Anabolic steroid: Difference between revisions
O.Koslowski (talk | contribs) m Reverted edits by 158.59.240.166 (talk) to last revision by 112.198.244.107 (HG) |
|||
| Line 678: | Line 678: | ||
==== United States ==== |
==== United States ==== |
||
[[File:SteroidpillsDEA.jpg|thumb|right |200px| Steroid pills intercepted by the US Drug Enforcement Administration during the "Operation raw deal" bust in September 2007.]] |
[[File:SteroidpillsDEA.jpg|thumb|right |200px| Steroid pills intercepted by the US Drug Enforcement Administration during the "Operation raw deal" bust in September 2007.]] |
||
The history of the U.S. legislation on anabolic steroids goes back to the late 1980s, when the [[U.S. Congress]] considered placing anabolic steroids under the Controlled Substances Act following the controversy over [[Ben Johnson (sprinter)|Ben Johnson's]] victory at the [[1988 Summer Olympics]] in [[Seoul]]. During deliberations, the [[American Medical Association]] (AMA), [[Drug Enforcement Administration]] (DEA), [[Food and Drug Administration]] (FDA) as well as the [[National Institute on Drug Abuse]] (NIDA) all opposed listing anabolic steroids as controlled substances, citing the fact that use of these hormones does not lead to the |
The history of the U.S. legislation on anabolic steroids goes back to the late 1980s, when the [[U.S. Congress]] considered placing anabolic steroids under the Controlled Substances Act following the controversy over [[Ben Johnson (sprinter)|Ben Johnson's]] victory at the [[1988 Summer Olympics]] in [[Seoul]]. During deliberations, the [[American Medical Association]] (AMA), [[Drug Enforcement Administration]] (DEA), [[Food and Drug Administration]] (FDA) as well as the [[National Institute on Drug Abuse]] (NIDA) all opposed listing anabolic steroids as controlled substances, citing the fact that use of these hormones does not lead to the phtytytytytyttytytyysical or psychological dependence required for such scheduling under the Controlled Substance Act. Nevertheless, anabolic steroids were added to Schedule III of the Controlled Substances Act in the Anabolic Steroid Control Act of 1990.<ref name="congress">{{USBill|101|HR|4658}}</ref> |
||
The same act also introduced more stringent controls with higher criminal penalties for offenses involving the illegal distribution of anabolic steroids and human growth hormone. By the early 1990s, after anabolic steroids were scheduled in the U.S., several pharmaceutical companies stopped manufacturing or marketing the products in the U.S., including Ciba, Searle, Syntex, and others. In the Controlled Substances Act, anabolic steroids are defined to be any drug or hormonal substance chemically and pharmacologically related to testosterone (other than [[estrogen]]s, [[progestin]]s, and [[corticosteroid]]s) that promote muscle growth. The act was amended by the Anabolic Steroid Control Act of 2004, which added [[prohormone]]s to the list of [[controlled substance]]s, with effect from January 20, 2005.<ref name="usdoj">{{cite web | url=http://www.usdoj.gov/dea/pubs/cngrtest/ct031604.html | title=News from DEA, Congressional Testimony, 03/16/04 | accessdate=2007-04-24}}</ref> |
The same act also introduced more stringent controls with higher criminal penalties for offenses involving the illegal distribution of anabolic steroids and human growth hormone. By the early 1990s, after anabolic steroids were scheduled in the U.S., several pharmaceutical companies stopped manufacturing or marketing the products in the U.S., including Ciba, Searle, Syntex, and others. In the Controlled Substances Act, anabolic steroids are defined to be any drug or hormonal substance chemically and pharmacologically related to testosterone (other than [[estrogen]]s, [[progestin]]s, and [[corticosteroid]]s) that promote muscle growth. The act was amended by the Anabolic Steroid Control Act of 2004, which added [[prohormone]]s to the list of [[controlled substance]]s, with effect from January 20, 2005.<ref name="usdoj">{{cite web | url=http://www.usdoj.gov/dea/pubs/cngrtest/ct031604.html | title=News from DEA, Congressional Testimony, 03/16/04 | accessdate=2007-04-24}}</ref> |
||
Revision as of 17:06, 3 October 2012
Anabolic steroids, technically known as anabolic-androgen steroids (AAS) or colloquially as "steroids", are drugs that mimic the effects of testosterone and dihydrotestosterone in the body. They increase protein synthesis within cells, which results in the buildup of cellular tissue (anabolism), especially in muscles. Anabolic steroids also have androgenic and virilizing properties, including the development and maintenance of masculine characteristics such as the growth of the vocal cords, testicles, and body hair (secondary sexual characteristics). The word anabolic comes from the Greek ἀναβολή anabole, "that which is thrown up, mound", and the word androgenic from the Greek ἀνδρός andros, "of a man" + -γενής -genes, "born".
Anabolic steroids were first isolated, identified, and synthesized in the 1930s, and are now used therapeutically in medicine to stimulate bone growth and appetite, induce male puberty, and treat chronic wasting conditions, such as cancer and AIDS. The American College of Sports Medicine acknowledges that AAS, in the presence of adequate diet, can contribute to increases in body weight, often as lean mass increases, and that the gains in muscular strength achieved through high-intensity exercise and proper diet can be additionally increased by the use of AAS in some individuals.[1]
Health risks can be produced by long-term use or excessive doses of anabolic steroids.[2][3] These effects include harmful changes in cholesterol levels (increased low-density lipoprotein and decreased high-density lipoprotein), acne, high blood pressure, liver damage (mainly with oral steroids), dangerous changes in the structure of the left ventricle of the heart.[4] Conditions pertaining to hormonal imbalances such as gynecomastia and testicular atrophy may also be caused by anabolic steroids.
Ergogenic uses for anabolic steroids in sports, racing, and bodybuilding are controversial because of their adverse effects and the potential to gain an advantage conventionally considered "cheating." Their use is referred to as doping and banned by all major sporting bodies. For many years, AAS have been by far the most detected doping substances in IOC-accredited laboratories.[5][6] In countries where AAS are controlled substances, there is often a black market in which smuggled, clandestinely manufactured, or even counterfeit drugs are sold to users.
