PMID 38852786.
Among these advancements is lauflumide (NLS-4), a forward step from earlier substances initially envisioned by Lafon Laboratories yet not realized (Dowling et al., 2017). Developed by NLS Pharmaceutics AG, lauflumide represents a cutting-edge development as a selective dopamine reuptake inhibitor. It is an enantiomerically pure R-isomer, with an enantiomeric excess exceeding 95 %, of a bis(p-fluoro) phenyl ring-substituted derivative of modafinil, showcasing the innovative work of inventor Eric Konofal (USPTO Patent 2017, US9637447B2) (Konofal, 2017). Unlike modafinil, which induces hepatic enzyme activity with repeated doses, lauflumide does not act as an inducer of cytochrome P450 (CYP) enzymes, including CYP3A4/5. In mouse models, lauflumide has demonstrated potent wake-promoting effects without the risk of hypersomnia rebound (unpublished data). Moreover, the recovery sleep following lauflumide administration is marked by a reduced amount of NREM sleep and delta wave activity, indicating a decreased need for recovery sleep despite extended periods of wakefulness induced by the drug (Luca et al., 201 ).
- ^ Luca G, Bandarabadi M, Konofal E, Lecendreux M, Ferrié L, Figadère B, et al. (2018). "Lauflumide (NLS-4) Is a New Potent Wake-Promoting Compound". Front Neurosci. 12: 519. doi:10.3389/fnins.2018.00519. PMC 6104159. PMID 30158846.
Preliminary findings suggest that NLS-4 is a selective dopamine reuptake inhibitor, blocking (83%) dopamine transporter (DAT), higher than methylphenidate and without deleterious effects on peripheral adrenergic systems involved in hypertension (Study 100014859 CEREP 20/03/14, unpublished data).
- ^ a b c Giancola JB, Bonifazi A, Cao J, Ku T, Haraczy AJ, Lam J, et al. (2020). "Structure-activity relationships for a series of (Bis(4-fluorophenyl)methyl)sulfinylethyl-aminopiperidines and -piperidine amines at the dopamine transporter: Bioisosteric replacement of the piperazine improves metabolic stability". European Journal of Medicinal Chemistry. 208: 112674. doi:10.1016/j.ejmech.2020.112674. ISSN 0223-5234. PMC 7680422. PMID 32947229.
- ^ "Lauflumide - NLS Pharmaceutics Ltd". AdisInsight. January 28, 2024. Retrieved September 17, 2024.
- ^ a b Keighron JD, Giancola JB, Shaffer RJ, DeMarco EM, Coggiano MA, Slack RD, et al. (August 2019). "Distinct effects of (R)-modafinil and its (R)- and (S)-fluoro-analogues on mesolimbic extracellular dopamine assessed by voltammetry and microdialysis in rats". Eur J Neurosci. 50 (3): 2045–2053. doi:10.1111/ejn.14256. PMC 8294075. PMID 30402972.
- ^ a b c Camacho-Hernandez GA, Gopinath A, Okorom AV, Khoshbouei H, Newman AH (February 2024). "Development of a Fluorescently Labeled Ligand for Rapid Detection of DAT in Human and Mouse Peripheral Blood Monocytes". JACS Au. 4 (2): 657–665. doi:10.1021/jacsau.3c00719. PMC 10900201. PMID 38425927.
- ^ a b Hernandez GC, Gopinath A, Okorom A, Khoshbouei H, Newman AH (2024). "Fluorescently Labelled Ligand Allow Detection of DAT in Human and Mouse Peripheral Blood Monocytes by Flow Cytometry". ASPET 2024 Annual Meeting Abstract - Neuropharmacology. American Society for Pharmacology and Experimental Therapeutics. p. 520. doi:10.1124/jpet.520.939780.
- ^ Hersey M, Bartole MK, Jones CS, Newman AH, Tanda G (July 2023). "Are There Prevalent Sex Differences in Psychostimulant Use Disorder? A Focus on the Potential Therapeutic Efficacy of Atypical Dopamine Uptake Inhibitors". Molecules. 28 (13): 5270. doi:10.3390/molecules28135270. PMC 10343811. PMID 37446929.
- ^ a b Wisor J (October 2013). "Modafinil as a catecholaminergic agent: empirical evidence and unanswered questions". Front Neurol. 4: 139. doi:10.3389/fneur.2013.00139. PMC 3791559. PMID 24109471.
- ^ Garnock-Jones KP, Dhillon S, Scott LJ (September 2009). "Armodafinil". CNS Drugs. 23 (9): 793–803. doi:10.2165/11203290-000000000-00000. PMID 19689169.
- ^ a b Robertson P, Hellriegel ET (2003). "Clinical pharmacokinetic profile of modafinil". Clin Pharmacokinet. 42 (2): 123–137. doi:10.2165/00003088-200342020-00002. PMID 12537513.
- ^ Ecevitoglu A, Meka N, Rotolo RA, Edelstein GA, Srinath S, Beard KR, et al. (July 2024). "Potential therapeutics for effort-related motivational dysfunction: assessing novel atypical dopamine transport inhibitors". Neuropsychopharmacology. 49 (8): 1309–1317. doi:10.1038/s41386-024-01826-1. PMC 11224370. PMID 38429498.
