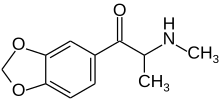
Methylone
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 3,4-Methylenedioxy-N-methylcathinone; Methylenedioxymethcathinone; MDMC; β-Keto-MDMA; βk-MDMA; M1; TSND-201; TSND201 |
| Routes of administration | Common: oral, insufflation Uncommon: IV or IM injection, rectal |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Onset of action | 0.5 hours[3] |
| Elimination half-life | 5.8–6.9 hours[3] |
| Duration of action | 2.5–3.0 hours[3] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C11H13NO3 |
| Molar mass | 207.229 g·mol−1 |
| 3D model (JSmol) | |
| Solubility in water | 357 mg/mL (20 °C) |
| |
| |
| | |
Methylone, also known as 3,4-methylenedioxy-N-methylcathinone (MDMC), is an empathogen and stimulant psychoactive drug. It is a member of the amphetamine, cathinone and methylenedioxyphenethylamine classes.
Methylone is a slight modification of 3,4-methylenedioxymethamphetamine (MDMA, also known as ecstasy). It was first synthesized by the chemists Peyton Jacob III and Alexander Shulgin in 1996 for potential use as an antidepressant.[4] Methylone has been sold for recreational use, taking advantage of the absence of legal prohibition of this compound in many countries.[citation needed]
Chemistry
[edit]Methylone is the substituted cathinone analogue of 3,4-methylenedioxymethamphetamine (MDMA) and the 3,4-methylenedioxy analog of methcathinone. The only structural difference of methylone with respect to MDMA is the substitution of 2 hydrogen atoms by 1 oxygen atom in the β position of the phenethylamine core, forming a ketone group.[5]
Effects
[edit]Resemblance to MDMA
[edit]
Left: amphetamine, methamphetamine and methcathinone.
Right: MDA, MDMA, and methylone
Methylone substitutes for MDMA in rats trained to discriminate MDMA from saline. Methylone does not substitute for amphetamine or for the hallucinogenic DOM in animals trained to discriminate between these drugs and saline.[6] Further, also in common with MDMA, methylone acts on monoaminergic systems. In vitro, methylone has one third the potency of MDMA at inhibiting platelet serotonin accumulation and about the same in its inhibiting effects on the dopamine and noradrenaline transporters.[7][8][5]
In spite of these behavioral and pharmacological similarities between methylone and MDMA, the observed subjective effects of both drugs are not completely identical. Alexander Shulgin wrote of the former:[9]
"[Methylone] has almost the same potency of MDMA, but it does not produce the same effects. It has an almost antidepressant action, pleasant and positive, but not the unique magic of MDMA."
In acute pharmacological studies of methylone (50–300 mg) in humans, the drug produced physiological and psychological effects including increased blood pressure, heart rate, body temperature, pupil dilation, stimulation, euphoria, feelings of well-being, enhanced empathy, increased sociability, and altered perception.[10][11] The studies found that the effects of methylone were similar to or milder than those of MDMA.[10][11] Methylone had a faster onset of action and its subjective effects wore off sooner than MDMA, which might lead to a redosing pattern of use.[10] The misuse potential of methylone, as measured by for instance drug liking responses, appeared to be similar to that of MDMA.[10] However it also has less off-target effects than MDMA which may be an advantage for medical applications.[12][10][13]
Pharmacology
[edit]Pharmacodynamics
[edit]Methylone acts as a mixed reuptake inhibitor and releasing agent of serotonin, norepinephrine, and dopamine.[5][14] In comparison to MDMA, it has approximately 3-fold lower affinity for the serotonin transporter, while its affinity for the norepinephrine and dopamine transporters is similar.[5][14] Notably, methylone's affinity for the vesicular monoamine transporter 2 (VMAT2) is about 13-fold lower than that of MDMA.[5] The results of these differences in pharmacology relative to MDMA are that methylone is less potent in terms of dose, has more balanced catecholaminergic effects relative to serotonergic, and behaves more like a reuptake inhibitor like methylphenidate than a releaser like amphetamine; however, methylone still has relatively robust releasing capabilities,[14] perhaps due to its ability to phosphorylate the monoamine transporters being similar in potency relative to MDMA.[citation needed]
In contrast to MDMA, methylone and its metabolites lack significant affinity for the serotonin 5-HT2A and 5-HT2C receptors and similarly do not activate the serotonin 5-HT2B receptor.[15][16][17] This may make methylone safer than MDMA, for instance in terms of long-term cardiac valvulopathy risk.[18][19][20]