History
Isolation of gonadal AAS
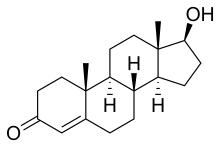
The use of gonadal steroids pre-dates their identification and isolation. Medical use of testicle extract began in the late 19th century while its effects on strength were still being studied.[7] The isolation of gonadal steroids can be traced back to 1931, when Adolf Butenandt, a chemist in Marburg, purified 15 milligrams of the male hormone androstenone from tens of thousands of litres of urine. This steroid was subsequently synthesized in 1934 by Leopold Ruzicka, a chemist in Zurich.[8]
In the 1930s, it was already known that the testes contain a more powerful androgen than androstenone, and three groups of scientists, funded by competing pharmaceutical companies in the Netherlands, Germany, and Switzerland, raced to isolate it.[8][9] This hormone was first identified by Karoly Gyula David, E. Dingemanse, J. Freud and Ernst Laqueur in a May 1935 paper "On Crystalline Male Hormone from Testicles (Testosterone)."[10] They named the hormone testosterone, from the stems of testicle and sterol, and the suffix of ketone. The chemical synthesis of testosterone was achieved in August that year, when Butenandt and G. Hanisch published a paper describing "A Method for Preparing Testosterone from Cholesterol."[11] Only a week later, the third group, Ruzicka and A. Wettstein, announced a patent application in a paper "On the Artificial Preparation of the Testicular Hormone Testosterone (Androsten-3-one-17-ol)."[12] Ruzicka and Butenandt were offered the 1939 Nobel Prize in Chemistry for their work, but the Nazi government forced Butenandt to decline the honor, although he accepted the prize after the end of World War II.[8][9]
Clinical trials on humans, involving either oral doses of methyltestosterone or injections of testosterone propionate, began as early as 1937.[8] Testosterone propionate is mentioned in a letter to the editor of Strength and Health magazine in 1938; this is the earliest known reference to an anabolic steroid in a U.S. weightlifting or bodybuilding magazine.[8] There are often reported rumors that German soldiers were administered anabolic steroids during the Second World War, the aim being to increase their aggression and stamina, but these are, as yet, unproven.[13] Adolf Hitler himself, according to his physician, was injected with testosterone derivatives to treat various ailments.[14] AAS were used in experiments conducted by the Nazis on concentration camp inmates,[14] and later by the allies attempting to treat the malnourished victims that survived Nazi camps.[13]
Development of synthetic AAS

The development of muscle-building properties of testosterone was pursued in the 1940s, in the Soviet Union and in Eastern Bloc countries such as East Germany, where steroid programs were used to enhance the performance of Olympic and other amateur weight lifters. In response to the success of Russian weightlifters, the U.S. Olympic Team physician Dr. John Ziegler worked with synthetic chemists to develop an anabolic steroid with reduced androgenic effects.[15] Ziegler's work resulted in the production of methandrostenolone, which Ciba Pharmaceuticals marketed as Dianabol. The new steroid was approved for use in the U.S. by the Food and Drug Administration (FDA) in 1958. It was most commonly administered to burn victims and the elderly. The drug's off-label users were mostly bodybuilders and weight lifters. Although Ziegler prescribed only small doses to athletes, he soon discovered that those having abused Dianabol suffered from enlarged prostates and atrophied testes.[16] AAS were placed on the list of banned substances of the IOC in 1976, and a decade later the committee introduced 'out-of-competition' doping tests because many athletes used AAS in their training period rather than during competition.[5]
Three major ideas governed modifications of testosterone into a multitude of AAS: Alkylation at 17-alpha position with methyl or ethyl group created orally active compounds because it slows the degradation of the drug by the liver; esterification of testosterone and nortestosterone at the 17-beta position allows the substance to be administered parenterally and increases the duration of effectiveness because agents soluble in oily liquids may be present in the body for several months; and alterations of the ring structure were applied for both oral and parenteral agents to seeking to obtain different anabolic to androgenic effect ratios.[17]
Pharmacology
Routes of administrations

There are four common forms in which anabolic steroids are administered: oral pills, injectable steroids, creams/gels for topical application, and skin patches. Oral administration is the most convenient. Testosterone administered by mouth is rapidly absorbed, but it is largely converted to inactive metabolites, and only about 1/6 is available in active form. In order to be sufficiently active when given by mouth, testosterone derivatives are alkylated at the 17 position, e.g. methyltestosterone and fluoxymesterone. This modification reduces the liver's ability to break down these compounds before they reach the systemic circulation.
Testosterone can be administered parenterally, but it has more irregular prolonged absorption time and greater activity in propionate, enanthate, undecanoate, or cypionate ester form. These derivatives are hydrolyzed to release free testosterone at the site of injection; absorption rate (and thus injection schedule) varies among different esters, but medical injections are normally done anywhere between semi-weekly to once every 12 weeks. A more frequent schedule may be desirable in order to maintain a more constant level of hormone in the system.[18] Injectable steroids are typically administered into the muscle, not into the vein, to avoid sudden changes in the amount of the drug in the bloodstream. In addition, because estered testosterone is dissolved in oil, intravenous injection has the potential to cause a dangerous embolism (clot) in the bloodstream.