- ^ Lee KH, Camacho-Hernandez GA, Newman AH, Shi L (June 17, 2024). "The Structural Basis of the Activity Cliff in Modafinil-Based Dopamine Transporter Inhibitors". Biomolecules. 14 (6): 713. doi:10.3390/biom14060713. ISSN 2218-273X. PMC 11202288. PMID 38927116.
- ^ Hsin LW, Prisinzano T, Wilkerson CR, Dersch CM, Horel R, Jacobson AE, et al. (February 2003). "Synthesis and dopamine transporter affinity of chiral 1-[2-[bis(4-fluorophenyl)methoxy]ethyl]-4-(2-hydroxypropyl)piperazines as potential cocaine abuse therapeutic agents". Bioorg Med Chem Lett. 13 (3): 553–556. doi:10.1016/s0960-894x(02)00940-x. PMID 12565970.
- ^ De Risi C, Ferraro L, Pollini GP, Tanganelli S, Valente F, Veronese AC (December 2008). "Efficient synthesis and biological evaluation of two modafinil analogues". Bioorg Med Chem. 16 (23): 9904–9910. doi:10.1016/j.bmc.2008.10.027. PMID 18954992.
- ^ Cao J, Prisinzano TE, Okunola OM, Kopajtic T, Shook M, Katz JL, et al. (October 2010). "Structure-Activity Relationships at the Monoamine Transporters for a Novel Series of Modafinil (2-[(diphenylmethyl)sulfinyl]acetamide) Analogues". ACS Med Chem Lett. 2 (1): 48–52. doi:10.1021/ml1002025. PMC 3041981. PMID 21344069.
- ^ Dunn D, Hostetler G, Iqbal M, Messina-McLaughlin P, Reiboldt A, Lin YG, et al. (March 2012). "Wake-promoting agents: search for next generation modafinil: part I". Bioorg Med Chem Lett. 22 (6): 2312–2314. doi:10.1016/j.bmcl.2011.12.099. PMID 22264475.
- ^ Dunn D, Hostetler G, Iqbal M, Messina-McLaughlin P, Reiboldt A, Lin YG, et al. (March 2012). "Wake-promoting agents: search for next generation modafinil: part II". Bioorg Med Chem Lett. 22 (6): 2315–2317. doi:10.1016/j.bmcl.2012.01.064. PMID 22341942.
- ^ Dunn D, Hostetler G, Iqbal M, Marcy VR, Lin YG, Jones B, et al. (June 2012). "Wake promoting agents: search for next generation modafinil, lessons learnt: part III". Bioorg Med Chem Lett. 22 (11): 3751–3753. doi:10.1016/j.bmcl.2012.04.031. PMID 22546675.
- ^ Louvet P, Schweizer D, Gourdel ME, Riguet E, Yue C, Marcy VR, et al. (August 2012). "Wake-promoting agents: search for next generation modafinil: part IV". Eur J Med Chem. 54: 949–951. doi:10.1016/j.ejmech.2012.05.038. PMID 22749190.
- ^ Lesur B, Lin YG, Marcy VR, Aimone LD, Gruner J, Bacon ER, et al. (March 2013). "Aryl-heteroaryl derivatives as novel wake-promoting agents". Chem Biol Drug Des. 81 (3): 429–432. doi:10.1111/cbdd.12083. PMID 23110414.
- ^ Okunola-Bakare OM, Cao J, Kopajtic T, Katz JL, Loland CJ, Shi L, et al. (February 2014). "Elucidation of structural elements for selectivity across monoamine transporters: novel 2-[(diphenylmethyl)sulfinyl]acetamide (modafinil) analogues". J Med Chem. 57 (3): 1000–1013. doi:10.1021/jm401754x. PMC 3954497. PMID 24494745.
- ^ Yoham J (May 12, 2015). "High Potency Eugeroics—Wake-Promoting Agents Beyond Modafinil". Biomedical Engineering. Retrieved September 25, 2024.
- ^ Cao J, Slack RD, Bakare OM, Burzynski C, Rais R, Slusher BS, et al. (December 2016). "Novel and High Affinity 2-[(Diphenylmethyl)sulfinyl]acetamide (Modafinil) Analogues as Atypical Dopamine Transporter Inhibitors". J Med Chem. 59 (23): 10676–10691. doi:10.1021/acs.jmedchem.6b01373. PMC 5161041. PMID 27933960.
- ^ Kalaba P, Aher NY, Ilić M, Dragačević V, Wieder M, Miklosi AG, et al. (November 2017). "Heterocyclic Analogues of Modafinil as Novel, Atypical Dopamine Transporter Inhibitors". J Med Chem. 60 (22): 9330–9348. doi:10.1021/acs.jmedchem.7b01313. PMID 29091428.
- ^ Kalaba P, Pacher K, Neill PJ, Dragacevic V, Zehl M, Wackerlig J, et al. (September 2023). "Chirality Matters: Fine-Tuning of Novel Monoamine Reuptake Inhibitors Selectivity through Manipulation of Stereochemistry". Biomolecules. 13 (9): 1415. doi:10.3390/biom13091415. PMC 10527105. PMID 37759815.