Similarly to MDMA, methylone has been found to be a monoaminergic neurotoxin in animals.[21] It has specifically been found to produce serotonergic and dopaminergic neurotoxicity in rodents.[21] However, in one study, moderate doses of MDMA produced serotonergic neurotoxicity in rodents whereas methylone and mephedrone did not do so, suggesting that cathinones like methylone may be less neurotoxic than their corresponding amphetamine counterparts like MDMA.[15][22]
Pharmacokinetics
[edit]The two major metabolic pathways in mammals for methylone are N-demethylation to methylenedioxycathinone (MDC), and demethylation followed by O-methylation of the 3- or 4-hydroxy group to 4-hydroxy-3-methoxymethcathinone (HMMC) or 3-hydroxy-4-methoxymethcathinone (3-OH-4-MeO-MC). When 5 mg/kg of methylone was administered to rats, it was found that around 26% was excreted as HMMC within the first 48 hours (less than 3% excreted unchanged).[23] The mean elimination half-lives of methylone in humans following oral administration of doses of 50 to 200 mg ranged from 5.8 to 6.9 hours.[3] The onset of action and duration of action of methylone in humans are 0.5 hours and 2.5 to 3.0 hours, respectively.[3]
Commercial distribution
[edit]
Analysis of "Explosion" has confirmed that the active ingredient is methylone.[24][unreliable source?] Many other formulations marketed as household chemicals, as well as the pure powder, have been sold.
Legal status
[edit]Netherlands
[edit]In the Netherlands, methylone is not yet listed under the Opium Law, but is covered under the medicine act. Because methylone is not registered officially, it is forbidden to trade in methylone. The Minister of Health has asked the Coordination point Assessment and Monitoring new drugs group (CAM) to gather information about this substance, resulting possibly in an official risk assessment.[25] Until now, no research has been conducted on the toxicity of methylone, so nothing is known about the harmfulness of this new drug.
New Zealand
[edit]In New Zealand, although methylone is not explicitly scheduled and falls outside the strict definitions of an "amphetamine analogue" in the Misuse of Drugs Act, it is considered to be "substantially similar" to methcathinone and is thus considered by law enforcement authorities to be a Class C illegal drug. Methylone was sold in New Zealand for around 6 months from November 2005 to April 2006 as an MDMA substitute, under the name "Ease". The product was withdrawn after legal disputes with the government.[26][27]
UK
[edit]In the UK, methylone is illegal since the 16/04/2010 revision of the misuse of drugs act. Before this it was not specifically mentioned in United Kingdom (U.K.) law as the β-ketone was not covered under the Misuse of Drugs Act. In March 2010, plans were announced to make methylone and other cathinones, Class B drugs, "within weeks". While delayed by dissatisfaction in the Advisory Council on the Misuse of Drugs, the revision was rushed through by the government with little regard for the views of the council. The importation of the compound was banned immediately.[28]
Sweden
[edit]Sveriges riksdag added methylone to schedule I ("substances, plant materials and fungi which normally do not have medical use") as narcotics in Sweden as of Oct 1, 2010, published by Medical Products Agency in their regulation LVFS 2010:23 listed as Metylon, 2-metylamino-1-(3,4-metylendioxifenyl)propan-1-on.[29] Methylone was first classified by Sveriges riksdags health ministry Statens folkhälsoinstitut as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health) as of Nov 1, 2005, in their regulation SFS 2005:733 listed as 3,4-metylendioximetkatinon (Metylon).[30]
Canada
[edit]Although not listed as a Schedule 1[31] substance, Health Canada reports that methylone falls under the scheduling as an analogue of amphetamine. However, Methylone bears the exact chemical difference between amphetamine and cathinone – and cathinone is listed as not being an analogue of amphetamine, possibly implying that methylone is unscheduled in Canada.[32] The CDSA was updated as a result of the Safe Streets Act changing amphetamines from Schedule 3 to Schedule 1; however, methylone was not added.[33]
United States
[edit]In October 2011, the DEA issued an emergency ban on methylone. It was made illegal to possess and distribute.[34][35] On April 4, 2013, the DEA placed methylone as a Schedule 1 substance under the CSA.[36]
- Arizona:
- Effective February 16, 2012, methylenedioxymethcathinone (methylone) was classified as a dangerous drug, making it a felony to knowingly possess, use, possess for sale, manufacture, administer, transport for sale, import into the state, or offer to transport for sale or import into this state, sell, transfer or offer to sell or transfer. A.R.S. 13-3401(6)(c)(xliii), 2012 Ariz. Legis. Serv. Ch. 1 (H.B. 2356).