Transdermal patches (adhesive patches placed on the skin) may also be used to deliver a steady dose through the skin and into the bloodstream. Testosterone-containing creams and gels that are applied daily to the skin are also available, but absorption is inefficient (roughly 10%, varying between individuals) and these treatments tend to be more expensive. Individuals who are especially physically active and/or bathe often may not be good candidates, since the medication can be washed off and may take up to six hours to be fully absorbed. There is also the risk that an intimate partner or child may come in contact with the application site and inadvertently dose himself or herself; children and women are highly sensitive to testosterone and can suffer unintended masculinization and health effects, even from small doses. Injection is the most common method used by individuals administering anabolic steroids for non-medical purposes.[19]
The traditional routes of administration do not have differential effects on the efficacy of the drug. Studies indicate that the anabolic properties of anabolic steroids are relatively similar despite the differences in pharmacokinetic principles such as first-pass metabolism. However, the orally available forms of AAS may cause liver damage in high doses.[6][20]
Mechanism of action

The pharmacodynamics of anabolic steroids are unlike peptide hormones. Water-soluble peptide hormones cannot penetrate the fatty cell membrane and only indirectly affect the nucleus of target cells through their interaction with the cell’s surface receptors. However, as fat-soluble hormones, anabolic steroids are membrane-permeable and influence the nucleus of cells by direct action. The pharmacodynamic action of anabolic steroids begin when the exogenous hormone penetrates the membrane of the target cell and binds to an androgen receptor located in the cytoplasm of that cell. From there, the compound hormone-receptor diffuses into the nucleus, where it either alters the expression of genes[22] or activates processes that send signals to other parts of the cell.[23] Different types of anabolic steroids bind to the androgen receptor with different affinities, depending on their chemical structure.[5] Some anabolic steroids such as methandrostenolone bind weakly to this receptor in vitro, but still exhibit androgenic effects in vivo. The reason for this discrepancy is not known.[24]
The effect of anabolic steroids on muscle mass is caused in at least two ways:[25] first, they increase the production of proteins; second, they reduce recovery time by blocking the effects of stress hormone cortisol on muscle tissue, so that catabolism of muscle is greatly reduced. It has been hypothesized that this reduction in muscle breakdown may occur through anabolic steroids inhibiting the action of other steroid hormones called glucocorticoids that promote the breakdown of muscles.[26] Anabolic steroids also affect the number of cells that develop into fat-storage cells, by favouring cellular differentiation into muscle cells instead.[27] Anabolic steroids can also decrease fat by increasing basal metabolic rate (BMR), since an increase in muscle mass increases BMR.
Anabolic and androgenic effects
| ||||||||||||||||
As the name suggests, anabolic-androgenic steroids have two different, but overlapping, types of effects: anabolic, meaning that they promote anabolism (cell growth), and androgenic (or virilising), meaning that they affect the development and maintenance of masculine characteristics.
Some examples of the anabolic effects of these hormones are increased protein synthesis from amino acids, increased appetite, increased bone remodeling and growth, and stimulation of bone marrow, which increases the production of red blood cells. Through a number of mechanisms anabolic steroids stimulate the formation of muscle cells and hence cause an increase in the size of skeletal muscles, leading to increased strength.[28][29][30]
The androgenic effects of AAS are numerous. Depending on the length of use, the side effects of the steroid can be irreversible. Processes affected include pubertal growth, sebaceous gland oil production, and sexuality (especially in fetal development). Some examples of virilizing effects are growth of the clitoris in females and the penis in male children (the adult penis does not grow, and will eventually get smaller even when exposed to high doses of androgens), increased growth of androgen-sensitive hair (pubic, beard, chest, and limb hair), increased vocal cord size, deepening the voice, increased libido, suppression of natural sex hormones, and impaired production of sperm.[31]
The androgenic:anabolic ratio of an AAS is an important factor when determining the clinical application of these compounds. Compounds with a high ratio of androgenic to an anabolic effects are the drug of choice in androgen-replacement therapy (e.g., treating hypogonadism in males), whereas compounds with a reduced androgenic:anabolic ratio are preferred for anemia and osteoporosis, and to reverse protein loss following trauma, surgery, or prolonged immobilization. Determination of androgenic:anabolic ratio is typically performed in animal studies, which has led to the marketing of some compounds claimed to have anabolic activity with weak androgenic effects. This disassociation is less marked in humans, where all anabolic steroids have significant androgenic effects.[18]
A commonly used protocol for determining the androgenic:anabolic ratio, dating back to the 1950s, uses the relative weights of ventral prostate (VP) and levator ani muscle (LA) of male rats. The VP weight is an indicator of the androgenic effect, while the LA weight is an indicator of the anabolic effect. Two or more batches of rats are castrated and given no treatment and respectively some AAS of interest. The LA/VP ratio for an AAS is calculated as the ratio of LA/VP weight gains produced by the treatment with that compound using castrated but untreated rats as baseline: (LAc,t–LAc)/(VPc,t–VPc). The LA/VP weight gain ratio from rat experiments is not unitary for testosterone (typically 0.3–0.4), but it is normalized for presentation purposes, and used as basis of comparison for other AAS, which have their androgenic:anabolic ratios scaled accordingly (as shown in the table above).[24][32] In the early 2000s, this procedure was standardized and generalized throughout OECD in what is now known as the Hershberger assay.
Body composition and strength improvements
A review spanning more than three decades of experimental studies in men found that body weight may increase by 2–5 kg as a result of short-term (<10 weeks) AAS use, which may be attributed mainly to an increase of lean mass. Animal studies also found that fat mass was reduced, but most studies in humans failed to elucidate significant fat mass decrements. The effects on lean body mass have been shown to be dose-dependent. Both muscle hypertrophy and the formation of new muscle fibers have been observed. The hydration of lean mass remains unaffected by AAS use, although small increments of blood volume cannot be ruled out.[33]
The upper region of the body (thorax, neck, shoulders, and upper arm) seems to be more susceptible for AAS than other body regions because of predominance of androgen receptors in the upper body. The largest difference in muscle fiber size between AAS users and non-users was observed in type I muscle fibers of the vastus lateralis and the trapezius muscle as a result of long-term AAS self-administration. After drug withdrawal, the effects fade away slowly, but may persist for more than 6–12 weeks after cessation of AAS use.[33]
The same review observed strength improvements in the range of 5-20% of baseline strength, depending largely on the drugs and dose used as well as the administration period. Overall, the exercise where the most significant improvements were observed is the bench press.[34] For almost two decades, it was assumed that AAS exerted significant effects only in experienced strength athletes, particularly based on the studies of Hervey and coworkers.[35][36] In 1996, a randomized controlled trial published in the New England Journal of Medicine demonstrated, however, that even in novice athletes a 10-week strength training program accompanied by testosterone enanthate at 600 mg/week may improve strength more than training alone does.[34][37] The same study found that dose to be sufficient to significantly improve lean muscle mass relative to placebo even in subjects that did not exercise at all.[37] A 2001 study by the same first author, showed that the anabolic effects of testosterone enanthate were highly dose dependent.[33][38]
Adverse effects
Anabolic steroids can cause many adverse effects. Depending on the length of drug abuse, there is a chance that the immune system can be damaged. Most of these side-effects are dose-dependent, the most common being elevated blood pressure, especially in those with pre-existing hypertension,[39] and harmful changes in cholesterol levels: Some steroids cause an increase in LDL "bad" cholesterol and a decrease in HDL "good" cholesterol.[40] Anabolic steroids have been shown to alter fasting blood sugar and glucose tolerance tests.[41] Anabolic steroids such as testosterone also increase the risk of cardiovascular disease[2] or coronary artery disease.[42][43] Acne is fairly common among anabolic steroid users, mostly due to stimulation of the sebaceous glands by increased testosterone levels.[44][45] Conversion of testosterone to dihydrotestosterone (DHT) can accelerate the rate of premature baldness for males genetically predisposed, but testosterone itself can produce baldness in females.[46]
High doses of oral anabolic steroid compounds can cause liver damage, as the steroids are metabolized (17α-alkylated) in the digestive system to increase their bioavailability and stability.[3]
There are also sex-specific side-effects of anabolic steroids. Development of breast tissue in males, a condition called gynecomastia (which is usually caused by high levels of circulating estradiol), may arise because of increased conversion of testosterone to estradiol by the enzyme aromatase.[47] Reduced sexual function and temporary infertility can also occur in males.[48][49][50] Another male-specific side-effect that can occur is testicular atrophy, caused by the suppression of natural testosterone levels, which inhibits production of sperm (most of the mass of the testes is developing sperm). This side-effect is temporary: The size of the testicles usually returns to normal within a few weeks of discontinuing anabolic steroid use as normal production of sperm resumes.[51] Female-specific side-effects include increases in body hair, permanent deepening of the voice, enlarged clitoris, and temporary decreases in menstrual cycles. When taken during pregnancy, anabolic steroids can affect fetal development by causing the development of male features in the female fetus and female features in the male fetus.[52]
A number of severe side-effects can occur if adolescents use anabolic steroids.
