Mepacrine
This article needs additional citations for verification. (August 2024) |
 | |
| Clinical data | |
|---|---|
| Trade names | Atabrine, Atebrin |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Pharmacokinetic data | |
| Protein binding | 80–90% |
| Elimination half-life | 5–14 days |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.001.371 |
| Chemical and physical data | |
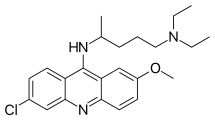
| Formula | C23H30ClN3O |
| Molar mass | 399.96 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Mepacrine, also called quinacrine or by the trade names Atabrine or Atebrin, is a medication with several uses. It is related to chloroquine and mefloquine. Although formerly available from compounding pharmacies, as of August 2020 it is totally unavailable in the United States.[1]
Medical uses
[edit]
The main uses of mepacrine are as an antiprotozoal, antirheumatic, and an intrapleural sclerosing agent.[2]
Mepacrine is used off label as a primary antimicrobial agent for patients with metronidazole-resistant giardiasis and patients who should not receive or cannot tolerate metronidazole. Giardiasis with a high level of drug resistance may even require a combination of mepacrine and metronidazole to cure.[2]
Mepacrine is also used off-label for the treatment of systemic lupus erythematosus,[3] indicated in the treatment of discoid and subcutaneous lupus manifestations, particularly in patients who are unable to take hydroxychloroquine[2]
As an sclerosing agent, it is used as pneumothorax prophylaxis in patients at high risk of recurrence, e.g., in those with cystic fibrosis.[2]
Mepacrine is not the drug of choice because side effects are common, including toxic psychosis, and may cause permanent damage. See mefloquine for more information.
In addition to medical applications, mepacrine is an effective in vitro research tool for the epifluorescent visualization of cells, especially platelets. Mepacrine is a green fluorescent dye taken up by most cells. Platelets store mepacrine in dense granules.[4]
Mechanism
[edit]Its mechanism of action against protozoa is uncertain, but it is thought to act against the protozoan's cell membrane. It is known to act as a histamine N-methyltransferase inhibitor. It also inhibits NF-κB and activates p53.
History
[edit]Antiprotozoal
[edit]
Mepacrine was initially approved in the 1930s as an antimalarial drug. It was used extensively during the second World War by Allied forces fighting in North Africa and the Far East to prevent malaria.[5]
This antiprotozoal is also approved for the treatment of giardiasis (an intestinal parasite),[6] and has been researched as an inhibitor of phospholipase A2.
Scientists at Bayer in Germany first synthesised mepacrine in 1931. The product was one of the first synthetic substitutes for quinine although later superseded by chloroquine.
Anthelmintics
[edit]In addition it has been used for treating tapeworm infections.[7]
Creutzfeldt–Jakob disease
[edit]Mepacrine has been shown to bind to the prion protein and prevent the formation of prion aggregates in vitro,[8] and full clinical trials of its use as a treatment for Creutzfeldt–Jakob disease are under way in the United Kingdom and the United States. Small trials in Japan have reported improvement in the condition of patients with the disease,[9] although other reports have shown no significant effect,[10] and treatment of scrapie in mice and sheep has also shown no effect.[11][12] Possible reasons for the lack of an in vivo effect include inefficient penetration of the blood–brain barrier, as well as the existence of drug-resistant prion proteins that increase in number when selected for by treatment with mepacrine.[13]
Non-surgical sterilization for women
[edit]The use of mepacrine for non-surgical sterilization for women has also been studied. The first report of this method claimed a first year failure rate of 3.1%.[14] However, despite a multitude of clinical studies on the use of mepacrine and female sterilization, no randomized, controlled trials have been reported to date and there is some controversy over its use.[2]
Pellets of mepacrine are inserted through the cervix into a woman's uterine cavity using a preloaded inserter device, similar in manner to IUCD insertion. The procedure is undertaken twice, first in the proliferative phase, 6 to 12 days following the first day of the menstrual cycle and again one month later. The sclerosing effects of the drugs at the utero-tubal junctions (where the Fallopian tubes enter the uterus) results in scar tissue forming over a six-week interval to close off the tubes permanently.
In the United States, this method has undergone Phase I clinical testing. The FDA has waived the necessity for Phase II clinical trials because of the extensive data pertaining to other uses of mepacrine. The next step in the FDA approval process in the United States is a Phase III large multi-center clinical trial. The method is currently used off-label.
Many peer reviewed studies suggest that[15] mepacrine sterilization (QS) is potentially safer than surgical sterilization.[16][17] Nevertheless, in 1998 the Supreme Court of India banned the import or use of the drug, allegedly based on reports that it could cause cancer or ectopic pregnancies.[18]
Skin dye
[edit]During the Second Sino-Japanese War, American Sino-American Cooperative Organization operatives yellowed their skin using mepacrine tablets in order to more closely match the skin color of their Chinese peers.[19]
See also
[edit]References
[edit]- ^ "Quinacrine Shortage & What the ACR Is Doing about It". 13 March 2019 [8 February 2019]. Retrieved 24 August 2020.
- ^ a b c d e Drugs.com: Quinacrine. Retrieved on August 24, 2009.