- Florida:
- In January 2011, it was reported that Florida Attorney General Pam Bondi issued an emergency ban on MDPV, Methylone, Mephedrone, 3-methoxymethcathinone, 3-fluoromethcathinone, and 4-fluoromethcathinone as media attention on products labeled as "bath salts" grew. These chemicals are now Schedule I under Florida law.[37]
- Louisiana:
- In January 2011, Louisiana Governor Bobby Jindal emergency scheduled 3,4-methylenedioxymethcathinone (methylone), 3,4-methyenedioxypyrovalerone (MDPV), 4-methylmethcathinone (mephedrone), 4-methoxymethcathinone (methedrone), 4-fluoromethcathinone (flephedrone), and 3-fluoromethcathinone (3-FMC).
- Tennessee:
- On May 5, 2011, Tennessee Governor Bill Haslam signed a law making it a crime to knowingly produce, manufacture, distribute, sell, offer for sale or possess with intent produce, manufacture, distribute, sell, or offer for sale any product containing 3,4-methylenedioxymethcathinone (methylone), 3,4-methyenedioxypyrovalerone (MDPV), 4-methylmethcathinone (mephedrone), 4-methoxymethcathinone (methedrone), 4-fluoromethcathinone (flephedrone), and 3-fluoromethcathinone (3-FMC).[38]
- Texas:
- In September 2011, Texas added 3,4-methylenedioxy-N-methylcathinone to the Penalty Group 2 listing of the Health and Safety Code. Possession of a substance in penalty group 2 is a minimum of a state jail felony.
- Michigan:
- Schedule 1 controlled substance in 2012.
Etymology
[edit]"Methylone" is also a trademarked brand name for an injectable form of methylprednisolone, a corticosteroid hormone used to treat arthritis and severe allergic reactions; hence, methylone may be confused with it. Aside from context, they can be distinguished by the fact that the name will usually be capitalized when referring to the prescription drug.
A proposed alternate name is βk-MDMA, or beta-keto-MDMA. While this nomenclature has not caught on because the name "methylone" became widely used before the conflicting Methylone trademark was noticed, the analogous names for related chemicals βk-MDEA and βk-MBDB have become the established names for those substances.
Research
[edit]Post-traumatic stress disorder
[edit]Under the developmental code name TSND-201, methylone is under development by Transcend Therapeutics for the treatment of post-traumatic stress disorder (PTSD).[39][40][41] As of July 2024, it is in phase 2 clinical trials for this indication.[39][40]
References
[edit]- ^ Anvisa (2023-07-24). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-07-25). Archived from the original on 2023-08-27. Retrieved 2023-08-27.
- ^ "Ustawa z dnia 15 kwietnia 2011 r. o zmianie ustawy o przeciwdziałaniu narkomanii ( Dz.U. 2011 nr 105 poz. 614 )". Internetowy System Aktów Prawnych. Retrieved 17 June 2011.
- ^ a b c d e Poyatos L, Lo Faro AF, Berardinelli D, Sprega G, Malaca S, Pichini S, et al. (November 2022). "Methylone and MDMA Pharmacokinetics Following Controlled Administration in Humans". International Journal of Molecular Sciences. 23 (23): 14636. doi:10.3390/ijms232314636. PMC 9736016. PMID 36498963.
- ^ WO 9639133, Jacob III P, Shulgin AT, "Novel N-Substituted-2-Amino-3',4'-Methylene-dioxypropiophenones", published 1996-12-12, assigned to Neurobiological Technologies Inc.
- ^ a b c d e Cozzi NV, Sievert MK, Shulgin AT, Jacob P, Ruoho AE (September 1999). "Inhibition of plasma membrane monoamine transporters by beta-ketoamphetamines". European Journal of Pharmacology. 381 (1): 63–69. doi:10.1016/S0014-2999(99)00538-5. PMID 10528135.
- ^ Dal Cason TA, Young R, Glennon RA (December 1997). "Cathinone: an investigation of several N-alkyl and methylenedioxy-substituted analogs". Pharmacology, Biochemistry, and Behavior. 58 (4). Elsevier BV: 1109–1116. doi:10.1016/s0091-3057(97)00323-7. PMID 9408221. S2CID 9704972.