For example, the steroids may prematurely stop the lengthening of bones (premature epiphyseal fusion through increased levels of estrogen metabolites), resulting in stunted growth. Other effects include, but are not limited to, accelerated bone maturation, increased frequency and duration of erections, and premature sexual development. Anabolic steroid use in adolescence is also correlated with poorer attitudes related to health.[53]
Other side-effects can include alterations in the structure of the heart, such as enlargement and thickening of the left ventricle, which impairs its contraction and relaxation.[4] Possible effects of these alterations in the heart are hypertension, cardiac arrhythmias, congestive heart failure, heart attacks, and sudden cardiac death.[54] These changes are also seen in non-drug-using athletes, but steroid use may accelerate this process.[55][56] However, both the connection between changes in the structure of the left ventricle and decreased cardiac function, as well as the connection to steroid use have been disputed.[57][58]
Psychiatric effects
A 2005 review in CNS Drugs determined that "significant psychiatric symptoms including aggression and violence, mania, and less frequently psychosis and suicide have been associated with steroid abuse. Long-term steroid abusers may develop symptoms of dependence and withdrawal on discontinuation of AAS".[59] High concentrations of AAS, comparable to those likely sustained by many recreational AAS users, produce apoptotic effects on neurons, raising the specter of possibly irreversible neuropsychiatric toxicity. Recreational AAS use appears to be associated with a range of potentially prolonged psychiatric effects, including dependence syndromes, mood disorders, and progression to other forms of substance abuse, but the prevalence and severity of these various effects remains poorly understood.[60] There is no evidence that steroid dependence develops from therapeutic use of anabolic steroids to treat medical disorders, but instances of AAS dependence have been reported among weightlifters and bodybuilders who chronically administered supraphysiologic doses.[61] Mood disturbances (e.g. depression, [hypo-]mania, psychotic features) are likely to be dose- and drug-dependent, but AAS dependence or withdrawal effects seem to occur only in a small number of AAS users.[62]
Large-scale long-term studies of psychiatric effects on AAS users are not currently available.[60] In 2003, the first naturalistic long-term study on ten users, seven of which having completed the study, found a high incidence of mood disorders and substance abuse, but few clinically relevant changes in physiological parameters or laboratory measures were noted throughout the study, and these changes were not clearly related to periods of reported AAS use.[63] A 13-month study, which was published in 2006 and which involved 320 body builders and athletes suggests that the wide range of psychiatric side-effects induced by the use of AAS is correlated to the severity of abuse.[64]
Aggression and hypomania
From the mid-1980s onward, the media reported "roid rage" as a side-effect of AAS.[65]
A 2005 review determined that some, but not all, randomized controlled studies have found that anabolic steroid use correlates with hypomania and increased aggressiveness, but pointed out that attempts to determine whether AAS use triggers violent behaviour have failed, primarily because of high rates of non-participation.[66] A 2008 study on a nationally representative sample of young adult males in the United States found an association between lifetime and past-year self-reported anabolic-androgenic steroid use and involvement in violent acts. Compared with individuals that did not use steroids, young adult males that used anabolic-androgenic steroids reported greater involvement in violent behaviors even after controlling for the effects of key demographic variables, previous violent behavior, and polydrug use.[67] A 1996 review examining the blind studies available at that time also found that these had demonstrated a link between aggression and steroid use, but pointed out that with estimates of over one million past or current steroid users in the United States at that time, an extremely small percentage of those using steroids appear to have experienced mental disturbance severe enough to result in clinical treatments or medical case reports.[68]
A 1996 randomized controlled trial, which involved 43 men, did not find an increase in the occurrence of angry behavior during 10 weeks of administration of testosterone enanthate at 600 mg/week, but this study screened out subjects that had previously abused steroids or had any psychiatric antecedents.[37][69] A trial conducted in 2000 using testosterone cypionate at 600 mg/week found that treatment significantly increased manic scores on the YMRS, and aggressive responses on several scales. The drug response was highly variable. However: 84% of subjects exhibited minimal psychiatric effects, 12% became mildly hypomanic, and 4% (2 subjects) became markedly hypomanic. The mechanism of these variable reactions could not be explained by demographic, psychological, laboratory, or physiological measures.[70]
A 2006 study of two pairs of identical twins, in which one twin used anabolic steroids and the other did not, found that in both cases the steroid-using twin exhibited high levels of aggressiveness, hostility, anxiety, and paranoid ideation not found in the "control" twin.[71] A small-scale study of 10 AAS users found that cluster B personality disorders were confounding factors for aggression.[72]
Depression and suicide
The relationship between AAS use and depression is inconclusive. There have been anecdotal reports of depression and suicide in teenage steroid users,[73] but little systematic evidence. A 1992 review found that anabolic-androgenic steroids may both relieve and cause depression, and that cessation or diminished use of anabolic-androgenic steroids may also result in depression, but called for additional studies due to disparate data.[74]
Addiction potential
In an animal study, male rats developed a conditioned place preference to testosterone injections into the nucleus accumbens, an effect blocked by dopamine antagonists, which suggests that androgen reinforcement is mediated by the brain. Moreover, testosterone appears to act through the mesolimbic dopamine system, a common substrate for drugs of abuse. Nonetheless, androgen reinforcement is not comparable to that of cocaine, nicotine, or heroin. Instead, testosterone resembles other mild reinforcers, such as caffeine, or benzodiazepines. The potential for androgen addiction remains to be determined.[75]
Medical and ergogenic uses
Medical uses

Since the discovery and synthesis of testosterone in the 1930s, anabolic steroids have been used by physicians for many purposes, with varying degrees of success, for the treatment of:
- Bone marrow stimulation: For decades, anabolic steroids were the mainstay of therapy for hypoplastic anemias due to leukemia or kidney failure, especially aplastic anemia.[76] Anabolic steroids have largely been replaced in this setting by synthetic protein hormones (such as epoetin alfa) that selectively stimulate growth of blood cell precursors.