- ^ Toubi E, Kessel A, Rosner I, Rozenbaum M, Paran D, Shoenfeld Y (2006). "The reduction of serum B-lymphocyte activating factor levels following quinacrine add-on therapy in systemic lupus erythematosus". Scand. J. Immunol. 63 (4): 299–303. doi:10.1111/j.1365-3083.2006.01737.x. PMID 16623930.
- ^ Wall JE, Buijs-Wilts M, Arnold JT, et al. (1995). "A flow cytometric assay using mepacrine for study of uptake and release of platelet dense granule contents". Br. J. Haematol. 89 (2): 380–385. doi:10.1111/j.1365-2141.1995.tb03315.x. PMID 7873389. S2CID 24132625.
- ^ Baird JK (2011). "Resistance to chloroquine unhinges vivax malaria therapeutics". Antimicrob. Agents Chemother. 55 (5): 1827–1830. doi:10.1128/aac.01296-10. PMC 3088196. PMID 21383088.
- ^ Canete R, Escobedo AA, Gonzalez ME, Almirall P (2006). "Randomized clinical study of five days apostrophe therapy with mebendazole compared to quinacrine in the treatment of symptomatic giardiasis in children". World J. Gastroenterol. 12 (39): 6366–70. doi:10.3748/wjg.v12.i39.6366. PMC 4088148. PMID 17072963.
- ^ "quinacrine" at Dorland's Medical Dictionary
- ^ Doh-Ura K, Iwaki T, Caughey B (May 2000). "Lysosomotropic Agents and Cysteine Protease Inhibitors Inhibit Scrapie-Associated Prion Protein Accumulation". J Virol. 74 (10): 4894–7. doi:10.1128/JVI.74.10.4894-4897.2000. PMC 112015. PMID 10775631.
- ^ Kobayashi Y, Hirata K, Tanaka H, Yamada T (July 2003). "[Quinacrine administration to a patient with Creutzfeldt–Jakob disease who received a cadaveric dura mater graft--an EEG evaluation]". Rinsho Shinkeigaku. 43 (7): 403–8. PMID 14582366.
- ^ Haïk S, Brandel J, Salomon D, Sazdovitch V, Delasnerie-Lauprêtre N, Laplanche J, Faucheux B, Soubrié C, Boher E, Belorgey C, Hauw J, Alpérovitch A (28 December 2004). "Compassionate use of quinacrine in Creutzfeldt–Jakob disease fails to show significant effects". Neurology. 63 (12): 2413–5. doi:10.1212/01.wnl.0000148596.15681.4d. PMID 15623716. S2CID 37534686.
- ^ Barret A, Tagliavini F, Forloni G, Bate C, Salmona M, Colombo L, De Luigi A, Limido L, Suardi S, Rossi G, Auvré F, Adjou K, Salès N, Williams A, Lasmézas C, Deslys J (August 2003). "Evaluation of Quinacrine Treatment for Prion Diseases". J. Virol. 77 (15): 8462–9. doi:10.1128/JVI.77.15.8462-8469.2003. PMC 165262. PMID 12857915.
- ^ Gayrard V, Picard-Hagen N, Viguié C, Laroute V, Andréoletti O, Toutain P (February 2005). "A possible pharmacological explanation for quinacrine failure to treat prion diseases: pharmacokinetic investigations in a ovine model of scrapie". Br. J. Pharmacol. 144 (3): 386–93. doi:10.1038/sj.bjp.0706072. PMC 1576015. PMID 15655516. - Abstract
- ^ Ghaemmaghami S, Ahn M, Lessard P, Giles K, Legname G, et al. (November 2009). Mabbott N (ed.). "Continuous Quinacrine Treatment Results in the Formation of Drug-Resistant Prions". PLOS Pathogens. 5 (11): 2413–5. doi:10.1371/journal.ppat.1000673. PMC 2777304. PMID 19956709.
- ^ Zipper J, Cole LP, Goldsmith A, Wheeler R, Rivera M (1980). "Quinacrine hydrochloride pellets: preliminary data on a nonsurgical method of female sterilisation". International Journal of Gynecology & Obstetrics. 18 (4): 275–90. doi:10.1002/j.1879-3479.1980.tb00496.x. PMID 6109672. S2CID 41946631.
- ^ "Quinacrine sterilization: reports on 40,252 cases". International Journal of Gynaecology and Obstetrics. 83 (Suppl 2): S1–159. October 2003. PMID 14763179.
- ^ Sokal, D.C., Kessel. E., Zipper. J., and King. T. (1994). "Quinacrine: Clinical experience". Background Paper for the World Health Organization Consultation on the Development of New Technologies for Female Sterilization: 25–7.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Peterson, H.B., Lubell, L., DeStefano, F., and Ory, H.W. (1983). "The safety and efficacy of tubal sterilization: an international overview". Int. J. Gynaecol. Obstet. 21 (2): 139–44. doi:10.1016/0020-7292(83)90051-6. PMID 6136433. S2CID 15179539.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ George, Nirmala (July 25, 1998). "Govt drags feet on quinacrine threat". Indian Express..
