Phendimetrazine
 | |
| Clinical data | |
|---|---|
| Trade names | Bontril |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Peak plasma levels occur within 1 to 3 hours. Absorption is usually complete by 4 to 6 hours |
| Metabolism | Hepatic |
| Elimination half-life | 19-24 hours |
| Excretion | Urinary elimination |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.010.186 |
| Chemical and physical data | |
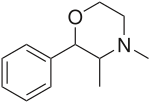
| Formula | C12H17NO |
| Molar mass | 191.27 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Phendimetrazine (Bontril, Adipost, Anorex-SR, Appecon, Melfiat, Obezine, Phendiet, Plegine, Prelu-2, Statobex) is a stimulant drug of the morpholine chemical class used as an appetite suppressant.[1]
Pharmacology
Phendimetrazine functions as a prodrug to phenmetrazine, and approximately 30% of any given oral dose is converted into it. Phendimetrazine can essentially be thought of as an extended release and less abusable version of phenmetrazine. Phenmetrazine (and therefore phendimetrazine as well) acts as a norepinephrine-dopamine releasing agent (NDRA).[2]
Its structure incorporates the backbone of methamphetamine, a potent CNS stimulant. Whilst adding an N-methyl group to the structure of amphetamine significantly increases its potency and bioavailability, in the case of phendimetrazine the added methyl group results in the compound becoming virtually inactive, at least until the methyl group is removed by the body's metabolism. This results in a steady, continued activation of the drug in the body, both lowering abuse potential and allowing for once-daily administration.
Legality
Phendimetrazine is as potent as amphetamine, and is a Schedule III drug under the Convention on Psychotropic Substances. In the United States, phendimetrazine is a Schedule III controlled substance under the Uniform Controlled Substances Act of 1970.
According to the "List of psychotropic substances under international control", phendimetrazine is a Schedule IV controlled substance.[3] Phendimetrazine is listed as a Schedule III substance under the U.S. Controlled Substance Act in the 2007 Drug Identification Bible, pg 615 and under Section 11056(b)(6) California Health and Safety Code.
See also
- 2-Phenyl-3,6-dimethylmorpholine
- 3-Fluorophenmetrazine
- Fenbutrazate
- G-130
- Morazone
- Manifaxine
- Radafaxine
- Phenmetrazine
- Fenmetramide
References
- ^ Landau D, Jackson J, Gonzalez G (2008). "A case of demand ischemia from phendimetrazine". Cases J. 1 (1): 105. doi:10.1186/1757-1626-1-105. PMC 2531092. PMID 18710555.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Rothman RB, Baumann MH (2006). "Therapeutic potential of monoamine transporter substrates". Current Topics in Medicinal Chemistry. 6 (17): 1845–59. doi:10.2174/156802606778249766. PMID 17017961.
- ^ List of psychotropic substances under international control