- ^ Cozzi NV, Sievert MK, Shulgin AT, Jacob III P, Ruoho AE (1998). "Methcathinone and 2 methylamino-1-(3,4-methylenedioxyphenyl)propan-1-one (methylone) selectively inhibit plasma membrane catecholamine reuptake transporters". Soc. Neurosci. Abs. 24 (341.8).
- ^ Cozzi NV, Shulgin AT, Ruoho AE (1998). "Methcathinone (MCAT) and 2-methylamino-1-(3,4 methylenedioxyphenyl)propan-1-one (MDMCAT) inhibit [3H]serotonin uptake into human platelets". Amer. Chem. Soc. Div. Med. Chem. Abs. 215 (152).
- ^ "Cathinone | Ask Dr. Shulgin Online". Archived from the original on 2010-04-13. Retrieved 2010-01-17.
- ^ a b c d e Poyatos L, Pérez-Mañá C, Hladun O, Núñez-Montero M, de la Rosa G, Martín S, et al. (2023). "Pharmacological effects of methylone and MDMA in humans". Frontiers in Pharmacology. 14: 1122861. doi:10.3389/fphar.2023.1122861. PMC 9981643. PMID 36873994.
- ^ a b Poyatos L, Papaseit E, Olesti E, Pérez-Mañá C, Ventura M, Carbón X, et al. (August 2021). "A Comparison of Acute Pharmacological Effects of Methylone and MDMA Administration in Humans and Oral Fluid Concentrations as Biomarkers of Exposure". Biology. 10 (8): 788. doi:10.3390/biology10080788. PMC 8389614. PMID 34440023.
- ^ Warner-Schmidt J, Pittenger C, Stogniew M, Mandell B, Olmstead SJ, Kelmendi B (2022). "Methylone, a rapid acting entactogen with robust anxiolytic and antidepressant-like activity". Frontiers in Psychiatry. 13: 1041277. doi:10.3389/fpsyt.2022.1041277. PMC 9873307. PMID 36704743.
- ^ Warner-Schmidt J, Stogniew M, Mandell B, Rowland RS, Schmidt EF, Kelmendi B (2024-02-07). "Methylone is a rapid-acting neuroplastogen with less off-target activity than MDMA". Frontiers in Neuroscience. 18: 1353131. doi:10.3389/fnins.2024.1353131. PMC 10882719. PMID 38389788.
- ^ a b c Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology. 559 (2–3): 132–137. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ^ a b López-Arnau R, Camarasa J, Carbó ML, Nadal-Gratacós N, Puigseslloses P, Espinosa-Velasco M, et al. (2022). "3,4-Methylenedioxy methamphetamine, synthetic cathinones and psychedelics: From recreational to novel psychotherapeutic drugs". Front Psychiatry. 13: 990405. doi:10.3389/fpsyt.2022.990405. PMC 9574023. PMID 36262632.
- ^ Warner-Schmidt J, Stogniew M, Mandell B, Rowland RS, Schmidt EF, Kelmendi B (2024). "Methylone is a rapid-acting neuroplastogen with less off-target activity than MDMA". Front Neurosci. 18: 1353131. doi:10.3389/fnins.2024.1353131. PMC 10882719. PMID 38389788.
- ^ Luethi D, Kolaczynska KE, Walter M, Suzuki M, Rice KC, Blough BE, et al. (July 2019). "Metabolites of the ring-substituted stimulants MDMA, methylone and MDPV differentially affect human monoaminergic systems". J Psychopharmacol. 33 (7): 831–841. doi:10.1177/0269881119844185. PMC 8269116. PMID 31038382.
- ^ Tagen M, Mantuani D, van Heerden L, Holstein A, Klumpers LE, Knowles R (September 2023). "The risk of chronic psychedelic and MDMA microdosing for valvular heart disease". J Psychopharmacol. 37 (9): 876–890. doi:10.1177/02698811231190865. PMID 37572027.
- ^ Rouaud A, Calder AE, Hasler G (March 2024). "Microdosing psychedelics and the risk of cardiac fibrosis and valvulopathy: Comparison to known cardiotoxins". J Psychopharmacol. 38 (3): 217–224. doi:10.1177/02698811231225609. PMC 10944580. PMID 38214279.
