Oleamide
| |
| Names | |
|---|---|
| IUPAC name
(Z)-Octadec-9-enamide
| |
| Other names
Oleylamide
9-Octadecenamide (Z)-9-Octadecenamide 9,10-Octadecenoamide Oleic acid amide Cis-9,10-octadecenoamide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.005.550 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H35NO | |
| Molar mass | 281.484 g·mol−1 |
| Appearance | Creamy solid[1] |
| Density | 0.879 g/cm3 |
| Melting point | 70 °C (158 °F; 343 K)[2][3] |
| Boiling point | > 200 °C (392 °F; 473 K)[1] |
| Insoluble[1] | |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Flash point | > 200 °C (392 °F; 473 K)[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
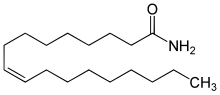
Oleamide is an organic compound with the formula CH3(CH2)7CH=CH(CH2)7CONH2(.[4] It is the amide derived from the fatty acid oleic acid. It is a colorless waxy solid and occurs in nature. Sometimes labeled as a fatty acid primary amide (FAPA), it is biosynthesized from N-oleoylglycine.[5]
Biochemical and medical aspects
In terms of natural occurrence, oleamide was first detected in human plasma. It was later shown to accumulate in the cerebrospinal fluid during sleep deprivation and induces sleep in animals.[5][6]
It has been considered as a potential treatment for mood and sleep disorders, as well as cannabinoid-regulated depression.[7][8]
In terms of its sleep inducing effects, it is speculated that oleamide interacts with multiple neurotransmitter systems.[9]
Other occurrences
Synthetic oleamide has a variety of industrial uses including as a slip agent, a lubricant, and a corrosion inhibitor.[10]
Oleamide was found to be leaking out of polypropylene plastics in laboratory experiments, affecting experimental results.[11] Since polypropylene is used in a wide number of food containers such as those for yogurt, the problem is being studied.[12]
Oleamide is "one of the most frequent non-cannabinoid ingredients associated with Spice products."[13] Analysis of 44 products synthetic cannabinoid revealed oleamide in 7 of the products tested.[14]
See also
References
- ^ a b c d Oleamide at chemicalland21.com
- ^ "Oleamide CAS#: 301-02-0".
- ^ "(9Z)-9-Octadecenamide | C18H35NO | ChemSpider".
- ^ "Oleamide".
- ^ a b McKinney, Michele K.; Cravatt, Benjamin F. (June 2005). "Structure and Function of Fatty Acid Amide Hydrolase". Annual Review of Biochemistry. 74 (1): 411–432. doi:10.1146/annurev.biochem.74.082803.133450. PMID 15952893.
- ^ Cravatt, B.; Prospero-Garcia, O; Siuzdak, G; Gilula, N.; Henriksen, S.; Boger, D.; Lerner, R. (9 June 1995). "Chemical characterization of a family of brain lipids that induce sleep". Science. 268 (5216): 1506–1509. Bibcode:1995Sci...268.1506C. doi:10.1126/science.7770779. PMID 7770779.
- ^ Methods of treating anxiety and mood disorders with oleamide – US Patent 6359010 Archived 2011-06-12 at the Wayback Machine
- ^ Mechoulam, Raphael; Fride, Ester; Hanu, Lumir; Sheskin, Tzviel; Bisogno, Tiziana; Di Marzo, Vincenzo; Bayewitch, Michael; Vogel, Zvi (September 1997). "Anandamide may mediate sleep induction". Nature. 389 (6646): 25–26. Bibcode:1997Natur.389R..25M. doi:10.1038/37891. PMID 9288961.
- ^ Fedorova, I; Hashimoto, A; Fecik, RA; Hedrick, MP; Hanus, LO; Boger, DL; Rice, KC; Basile, AS (October 2001). "Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems". The Journal of Pharmacology and Experimental Therapeutics. 299 (1): 332–42. PMID 11561096.
- ^ Surfactants : Westco Oleamide a Slip Agent In Polyethylene Films Archived January 27, 2007, at the Wayback Machine
- ^ McDonald, G. R.; Hudson, A. L.; Dunn, S. M. J.; You, H.; Baker, G. B.; Whittal, R. M.; Martin, J. W.; Jha, A.; Edmondson, D. E.; Holt, A. (7 November 2008). "Bioactive Contaminants Leach from Disposable Laboratory Plasticware". Science. 322 (5903): 917–917. Bibcode:2008Sci...322..917M. doi:10.1126/science.1162395. PMID 18988846.
- ^ Mittelstaedt, Martin (6 November 2008). "Researchers Raise Alarm After Chemical Leak Found In Common Plastic". Globe and Mail. Retrieved 10 June 2013.
- ^ Fattore, Liana; Fratta, Walter (21 September 2011). "Beyond THC: The New Generation of Cannabinoid Designer Drugs". Frontiers in Behavioral Neuroscience. 5: 60. doi:10.3389/fnbeh.2011.00060. PMC 3187647. PMID 22007163.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Uchiyama, Nahoko; Kikura-Hanajiri, Ruri; Ogata, Jun; Goda, Yukihiro (May 2010). "Chemical analysis of synthetic cannabinoids as designer drugs in herbal products". Forensic Science International. 198 (1–3): 31–38. doi:10.1016/j.forsciint.2010.01.004. PMID 20117892.

