Norgestrel
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ovral, others |
| Other names | dl-Norgestrel; DL-Norgestrel; (±)-Norgestrel; WY-3707; SH-70850; SH-850; FH 122-A; rac-13-Ethyl-17α-ethynyl-19-nortestosterone; rac-13-Ethyl-17α-ethynylestr-4-en-17β-ol-3-one |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| MedlinePlus | a602008 |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.026.758 |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.453 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Norgestrel, sold under the brand name Ovral among others, is a progestin medication which is used in birth control pills and in menopausal hormone therapy.[1][2][3][4] It is available both in combination with an estrogen and alone.[5] It is taken by mouth.[3][4]
Side effects of norgestrel include menstrual irregularities, headaches, nausea, breast tenderness, mood changes, acne, increased hair growth, and others.[citation needed] Norgestrel is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[4] It has weak androgenic activity and no other important hormonal activity.[4]
Norgestrel was patented in 1961 and came into medical use, specifically in birth control pills, in 1966.[6][7][8] It was subsequently introduced for use in menopausal hormone therapy as well.[5] Norgestrel is sometimes referred to as a "second-generation" progestin.[9] It is marketed widely throughout the world.[5][2] Norgestrel is available as a generic medication.[10] In 2017, the version with ethinylestradiol was the 284th most commonly prescribed medication in the United States, with more than one million prescriptions.[11][12]
Medical uses
Norgestrel is used in combination with ethinylestradiol or quinestrol in combined birth control pills, alone in progestogen-only birth control pills, and in combination with estradiol or conjugated estrogens in menopausal hormone therapy.[5] It has also been used as an emergency contraceptive in the Yuzpe regimen.[13]
Side effects
Pharmacology
Pharmacodynamics
Norgestrel is a progestogen, or an agonist of the progesterone receptor.[4] The biological activity of norgestrel lies in the levo enantiomer, levonorgestrel, whereas the dextro isomer is inactive.[4] As such, norgestrel is identical in its hormonal activity to levonorgestrel except that it is half as potent by weight.[4] Levonorgestrel, and by extension norgestrel, have some androgenic activity, but no estrogenic, antimineralocorticoid, or glucocorticoid activity.[4]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|
| Levonorgestrel | 150–162 | 34a, 45 | 0 | 1–8 | 17–75 | 50 | 0 |
| 5α-Dihydrolevonorgestrel | 50 | 38a | 0 | ? | ? | ? | ? |
| 3α,5α-Tetrahydrolevonorgestrel | ? | ? | 0.4 | ? | ? | ? | ? |
| 3β,5α-Tetrahydrolevonorgestrel | ? | ? | 2.4 | ? | ? | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone (a = mibolerone) for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources: See template. | |||||||
The ovulation-inhibiting dose of norgestrel appears to be greater than 75 μg/day, as ovulation occurred in 50 to 75% of cycles with this dosage of norgestrel in studies.[14] The ovulation-inhibiting dosage of levonorgestrel, which is twice as potent as norgestrel, is approximately 50 to 60 μg/day.[4][15][14] One review lists the ovulation-inhibiting dose of norgestrel as 100 μg/day.[16] The endometrial transformation dose of norgestrel is listed as 12 mg per cycle and the menstrual delay test dose of norgestrel is listed as 0.5 to 2 mg/day.[16][17]
Pharmacokinetics
The pharmacokinetics of norgestrel have been reviewed.[18]
Chemistry
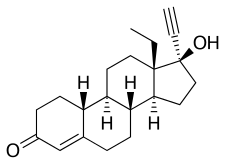
Norgestrel, also known as rac-13-ethyl-17α-ethynyl-19-nortestosterone or as rac-13-ethyl-17α-ethynylestr-4-en-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone.[1][2] It is a racemic mixture of stereoisomers dextronorgestrel (the C13α isomer; l-norgestrel, L-norgestrel, or (+)-norgestrel) and levonorgestrel (the C13β isomer; d-norgestrel, D-norgestrel, or (–)-norgestrel), the former of which is inactive (making norgestrel exactly half as potent as levonorgestrel).[19][20] Norgestrel is more specifically a derivative of norethisterone (17α-ethynyl-19-nortestosterone) and is a member of the gonane (18-methylestrane) subgroup of the 19-nortestosterone family of progestins.[21]
Synthesis
Chemical syntheses of norgestrel have been published.[18]
History
Norgestrel was first introduced, as a birth control pill in combination with ethinylestradiol, under the brand name Eugynon in Germany in 1966.[6][7] It was subsequently marketed as a combined birth control pill with ethinylestradiol in the United States under the brand name Ovral in 1968, and was marketed in many other countries as well.[22][23][5]
Society and culture
Generic names
Norgestrel is the generic name of the drug and its INN, USAN, USP, BAN, DCF, DCIT, and JAN.[1][2][3][5] It is also known as dl-norgestrel, DL-norgestrel, or (±)-norgestrel.[1][2][3][5]
Brand names
Norgestrel has been marketed under a variety of brand names including Cyclacur, Cryselle, Cyclo-Progynova, Duoluton, Elinest, Eugynon, Microgynon, Lo/Ovral, Low-Ogestrel, Logynon, Microlut, Minicon, Nordette, Neogest, Ogestrel, Ovral, Ovran, Ovranette, Ovrette, Planovar, Prempak, Progyluton, and Trinordiol among others.[1][2][5][22]
See also
References
- ^ a b c d e J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 887–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 751–. ISBN 978-3-88763-075-1.
