Nirmatrelvir: Difference between revisions
consolidate refs; Nirmatrelvir doesn't have a trade name; move research; remove text for wrong audience WP:MEDMOS |
Citation bot (talk | contribs) Alter: template type. | Use this bot. Report bugs. | Suggested by RoanokeVirginia | #UCB_toolbar |
||
| Line 146: | Line 146: | ||
== Research == |
== Research == |
||
In February 2021, Pfizer began their first [[Phases of clinical research#Phase I|phase I trial]] of PF-07321332 (nirmatrelvir).<ref>{{cite |
In February 2021, Pfizer began their first [[Phases of clinical research#Phase I|phase I trial]] of PF-07321332 (nirmatrelvir).<ref>{{cite document |title=Study Of PF-07321332 In Healthy Participants |url=https://clinicaltrials.gov/ct2/show/NCT04756531 |publisher=clinicaltrials.gov |date=18 October 2021}}</ref> |
||
In September 2021, Pfizer began a [[Phases of clinical research#Phase III|phase II/III trial]] of nirmatrelvir combined with ritonavir.<ref name="trials">{{Cite web |date=2 September 2021 |title=Pfizer begins dosing in Phase II/III trial of antiviral drug for Covid-19. |url=https://www.clinicaltrialsarena.com/news/pfizer-antiviral-covid-trial/ |website=Clinical Trials Arena}}</ref> |
In September 2021, Pfizer began a [[Phases of clinical research#Phase III|phase II/III trial]] of nirmatrelvir combined with ritonavir.<ref name="trials">{{Cite web |date=2 September 2021 |title=Pfizer begins dosing in Phase II/III trial of antiviral drug for Covid-19. |url=https://www.clinicaltrialsarena.com/news/pfizer-antiviral-covid-trial/ |website=Clinical Trials Arena}}</ref> |
||
In December 2021, Pfizer completed a Phase III study of nirmatrelvir combined with ritonavir.<ref>{{cite |
In December 2021, Pfizer completed a Phase III study of nirmatrelvir combined with ritonavir.<ref>{{cite document |title=EPIC-HR: Study of Oral PF-07321332/Ritonavir Compared With Placebo in Nonhospitalized High Risk Adults With COVID-19 |url=https://clinicaltrials.gov/ct2/show/NCT04960202 |publisher=clinicaltrials.gov |date=19 November 2021}}</ref> |
||
On 14 December 2021, Pfizer announced that the result of the Phase II/III study of nirmatrelvir combined with ritonavir results showed a reduced risk of hospitalization or death.<ref name="pfizer2">{{Cite press release |date=14 December 2021 |title=Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death | url = https://www.businesswire.com/news/home/20211214005548/en/Pfizer-Announces-Additional-Phase-23-Study-Results-Confirming-Robust-Efficacy-of-Novel-COVID-19-Oral-Antiviral-Treatment-Candidate-in-Reducing-Risk-of-Hospitalization-or-Death | publisher=[[Pfizer]] | via=Business Wire | access-date=25 December 2021}}</ref> |
On 14 December 2021, Pfizer announced that the result of the Phase II/III study of nirmatrelvir combined with ritonavir results showed a reduced risk of hospitalization or death.<ref name="pfizer2">{{Cite press release |date=14 December 2021 |title=Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death | url = https://www.businesswire.com/news/home/20211214005548/en/Pfizer-Announces-Additional-Phase-23-Study-Results-Confirming-Robust-Efficacy-of-Novel-COVID-19-Oral-Antiviral-Treatment-Candidate-in-Reducing-Risk-of-Hospitalization-or-Death | publisher=[[Pfizer]] | via=Business Wire | access-date=25 December 2021}}</ref> |
||
Revision as of 15:39, 1 January 2022
 | |
| Clinical data | |
|---|---|
| Other names | PF-07321332 |
| License data |
|
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
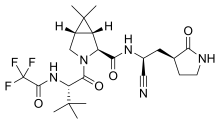
| Formula | C23H32F3N5O4 |
| Molar mass | 499.535 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 192.9 °C (379.2 °F) [6] |
| |
| |

Nirmatrelvir is an antiviral medication developed by Pfizer which acts as an orally active 3CL protease inhibitor.[7][8][9][10]
In December 2021, the combination of nirmatrelvir co-packaged with ritonavir was granted emergency use authorization by the US Food and Drug Administration (FDA) for the treatment of coronavirus disease COVID-19.[3][4][11] The co-packaged medications are sold under the brand name Paxlovid.[4][11][12] Paxlovid is not authorized for the pre-exposure or post-exposure prevention of COVID-19 or for initiation of treatment in those requiring hospitalization due to severe or critical COVID-19.[4]
On 31 December 2021, the UK Medicines and Healthcare products Regulatory Agency (MHRA) approved the combination of nirmatrelvir co-packaged with ritonavir "for people with mild to moderate COVID-19 who are at high risk of developing severe COVID-19".[13][14]