- ^ Rothman RB, Baumann MH (May 2009). "Serotonergic drugs and valvular heart disease". Expert Opin Drug Saf. 8 (3): 317–329. doi:10.1517/14740330902931524. PMC 2695569. PMID 19505264.
- ^ a b Daziani G, Lo Faro AF, Montana V, Goteri G, Pesaresi M, Bambagiotti G, et al. (March 2023). "Synthetic Cathinones and Neurotoxicity Risks: A Systematic Review". Int J Mol Sci. 24 (7): 6230. doi:10.3390/ijms24076230. PMC 10093970. PMID 37047201.
- ^ Baumann MH, Ayestas MA, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. (April 2012). "The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue". Neuropsychopharmacology. 37 (5): 1192–1203. doi:10.1038/npp.2011.304. PMC 3306880. PMID 22169943.
- ^ Kamata HT, Shima N, Zaitsu K, Kamata T, Miki A, Nishikawa M, et al. (August 2006). "Metabolism of the recently encountered designer drug, methylone, in humans and rats". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 36 (8): 709–723. doi:10.1080/00498250600780191. PMID 16891251. S2CID 10875717.
- ^ "Methylone sold under "Explosion" and "Inpact" brand names in the Netherlands and Japan". www.erowid.org. Apr 2005. Archived from the original on 2009-03-04.
- ^ van Amsterdam JG, Best W, Opperhuizen A, de Wolff FA (February 2004). "Evaluation of a procedure to assess the adverse effects of illicit drugs". Regulatory Toxicology and Pharmacology. 39 (1). Elsevier BV: 1–4. doi:10.1016/j.yrtph.2003.09.001. hdl:10029/12622. PMID 14746774.
- ^ "Party pill sparks official concern". One News. 7 April 2006. Archived from the original on 9 February 2012. Retrieved 23 October 2011.
- ^ "EASE trial terminated after conflicting advice". scoop.co.nz. April 9, 2006. Archived from the original on 2012-09-29.
- ^ "Suspected mephedrone-type compound seized at airport". BBC News. 1 April 2010. Retrieved 3 April 2010.
- ^ "Läkemedelsverkets föreskrifter - LVFS och HSLF-FS" (PDF). Läkemedelsverket - Swedish Medical Products Agency. Archived (PDF) from the original on 2011-02-16. Retrieved 2010-10-07.
- ^ "Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor;" (PDF). notisum.se (in Swedish). Archived (PDF) from the original on 2016-03-04. Retrieved 2015-10-10.
- ^ "Controlled Drugs and Substances Act : Legislative history · Schedule I · Section 19: Tramadol [Proposed]; Amphetamines". isomerdesign.com. Archived from the original on 10 November 2013. Retrieved 28 March 2018.
- ^ "Controlled Drugs and Substances Act : Definitions and Interpretations". isomerdesign.com. Archived from the original on 10 November 2013. Retrieved 28 March 2018.
- ^ "The Safe Streets and Communities Act Four Components Coming into Force". 18 October 2012. Archived from the original on 18 October 2012. Retrieved 28 March 2018.
- ^ "Chemicals Used in 'Bath Salts' Now Under Federal Control and Regulation". USA Dept of Justice. Archived from the original on 25 April 2014. Retrieved 22 April 2014.
- ^ "Schedules of Controlled Substances: Placement of Methylone Into Schedule I". usdoj.gov. Archived from the original on 17 April 2014. Retrieved 22 April 2014.
- ^ "Schedules of Controlled Substances: Placement of Methylone Into Schedule I". federalregister.gov. 2014-04-12. Archived from the original on 15 December 2014. Retrieved 22 April 2014.
- ^ "Florida Synthetic Drug Scheduling Actions - 2011-2014" (PDF). myfloridalegal.com. Archived (PDF) from the original on 2015-03-20. Retrieved 2017-04-08.
- ^ "Welcome to the Tennessee Secretary of State's Website – Tennessee Secretary of State" (PDF). state.tn.us. Archived (PDF) from the original on 1 September 2013. Retrieved 28 March 2018.
- ^ a b "Methylone - Transcend Therapeutics". AdisInsight. 24 July 2024. Retrieved 24 October 2024.
- ^ a b "Delving into the Latest Updates on Methylone with Synapse". Synapse. 20 September 2024. Retrieved 24 October 2024.
- ^ Bayer M (5 December 2023). "PTSD treatment is on the cusp of a paradigm shift. This biotech hopes to Transcend the competition". Fierce Biotech. Retrieved 24 October 2024.