- Growth stimulation: Anabolic steroids can be used by pediatric endocrinologists to treat children with growth failure.[77] However, the availability of synthetic growth hormone, which has fewer side effects, makes this a secondary treatment.
- Stimulation of appetite and preservation and increase of muscle mass: Anabolic steroids have been given to people with chronic wasting conditions such as cancer and AIDS.[78][79]
- Induction of male puberty: Androgens are given to many boys distressed about extreme delay of puberty. Testosterone is now nearly the only androgen used for this purpose and has been shown to increase height, weight, and fat-free mass in boys with delayed puberty.[80]
- Male contraception, in the form of testosterone enanthate; potential for use in the near-future as a safe, reliable, and reversible male contraceptive.[50][81]
- Stimulation of lean body mass and prevention of bone loss in elderly men, as some studies indicate.[82][83][84] However, a 2006 placebo-controlled trial of low-dose testosterone supplementation in elderly men with low levels of testosterone found no benefit on body composition, physical performance, insulin sensitivity, or quality of life.[85]
- Hormone replacement for men with low levels of testosterone; also effective in improving libido for elderly males.[86][87][88][89]
- Gender Identity Disorder, by producing secondary male characteristics, such as a deeper voice, increased bone and muscle mass, facial hair, increased levels of red blood cells, and clitoral enlargement in female-to-male patients.[90]
It should be noted that although steroids do have medical uses, they may also have side effects - for example, the potential risk of osteoporosis[citation needed].
Ergogenic use and abuse

Between 1 million and 3 million people (1% of the population) are thought to have misused AAS in the United States.[91] Studies in the United States have shown that anabolic steroid users tend to be mostly middle-class heterosexual men with a median age of about 25 who are noncompetitive bodybuilders and non-athletes and use the drugs for cosmetic purposes.[92] "Among 12- to 17-year-old boys, use of steroids and similar drugs jumped 25 percent from 1999 to 2000, with 20 percent saying they use them for looks rather than sports, a study by insurer Blue Cross Blue Shield found."(Eisenhauer)Cite error: The <ref> tag has too many names (see the help page). According to a recent survey, 78.4% of steroid users were noncompetitive bodybuilders and non-athletes, while about 13% reported unsafe injection practices such as reusing needles, sharing needles, and sharing multidose vials,[93] though a 2007 study found that sharing of needles was extremely uncommon among individuals using anabolic steroids for non-medical purposes, less than 1%.[19] Another 2007 study found that 74% of non-medical anabolic steroid users had secondary college degrees and more had completed college and fewer had failed to complete high school than is expected from the general populace.[19] The same study found that individuals using anabolic steroids for non-medical purposes had a higher employment rate and a higher household income than the general population.[19] Anabolic steroid users tend to research the drugs they are taking more than other controlled-substance users; however, the major sources consulted by steroid users include friends, non-medical handbooks, internet-based forums, blogs, and fitness magazines, which can provide questionable or inaccurate information.[94]
Anabolic steroid users tend to be disillusioned by the portrayal of anabolic steroids as deadly in the media and in politics.[95] According to one study, AAS users also distrust their physicians and in the sample 56% had not disclosed their AAS use to their physicians.[96] Another 2007 study had similar findings, showing that, while 66% of individuals using anabolic steroids for non-medical purposes were willing to seek medical supervision for their steroid use, 58% lacked trust in their physicians, 92% felt that the medical community's knowledge of non-medical anabolic steroid use was lacking, and 99% felt that the public has an exaggerated view of the side-effects of anabolic steroid use.[19] A recent study has also shown that long term AAS users were more likely to have symptoms of muscle dysmorphia and also showed stronger endorsement of more conventional male roles.[97]
Anabolic steroids have been used by men and women in many different kinds of professional sports to attain a competitive edge or to assist in recovery from injury. These sports include bodybuilding, weightlifting, shot put and other track and field, cycling, baseball, wrestling, mixed martial arts, boxing, football, and cricket. Such use is prohibited by the rules of the governing bodies of most sports. Anabolic steroid use occurs among adolescents, especially by those participating in competitive sports. It has been suggested that the prevalence of use among high-school students in the U.S. may be as high as 2.7%.[98] Male students used anabolic steroids more frequently than female students and, on average, those that participated in sports used steroids more often than those that did not.
Legal and sport restrictions
Legal status
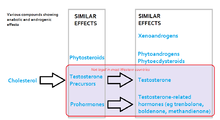
The legal status of anabolic steroids varies from country to country: some have stricter controls on their use or prescription than others though in many countries they are not illegal. In the U.S., anabolic steroids are currently listed as Schedule III controlled substances under the Controlled Substances Act, which makes the first offense simple possession of such substances without a prescription a federal crime punishable by up to one year in prison, and the unlawful distribution or possession with intent to distribute anabolic steroids punishable as a first offense by up to ten years in prison.[99] In Canada, anabolic steroids and their derivatives are part of the Controlled drugs and substances act and are Schedule IV substances, meaning that it is illegal to obtain or sell them without a prescription; however, possession is not punishable, a consequence reserved for schedule I, II, or III substances. Those guilty of buying or selling anabolic steroids in Canada can be imprisoned for up to 18 months. Import and export also carry similar penalties.[100] In Canada, researchers have concluded that steroid use among student athletes is extremely widespread. A study conducted in 1993 by the Canadian Centre for Drug-Free Sport found that nearly 83,000 Canadians between the ages of 11 and 18 use steroids.[101] Anabolic steroids are also illegal without prescription in Australia,[102] Argentina, Brazil and Portugal,[103] and are listed as Class C Controlled Drugs in the United Kingdom. Anabolic steroids are readily available without a prescription in some countries such as Mexico and Thailand.