Medical uses
Nirmatrelvir, co-packaged with ritonavir, is indicated for the treatment of mild-to-moderate coronavirus disease (COVID-19) in people aged twelve years of age and older weighing at least 40 kilograms (88 lb) with positive results of direct SARS-CoV-2 testing, and who are at high risk for progression to severe COVID-19, including hospitalization or death.[3][4] The co-packaged medication is not authorized for the pre-exposure or post-exposure prevention of COVID-19 or for initiation of treatment in those requiring hospitalization due to severe or critical COVID-19.[4]
Adverse effects
There are no human data on the use of nirmatrelvir during pregnancy related to the risk of birth defects, miscarriage or adverse outcomes. There are also no human data on the presence of nirmatrelvir in human milk, its effects on milk production or the infant. In pregnant rabbits, a reduction in fetal body weight was observed with systemic exposure 10 times higher than the authorized human dose of Paxlovid. A temporary reduction in body weight was observed in the offspring of nursing rats.[5]
Development
Pharmaceutical
Coronaviral proteases cleave multiple sites in the viral polyprotein, usually after glutamine residues. Early work on related human rhinoviruses showed that the flexible glutamine side chain could be replaced by a rigid pyrrolidone.[15][16] These drugs had been further developed prior to the SARS CoV2 pandemic for other diseases including SARS.[17] The utility of targeting the 3CL protease in a real world setting was first demonstrated in 2018 when GC376 (a prodrug of GC373) was used to treat the previously 100% lethal cat coronavirus disease, feline infectious peritonitis, caused by Feline coronavirus.[18]
Nirmatrelvir is an analog of GC373, where the aldehyde covalent cysteine acceptor has been replaced by a nitrile.[19][20]
Nirmatrelvir was developed by modification of the earlier clinical candidate lufotrelvir,[21][22] which is also a covalent 3CL protease inhibitor but its warhead is a phosphate prodrug of a hydroxyketone. Lufotrelvir needs to be administered intravenously limiting its use to a hospital setting. Stepwise modification of the tripeptide protein mimetic led to nirmatrelvir, which is suitable for oral administration.[6] Key changes include a reduction in the number of hydrogen bond donors, and the number of rotatable bonds by introducing a rigid bicyclic non-canonical amino acid, which mimics the leucine residue found in earlier inhibitors. This residue had previously been used in the synthesis of boceprevir.[23] Tert-leucine (abbreviation: Tle) used in the P3 position of nirmatrelvir was identified first as optimal non-canonical amino acid in potential drug targeting SARS-CoV-2-Mpro using combinatorial chemistry (Hybrid Combinatorial Substrate Library technology).[24][25]
Chemistry and pharmacology
Full details of the synthesis of nirmatrelvir were first published by scientists from Pfizer.
In the penultimate step, a synthetic homochiral amino acid is coupled with a homochiral amino amide using the water-soluble carbodiimide EDCI as coupling agent. The resulting intermediate is then treated with Burgess reagent, which dehydrates the amide group to the nitrile of the product.[6]
Nirmatrelvir is a covalent inhibitor, binding directly to the catalytic cysteine (Cys145) residue of the cysteine protease enzyme.[26]
In the co-packaged medication, ritonavir serves to slow down metabolism of nirmatrelvir by cytochrome enzymes to maintain higher circulating concentrations of the main drug.[27]
Society and culture
Economics
The UK placed an order for 250,000 courses in October 2021,[28][29] Australia pre-ordered 500,000 courses of the drug,[30] and the US secured 10 million courses for $5.295 billion.[31]
Licensing
In November 2021, Pfizer signed a license agreement with the United Nations–backed Medicines Patent Pool to allow nirmatrelvir to be manufactured and sold in 95 countries.[32] Pfizer stated that the agreement will allow local medicine manufacturers to produce the pill "with the goal of facilitating greater access to the global population". However, the deal excludes several countries with major COVID-19 outbreaks including Brazil, China, Russia, Argentina, and Thailand.[33][34]
Legal status
