Dinapsoline
Appearance
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
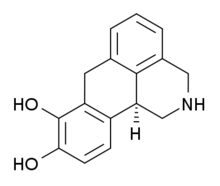
| Formula | C16H15NO2 |
| Molar mass | 253.295 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dinapsoline is a drug developed for the treatment of Parkinson's disease,[1] that acts as a selective full agonist at the dopamine D1 receptor.[2][3][4][5]
References
- ^ Gulwadi AG, Korpinen CD, Mailman RB, Nichols DE, Sit SY, Taber MT (February 2001). "Dinapsoline: characterization of a D1 dopamine receptor agonist in a rat model of Parkinson's disease". The Journal of Pharmacology and Experimental Therapeutics. 296 (2): 338–44. PMID 11160615.
- ^ Ghosh D, Snyder SE, Watts VJ, Mailman RB, Nichols DE (January 1996). "9-Dihydroxy-2,3,7,11b-tetrahydro-1H-naph[1,2,3-de]isoquinoline: a potent full dopamine D1 agonist containing a rigid-beta-phenyldopamine pharmacophore". Journal of Medicinal Chemistry. 39 (2): 549–55. doi:10.1021/jm950707+. PMID 8558526.
- ^ Sit SY, Xie K, Jacutin-Porte S, Taber MT, Gulwadi AG, Korpinen CD, Burris KD, Molski TF, Ryan E, Xu C, Wong H, Zhu J, Krishnananthan S, Gao Q, Verdoorn T, Johnson G (August 2002). "(+)-Dinapsoline: an efficient synthesis and pharmacological profile of a novel dopamine agonist". Journal of Medicinal Chemistry. 45 (17): 3660–8. doi:10.1021/jm0101545. PMID 12166939.
- ^ Sit SY, Xie K, Jacutin-Porte S, Boy KM, Seanz J, Taber MT, Gulwadi AG, Korpinen CD, Burris KD, Molski TF, Ryan E, Xu C, Verdoorn T, Johnson G, Nichols DE, Mailman RB (February 2004). "Synthesis and SAR exploration of dinapsoline analogues". Bioorg. Med. Chem. 12 (4): 715–34. doi:10.1016/j.bmc.2003.11.015. PMID 14759732.
- ^ Gleason SD, Witkin JM (May 2006). "Effects of dopamine D1 receptor agonists in rats trained to discriminate dihydrexidine". Psychopharmacology. 186 (1): 25–31. doi:10.1007/s00213-006-0342-2. PMID 16575553.