United States

The history of the U.S. legislation on anabolic steroids goes back to the late 1980s, when the U.S. Congress considered placing anabolic steroids under the Controlled Substances Act following the controversy over Ben Johnson's victory at the 1988 Summer Olympics in Seoul. During deliberations, the American Medical Association (AMA), Drug Enforcement Administration (DEA), Food and Drug Administration (FDA) as well as the National Institute on Drug Abuse (NIDA) all opposed listing anabolic steroids as controlled substances, citing the fact that use of these hormones does not lead to the phtytytytytyttytytyysical or psychological dependence required for such scheduling under the Controlled Substance Act. Nevertheless, anabolic steroids were added to Schedule III of the Controlled Substances Act in the Anabolic Steroid Control Act of 1990.[104]
The same act also introduced more stringent controls with higher criminal penalties for offenses involving the illegal distribution of anabolic steroids and human growth hormone. By the early 1990s, after anabolic steroids were scheduled in the U.S., several pharmaceutical companies stopped manufacturing or marketing the products in the U.S., including Ciba, Searle, Syntex, and others. In the Controlled Substances Act, anabolic steroids are defined to be any drug or hormonal substance chemically and pharmacologically related to testosterone (other than estrogens, progestins, and corticosteroids) that promote muscle growth. The act was amended by the Anabolic Steroid Control Act of 2004, which added prohormones to the list of controlled substances, with effect from January 20, 2005.[105]
United Kingdom
In the United Kingdom, anabolic steroids are classified as class C drugs for their illegal abuse potential, which puts them in the same class as benzodiazepines. Anabolic steroids are in Schedule 4, which is divided in 2 parts; Part 1 contains most of the benzodiazepines and Part 2 contains the anabolic and androgenic steroids. There are no special controls on Schedule 4 drugs. Many prohormones or designer steroids remain legal in the UK;[citation needed] however, they may be banned by anti-doping regulations in sports.
Status in sports
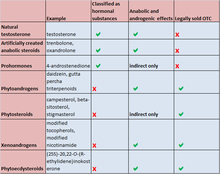
Anabolic steroids are banned by all major sports bodies including Fédération Internationale de Football Association[106] the Olympics,[107] the National Basketball Association,[108] the National Hockey League,[109] and the National Football League.[110] The World Anti-Doping Agency (WADA) maintains the list of performance-enhancing substances used by many major sports bodies and includes all anabolic agents, which includes all anabolic steroids and precursors as well as all hormones and related substances.[111][112] Spain has passed an anti-doping law creating a national anti-doping agency.[113] Italy passed a law in 2000 where penalties range up to three years in prison if an athlete has tested positive for banned substances.[114] In 2006, Russian President Vladimir Putin signed into law ratification of the International Convention Against Doping in Sport which would encourage cooperation with WADA. Many other countries have similar legislation prohibiting anabolic steroids in sports including Denmark,[115] France,[116] the Netherlands[117] and Sweden.[118]
Detection of use
The most commonly employed human physiological specimen for detecting anabolic steroid usage is urine, although both blood and hair have been investigated for this purpose. The anabolic steroids, whether of endogenous or exogenous origin, are subject to extensive hepatic biotransformation by a variety of enzymatic pathways. The primary urinary metabolites may be detectable for up to 30 days after the last use, depending on the specific agent, dose and route of administration. A number of the drugs have common metabolic pathways, and their excretion profiles may overlap those of the endogenous steroids, making interpretation of testing results a very significant challenge to the analytical chemist. Methods for detection of the substances or their excretion products in urine specimens usually involve gas chromatography–mass spectrometry or liquid chromatography-mass spectrometry.[119][120][121][122]
Illegal trade

Anabolic steroids are frequently produced in pharmaceutical laboratories, but, in nations where stricter laws are present, they are also produced in small home-made underground laboratories, usually from raw substances imported from abroad.[123] In these countries, the majority of steroids are obtained illegally through black market trade.[124][125] These steroids are usually manufactured in other countries, and therefore must be smuggled across international borders. As with most significant smuggling operations, organized crime is involved.[126]
In the late 2000s, the worldwide trade in illicit AAS increased significantly, and authorities announced record captures on three continents. In 2006, Finnish authorities announced a record seizure of 11.8 million AAS tablets. A year later, the DEA seized 11.4 million units of AAS in the largest U.S seizure ever. In the first three months of 2008, Australian customs reported a record 300 seizures of AAS shipments.[127]
In the U.S., Canada, and Europe, illegal steroids are sometimes purchased just as any other illegal drug, through dealers who are able to obtain the drugs from a number of sources. Illegal anabolic steroids are sometimes sold at gyms and competitions, and through the mail, but may also be obtained through pharmacists, veterinarians, and physicians.[128] In addition, a significant number of counterfeit products are sold as anabolic steroids, in particular via mail order from websites posing as overseas pharmacies. In the U.S., black-market importation continues from Mexico, Thailand, and other countries where steroids are more easily available, as they are legal.[129]
See also
131.Tygart, Travis T. "Steroids, the Media, and Youth." Prevention Researcher Integrated Research Services, Inc., Vol. 16 Supplement. December 2009: 7-9. SIRS Researcher. Web. 25 Oct 2010.
References
- ^ Michael Powers, "Performance-Enhancing Drugs" in Joel Houglum, in Gary L. Harrelson, Deidre Leaver-Dunn, "Principles of Pharmacology for Athletic Trainers", SLACK Incorporated, 2005, ISBN 1-55642-594-5, p. 330
- ^ a b Barrett-Connor E (1995). "Testosterone and risk factors for cardiovascular disease in men". Diabete Metab. 21 (3): 156–61. PMID 7556805.