On 16 November 2021, Pfizer submitted an application to the U.S. Food and Drug Administration (FDA) for emergency use authorization for nirmatrelvir in combination with ritonavir.[35][36][37] The authorization was granted on 22 December 2021.[4][12] The European Medicines Agency (EMA) issued guidance about the use of Paxlovid for the treatment of COVID-19 in the EU on 16 December 2021.[38] The Israeli Ministry of Health approved the use of Paxlovid on 26 December 2021.[39] South Korea approved the use of Paxlovid on 27 December 2021.[40] The UK Medicines and Healthcare products Regulatory Agency (MHRA) granted conditional approval of Paxlovid on 31 December 2021.[41][13]
Misleading comparison with ivermectin
The combination of nirmatrelvir with ritonavir is sometimes falsely claimed to be a "repackaged" version of the antiparasitic drug ivermectin, which has been promoted as a COVID-19 therapeutic. Such claims, sometimes using the nickname "Pfizermectin",[42] rely on superficial similarities between the pharmacokinetics of both drugs and the claim that Pfizer is suppressing the benefits of ivermectin.[43] If one assumes ivermectin is not administered near the site of infection or locally to vulnerable tissue, or rapid diffusion to a uniform concentration within the body, then, to be effective against COVID-19, the concentration of ivermectin in the blood would require a dose that is 10-20 times higher than is safe.[42][43]
Research
In February 2021, Pfizer began their first phase I trial of PF-07321332 (nirmatrelvir).[44]
In September 2021, Pfizer began a phase II/III trial of nirmatrelvir combined with ritonavir.[45]
In December 2021, Pfizer completed a Phase III study of nirmatrelvir combined with ritonavir.[46]
On 14 December 2021, Pfizer announced that the result of the Phase II/III study of nirmatrelvir combined with ritonavir results showed a reduced risk of hospitalization or death.[9]
On 31 December 2021, the United Kingdom's Medicines and Healthcare products Regulatory Agency (MHRA) approved the use of nirmatrelvir combined with ritonavir for adults who have mild to moderate infection and are at high risk of their illness worsening.[41][13]
The efficacy of the combination against hospitalization or death in adult outpatients when administered within five days of symptom onset is about 88% (95% CI, 75–94%).[5]
References
- ^ "Summary of Product Characteristics for Paxlovid". Medicines and Healthcare products Regulatory Agency (MHRA). 31 December 2021. Retrieved 31 December 2021.
- ^ "Regulatory approval of Paxlovid". Medicines and Healthcare products Regulatory Agency (MHRA). 31 December 2021. Retrieved 31 December 2021.
- ^ a b c "Paxlovid- nirmatrelvir and ritonavir kit". DailyMed. Retrieved 30 December 2021.
- ^ a b c d e f g "FDA Authorizes First Oral Antiviral for Treatment of COVID-19". U.S. Food and Drug Administration (FDA) (Press release). 22 December 2021. Retrieved 22 December 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ a b c Fact sheet for healthcare providers: Emergency Use Authorization for Paxlovid (PDF) (Technical report). Pfizer. 22 December 2021. LAB-1492-0.8. Archived from the original on 23 December 2021.
- ^ a b c Owen DR, Allerton CM, Anderson AS, Aschenbrenner L, Avery M, Berritt S, et al. (November 2021). "An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19". Science. 374 (6575): 1586–1593. doi:10.1126/science.abl4784. PMID 34726479. S2CID 240422219.
- ^ Şimşek-Yavuz S, Komsuoğlu Çelikyurt FI (August 2021). "Antiviral treatment of COVID-19: An update". Turkish Journal of Medical Sciences. 51 (SI-1): 3372–3390. doi:10.3906/sag-2106-250. PMID 34391321. S2CID 237054672.
- ^ Ahmad B, Batool M, Ain QU, Kim MS, Choi S (August 2021). "Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations". International Journal of Molecular Sciences. 22 (17): 9124. doi:10.3390/ijms22179124. PMC 8430524. PMID 34502033.
- ^ a b "Pfizer Announces Additional Phase 2/3 Study Results Confirming Robust Efficacy of Novel COVID-19 Oral Antiviral Treatment Candidate in Reducing Risk of Hospitalization or Death" (Press release). Pfizer. 14 December 2021. Retrieved 25 December 2021 – via Business Wire.
- ^ Vandyck K, Deval J (August 2021). "Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection". Current Opinion in Virology. 49: 36–40. doi:10.1016/j.coviro.2021.04.006. PMC 8075814. PMID 34029993.
- ^ a b "Pfizer Receives U.S. FDA Emergency Use Authorization for Novel COVID-19 Oral Antiviral Treatment" (Press release). Pfizer. 22 December 2021. Retrieved 22 December 2021 – via Business Wire.