- ^ a b c d I.K. Morton; Judith M. Hall (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 202–. ISBN 978-94-011-4439-1.
- ^ a b c d e f g h i Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. S2CID 24616324.
- ^ a b c d e f g h "Norgestrel - brand name list from". Drugs.com. Retrieved 2022-09-17.
- ^ a b Teresa Ortiz-Gómez; María Jesús Santesmases (22 April 2016). Gendered Drugs and Medicine: Historical and Socio-Cultural Perspectives. Taylor & Francis. pp. 175–. ISBN 978-1-317-12981-3.
The 1966 marketing campaign for Schering's second contraceptive, Eugynon, [...] (Schering AG Berline 1966, 11). [...] In 1970 [Schering] had already conducted an opinion poll among doctors in the run up to the marketing campaign for the newly introduced Neogynon. [...]
- ^ a b W. Gerhard Pohl (2004). Die wissenschaftliche Welt von gestern: die Preisträger des Ignaz L. Lieben-Preises 1865-1937 und des Richard Lieben-Preises 1912-1928 : ein Kapitel österreichischer Wissenschaftsgeschichte in Kurzbiografien. Böhlau Verlag Wien. pp. 150–. ISBN 978-3-205-77303-0.
[The contraceptive EUGYNON is launched in 1966. NEOGYNON follows in 1970.]
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 479. ISBN 9783527607495.
- ^ Howard J.A. Carp (9 April 2015). Progestogens in Obstetrics and Gynecology. Springer. p. 112. ISBN 978-3-319-14385-9.
- ^ "Generic Lo/Ovral-28 Availability".
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 11 April 2020.
- ^ "Ethinyl Estradiol; Norgestrel - Drug Usage Statistics". ClinCalc. Retrieved 11 April 2020.
- ^ "A Multicenter Clinical Investigation Employing Ethinyl Estradiol Combined with dl-Norgestrel as Postcoital Contraceptive Agent". Fertility and Sterility. 37 (4): 508–513. 1982. doi:10.1016/s0015-0282(16)46157-1. PMID 7040117.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b Endrikat J, Gerlinger C, Richard S, Rosenbaum P, Düsterberg B (December 2011). "Ovulation inhibition doses of progestins: a systematic review of the available literature and of marketed preparations worldwide". Contraception. 84 (6): 549–57. doi:10.1016/j.contraception.2011.04.009. PMID 22078182.
- ^ Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, Thijssen JH (December 2003). "Classification and pharmacology of progestins". Maturitas. 46 Suppl 1: S7–S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- ^ a b Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- ^ Freimut A. Leidenberger; Thomas Strowitzki; Olaf Ortmann (29 August 2009). Klinische Endokrinologie für Frauenärzte. Springer-Verlag. pp. 225, 227. ISBN 978-3-540-89760-6.
- ^ a b Die Gestagene. Springer-Verlag. 27 November 2013. pp. 16–17, 284–. ISBN 978-3-642-99941-3.
- ^ Brian K. Alldredge; Robin L. Corelli; Michael E. Ernst (1 February 2012). Koda-Kimble and Young's Applied Therapeutics: The Clinical Use of Drugs. Lippincott Williams & Wilkins. pp. 1072–. ISBN 978-1-60913-713-7.
- ^ J.P. Lavery; J.S. Sanfilippo (6 December 2012). Pediatric and Adolescent Obstetrics and Gynecology. Springer Science & Business Media. pp. 248–. ISBN 978-1-4612-5064-7.
- ^ Stefan Offermanns; W. Rosenthal (14 August 2008). Encyclopedia of Molecular Pharmacology. Springer Science & Business Media. pp. 390–. ISBN 978-3-540-38916-3.
- ^ a b William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 2935–. ISBN 978-0-8155-1856-3.
- ^ Lara Marks (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 73–. ISBN 978-0-300-16791-7.