- ^ a b Yamamoto Y, Moore R, Hess H, Guo G, Gonzalez F, Korach K, Maronpot R, Negishi M (2006). "Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity". J Biol Chem. 281 (24): 16625–31. doi:10.1074/jbc.M602723200. PMID 16606610.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ a b De Piccoli B, Giada F, Benettin A, Sartori F, Piccolo E (1991). "Anabolic steroid use in body builders: an echocardiographic study of left ventricle morphology and function". Int J Sports Med. 12 (4): 408–12. doi:10.1055/s-2007-1024703. PMID 1917226.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c Hartgens and Kuipers (2004), p. 515
- ^ a b Kicman AT, Gower DB (2003). "Anabolic steroids in sport: biochemical, clinical and analytical perspectives". Annals of Clinical Biochemistry. 40 (Pt 4): 321–56. doi:10.1258/000456303766476977. PMID 12880534.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Kuhn CM (2002). "Anabolic steroids". Recent Prog. Horm. Res. 57 (1): 411–34. doi:10.1210/rp.57.1.411. PMID 12017555.
- ^ a b c d e Hoberman JM, Yesalis CE (1995). "The history of synthetic testosterone". Scientific American. 272 (2): 76–81. doi:10.1038/scientificamerican0295-76. PMID 7817189.
- ^ a b Freeman ER, Bloom DA, McGuire EJ (2001). "A brief history of testosterone". Journal of Urology. 165 (2): 371–373. doi:10.1097/00005392-200102000-00004. PMID 11176375.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ David K, Dingemanse E, Freud J, Laqueur L (1935). "Uber krystallinisches mannliches Hormon aus Hoden (Testosteron) wirksamer als aus harn oder aus Cholesterin bereitetes Androsteron". Hoppe Seylers Z Physiol Chem. 233 (5–6): 281. doi:10.1515/bchm2.1935.233.5-6.281.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Butenandt A, Hanisch G. (1935). "A Method for Preparing Testosterone from Cholesterol". Chemische Berichte. 68: 1859.
- ^ Ruzicka L, Wettstein A (1935). "Sexualhormone VII. Uber die kunstliche Herstellung des Testikelhormons. Testosteron (Androsten-3-one-17-ol.)". Helvetica Chimica Acta. 18: 1264. doi:10.1002/hlca.193501801176.
- ^ a b Pat Lenehan, "Anabolic Steroids: And Other Performance-enhancing Drugs", CRC Press, 2003, ISBN 0-415-28030-3, page 6
- ^ a b Taylor, William N (January 1, 2002). Anabolic Steroids and the Athlete. McFarland & Company. p. 181. ISBN 0-7864-1128-7.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - ^ Calfee R, Fadale P (2006). "Popular ergogenic drugs and supplements in young athletes". Pediatrics. 117 (3): e577–89. doi:10.1542/peds.2005-1429. PMID 16510635.
- ^ Justin Peters The Man Behind the Juice, Slate Friday, Feb. 18, 2005, Accessed 29 April 2008
- ^ Hartgens and Kuipers (2004), p. 516
- ^ a b c George P. Chrousos, The gonadal hormones and inhibitors, in Bertram G. Katzung (Ed.), Basic and Clinical Pharmacology, McGraw-Hill Professional, 2006, ISBN 0-07-145153-6, p. 674–676
- ^ a b c d e Cohen, J. (2007). "A league of their own: demographics, motivations and patterns of use of 1,955 male adult non-medical anabolic steroid users in the United States". Feedback. 4: 12. doi:10.1186/1550-2783-4-12. PMC 2131752. PMID 17931410.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help)CS1 maint: unflagged free DOI (link) - ^ Mutzebaugh C (1998). "Does the choice of alpha-AAS really make a difference?". HIV Hotline. 8 (5–6): 10–1. PMID 11366379.
- ^ Pereira de Jésus-Tran K, Côté PL, Cantin L, Blanchet J, Labrie F, Breton R (2006). "Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity". Protein Sci. 15 (5): 987–99. doi:10.1110/ps.051905906. PMC 2242507. PMID 16641486.
{{cite journal}}: no-break space character in|title=at position 105 (help)CS1 maint: multiple names: authors list (link) - ^ Lavery DN, McEwan IJ (2005). "Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations". Biochem. J. 391 (Pt 3): 449–64. doi:10.1042/BJ20050872. PMC 1276946. PMID 16238547.
- ^ Cheskis B (2004). "Regulation of cell signalling cascades by steroid hormones". J. Cell. Biochem. 93 (1): 20–7. doi:10.1002/jcb.20180. PMID 15352158.
- ^ a b Roselli CE (1998). "The effect of anabolic-androgenic steroids on aromatase activity and androgen receptor binding in the rat preoptic area". Brain Res. 792 (2): 271–6. doi:10.1016/S0006-8993(98)00148-6. PMID 9593936.
- ^ Brodsky I, Balagopal P, Nair K (1996). "Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men—a clinical research center study". J. Clin. Endocrinol. Metab. 81 (10): 3469–75. doi:10.1210/jc.81.10.3469. PMID 8855787.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hickson R, Czerwinski S, Falduto M, Young A (1990). "Glucocorticoid antagonism by exercise and androgenic-anabolic steroids". Med Sci Sports Exerc. 22 (3): 331–40. PMID 2199753.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Singh R, Artaza J, Taylor W, Gonzalez-Cadavid N, Bhasin S (2003). "Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway". Endocrinology. 144 (11): 5081–8. doi:10.1210/en.2003-0741. PMID 12960001.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Schroeder E, Vallejo A, Zheng L; et al. (2005). "Six-week improvements in muscle mass and strength during androgen therapy in older men". J Gerontol a Biol Sci Med Sci. 60 (12): 1586–92. doi:10.1093/gerona/60.12.1586. PMID 16424293.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Grunfeld C, Kotler D, Dobs A, Glesby M, Bhasin S (2006). "Oxandrolone in the treatment of HIV-associated weight loss in men: a randomized, double-blind, placebo-controlled study". J Acquir Immune Defic Syndr. 41 (3): 304–14. doi:10.1097/01.qai.0000197546.56131.40. PMID 16540931.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Giorgi A, Weatherby R, Murphy P (1999). "Muscular strength, body composition and health responses to the use of testosterone enanthate: a double blind study". Journal of science and medicine in sport / Sports Medicine Australia. 2 (4): 341–55. PMID 10710012.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kuhn CM (2002). "Recent Progress in Hormone Research - Anabolic steroids". The Endocrine Society. 57 (57). Department of Pharmacology and Cancer Biology, Duke University Medical Center, Durham, North Carolina: 411–434. doi:10.1210/rp.57.1.411. PMID 12017555.