- ^ a b "Frequently Asked Questions on the Emergency Use Authorization for Paxlovid for Treatment of COVID-19" (PDF). U.S. Food and Drug Administration (FDA). 22 December 2021.
- ^ a b c "Oral COVID-19 antiviral, Paxlovid, approved by UK regulator" (Press release). Medicines and Healthcare products Regulatory Agency. 31 December 2021.
- ^ Reed J (31 December 2021). "Paxlovid: UK medicines regulator approves second Covid antiviral pill". BBC News Online.
- ^ Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R (June 2003). "Coronavirus Main Proteinase (3CLpro) Structure: Basis for Design of Anti-SARS Drugs". Science. 300 (5626): 1763–1767. Bibcode:2003Sci...300.1763A. doi:10.1126/science.1085658. PMID 12746549. S2CID 13031405.
- ^ Dragovich PS, Prins TJ, Zhou R, Webber SE, Marakovits JT, Fuhrman SA, et al. (April 1999). "Structure-based design, synthesis, and biological evaluation of irreversible human rhinovirus 3C protease inhibitors. 4. Incorporation of P1 lactam moieties as L-glutamine replacements". Journal of Medicinal Chemistry. 42 (7): 1213–1224. doi:10.1021/jm9805384. PMID 10197965.
- ^ Pillaiyar T, Manickam M, Namasivayam V, Hayashi Y, Jung SH (July 2016). "An Overview of Severe Acute Respiratory Syndrome-Coronavirus (SARS-CoV) 3CL Protease Inhibitors: Peptidomimetics and Small Molecule Chemotherapy". Journal of Medicinal Chemistry. 59 (14): 6595–6628. doi:10.1021/acs.jmedchem.5b01461. PMC 7075650. PMID 26878082.
- ^ Pedersen NC, Kim Y, Liu H, Galasiti Kankanamalage AC, Eckstrand C, Groutas WC, et al. (April 2018). "Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis". Journal of Feline Medicine and Surgery. 20 (4): 378–392. doi:10.1177/1098612X17729626. PMC 5871025. PMID 28901812.
- ^ Halford B (7 April 2021). "Pfizer unveils its oral SARS-CoV-2 inhibitor". Chemical & Engineering News. 99 (13): 7. doi:10.47287/cen-09913-scicon3. S2CID 234887434.
- ^ Vuong W, Khan MB, Fischer C, Arutyunova E, Lamer T, Shields J, et al. (August 2020). "Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication". Nature Communications. 11 (1): 4282. doi:10.1038/s41467-020-18096-2. PMC 7453019. PMID 32855413.
- ^ Clinical trial number NCT04535167 for "First-In-Human Study To Evaluate Safety, Tolerability, And Pharmacokinetics Following Single Ascending And Multiple Ascending Doses of PF-07304814 In Hospitalized Participants With COVID-19 " at ClinicalTrials.gov
- ^ Boras B, Jones RM, Anson BJ, Arenson D, Aschenbrenner L, Bakowski MA, et al. (2021). "Preclinical characterization of an intravenous coronavirus 3CL protease inhibitor for the potential treatment of COVID19". Nature Communications. 12 (1): 6055. Bibcode:2021NatCo..12.6055B. doi:10.1038/s41467-021-26239-2. PMC 8523698. PMID 34663813.
- ^ Njoroge FG, Chen KX, Shih NY, Piwinski JJ (January 2008). "Challenges in modern drug discovery: a case study of boceprevir, an HCV protease inhibitor for the treatment of hepatitis C virus infection". Accounts of Chemical Research. 41 (1): 50–59. doi:10.1021/ar700109k. PMID 18193821. S2CID 2629035.
- ^ Poreba M, Salvesen GS, Drag M (October 2017). "Synthesis of a HyCoSuL peptide substrate library to dissect protease substrate specificity". Nature Protocols. 12 (10): 2189–2214. doi:10.1038/nprot.2017.091. PMID 28933778. S2CID 23895951.
- ^ Rut W, Groborz K, Zhang L, Sun X, Zmudzinski M, Pawlik B, et al. (October 2020). "SARS-CoV-2 M pro inhibitors and activity-based probes for patient-sample imaging". Nature Chemical Biology. 17 (2): 222–228. doi:10.1038/s41589-020-00689-z. PMID 33093684. S2CID 224827220.
- ^ Pavan M, Bolcato G, Bassani D, Sturlese M, Moro S (December 2021). "Supervised Molecular Dynamics (SuMD) Insights into the mechanism of action of SARS-CoV-2 main protease inhibitor PF-07321332". J Enzyme Inhib Med Chem. 36 (1): 1646–1650. doi:10.1080/14756366.2021.1954919. PMC 8300928. PMID 34289752.
- ^ Woodley M (19 October 2021). "What is Australia's potential new COVID treatment?". The Royal Australian College of General Practitioners (RACGP). Retrieved 6 November 2021.
- ^ "Pfizer Covid pill 'can cut hospitalisations and deaths by nearly 90%'". The Guardian. 5 November 2021. Retrieved 17 November 2021.
- ^ Mahase E (October 2021). "Covid-19: UK stockpiles two unapproved antiviral drugs for treatment at home". BMJ. 375: n2602. doi:10.1136/bmj.n2602. PMID 34697079. S2CID 239770104.
- ^ "What are the two new COVID-19 treatments Australia has gained access to?". ABC News (Australia). 17 October 2021. Retrieved 5 November 2021.
- ^ "Biden Administration Secures 10 Million Courses of Pfizer's COVID-19 Oral Antiviral Medicine as Additional Tool to Reduce Hospitalizations and Save Lives" (Press release). HHS.gov. HHS Press Office. 18 November 2021. Retrieved 26 December 2021.
- ^ "Pfizer and The Medicines Patent Pool (MPP) Sign Licensing Agreement for COVID-19 Oral Antiviral Treatment Candidate to Expand Access in Low- and Middle-Income Countries" (Press release). Pfizer. 16 November 2021. Retrieved 17 November 2021 – via Business Wire.
- ^ "Covid-19: Pfizer to allow developing nations to make its treatment pill". BBC News. 16 November 2021. Archived from the original on 16 November 2021. Retrieved 17 November 2021.
- ^ "Pfizer Will Allow Its Covid Pill to Be Made and Sold Cheaply in Poor Countries". The New York Times. 16 November 2021. Retrieved 17 November 2021.
- ^ "Pfizer Seeks Emergency Use Authorization for Novel COVID-19 Oral Antiviral Candidate" (Press release). Pfizer. 16 November 2021. Retrieved 17 November 2021 – via Business Wire.
- ^ Kimball S (16 November 2021). "Pfizer submits FDA application for emergency approval of Covid treatment pill". CNBC. Retrieved 17 November 2021.
- ^ Robbins R (5 November 2021). "Pfizer Says Its Antiviral Pill Is Highly Effective in Treating Covid". The New York Times. ISSN 0362-4331. Archived from the original on 8 November 2021. Retrieved 9 November 2021.
- ^ "EMA issues advice on use of Paxlovid (PF-07321332 and ritonavir) for the treatment of COVID-19: rolling review starts in parallel" (Press release). European Medicines Agency (EMA). 16 December 2021.
- ^ "The Use of Pfizer's Anti-Viral Drug for the Treatment of COVID-19 Has Been Approved". GOV.IL. Retrieved 28 December 2021.
- ^ Reuters (27 December 2021). "S.Korea authorises emergency use of Pfizer's oral coronavirus treatment". Reuters. Retrieved 28 December 2021.
{{cite news}}:|last=has generic name (help) - ^ a b Aripaka P (31 December 2021). "Britain approves Pfizer's antiviral COVID-19 pill". Reuters. Retrieved 31 December 2021.
- ^ a b Bloom J (2 December 2021). "How Does Pfizer's Pavloxid Compare With Ivermectin?". American Council on Science and Health. Retrieved 12 December 2021.
- ^ a b Gorski D (15 November 2021). "Pfizer's new COVID-19 protease inhibitor drug is not just 'repackaged ivermectin'". Science-Based Medicine.
- ^ "Study Of PF-07321332 In Healthy Participants" (Document). clinicaltrials.gov. 18 October 2021.
{{cite document}}: Unknown parameter|url=ignored (help) - ^ "Pfizer begins dosing in Phase II/III trial of antiviral drug for Covid-19". Clinical Trials Arena. 2 September 2021.
- ^ "EPIC-HR: Study of Oral PF-07321332/Ritonavir Compared With Placebo in Nonhospitalized High Risk Adults With COVID-19" (Document). clinicaltrials.gov. 19 November 2021.
{{cite document}}: Unknown parameter|url=ignored (help)
External links
- "Nirmatrelvir". Drug Information Portal. U.S. National Library of Medicine.
- "Early Data Suggest Pfizer Pill May Prevent Severe COVID-19". National Institutes of Health. 16 November 2021.