- ^ L.G. Hershberger, E.G. Shipley, R.K. Meyer, Myotropic activity of 19-nortestosterone and other steroids determined by modified levator ani muscle method, Proc. Soc. Exp. Biol. Med. 83 (1953), 175-180
- ^ a b c Hartgens and Kuipers (2004), p. 519-527
- ^ a b Hartgens and Kuipers (2004), p. 528
- ^ Hervey GR, Hutchinson I, Knibbs AV; et al. (1976). ""Anabolic" effects of methandienone in men undergoing athletic training". Lancet. 2 (7988): 699–702. doi:10.1016/S0140-6736(76)90001-5. PMID 61389.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Hervey GR, Knibbs AV, Burkinshaw L; et al. (1981). "Effects of methandienone on the performance and body composition of men undergoing athletic training". Clin. Sci. 60 (4): 457–61. PMID 7018798.
{{cite journal}}: Explicit use of et al. in:|author=(help); Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ a b c Bhasin S, Storer T, Berman N; et al. (1996). "The effects of supraphysiologic doses of testosterone on muscle size and strength in normal men". N. Engl. J. Med. 335 (1): 1–7. doi:10.1056/NEJM199607043350101. PMID 8637535.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Bhasin S, Woodhouse L, Casaburi R; et al. (2001). "Testosterone dose-response relationships in healthy young men". Am J Physiol Endocrinol Metab. 281 (6): E1172–81. PMID 11701431.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Grace F, Sculthorpe N, Baker J, Davies B (2003). "Blood pressure and rate pressure product response in males using high-dose anabolic-androgenic steroids (AAS)". J Sci Med Sport. 6 (3): 307–12. doi:10.1016/S1440-2440(03)80024-5. PMID 14609147.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tokar, Steve (2006). "Liver Damage And Increased Heart Attack Risk Caused By Anabolic Steroid Use". University of California - San Francisco. Retrieved 2007-04-24.
{{cite web}}: Unknown parameter|month=ignored (help) - ^ "DailyMed: About DailyMed". Dailymed.nlm.nih.gov. Retrieved 2008-11-03.
- ^ Bagatell C, Knopp R, Vale W, Rivier J, Bremner W (1992). "Physiologic testosterone levels in normal men suppress high-density lipoprotein cholesterol levels". Ann Intern Med. 116 (12 Pt 1): 967–73. PMID 1586105.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mewis C, Spyridopoulos I, Kühlkamp V, Seipel L (1996). "Manifestation of severe coronary heart disease after anabolic drug abuse". Clinical Cardiology. 19 (2): 153–5. doi:10.1002/clc.4960190216. PMID 8821428.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hartgens and Kuipers (2004), p. 543
- ^ Melnik B, Jansen T, Grabbe S (2007). "Abuse of anabolic-androgenic steroids and bodybuilding acne: an underestimated health problem". Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 5 (2): 110–7. doi:10.1111/j.1610-0387.2007.06176.x. PMID 17274777.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Vierhapper H, Maier H, Nowotny P, Waldhäusl W (2003). "Production rates of testosterone and of dihydrotestosterone in female pattern hair loss". Metab. Clin. Exp. 52 (7): 927–9. doi:10.1016/S0026-0495(03)00060-X. PMID 12870172.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Marcus R, Korenman S (1976). "Estrogens and the human male". Annu Rev Med. 27: 357–70. doi:10.1146/annurev.me.27.020176.002041. PMID 779604.
- ^ Hoffman JR, Ratamess NA (June 1, 2006). "Medical Issues Associated with Anabolic Steroid Use: Are they Exaggerated?" (PDF). Journal of Sports Science and Medicine. Archived from the original (PDF) on 20 June 2007. Retrieved 2007-05-08.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Meriggiola M, Costantino A, Bremner W, Morselli-Labate A (2002). "Higher testosterone dose impairs sperm suppression induced by a combined androgen-progestin regimen". J. Androl. 23 (5): 684–90. PMID 12185103.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Matsumoto A (1990). "Effects of chronic testosterone administration in normal men: safety and efficacy of high dosage testosterone and parallel dose-dependent suppression of luteinizing hormone, follicle-stimulating hormone, and sperm production". J. Clin. Endocrinol. Metab. 70 (1): 282–7. doi:10.1210/jcem-70-1-282. PMID 2104626.
- ^ Alén M, Reinilä M, Vihko R (1985). "Response of serum hormones to androgen administration in power athletes". Medicine and science in sports and exercise. 17 (3): 354–9. PMID 2991700.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Manikkam M, Crespi E, Doop D; et al. (2004). "Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep". Endocrinology. 145 (2): 790–8. doi:10.1210/en.2003-0478. PMID 14576190.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Irving L, Wall M, Neumark-Sztainer D, Story M (2002). "Steroid use among adolescents: findings from Project EAT". The Journal of adolescent health : official publication of the Society for Adolescent Medicine. 30 (4): 243–52. doi:10.1016/S1054-139X(01)00414-1. PMID 11927236.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sullivan ML, Martinez CM, Gallagher EJ (1999). "Atrial fibrillation and anabolic steroids". The Journal of emergency medicine. 17 (5): 851–7. doi:10.1016/S0736-4679(99)00095-5. PMID 10499702.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dickerman RD, Schaller F, McConathy WJ (1998). "Left ventricular wall thickening does occur in elite power athletes with or without anabolic steroid Use". Cardiology. 90 (2): 145–8. doi:10.1159/000006834. PMID 9778553.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ George KP, Wolfe LA, Burggraf GW (1991). "The 'athletic heart syndrome'. A critical review". Sports medicine (Auckland, N.Z.). 11 (5): 300–30. doi:10.2165/00007256-199111050-00003. PMID 1829849.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Dickerman R, Schaller F, Zachariah N, McConathy W (1997). "Left ventricular size and function in elite bodybuilders using anabolic steroids". Clin J Sport Med. 7 (2): 90–3. doi:10.1097/00042752-199704000-00003. PMID 9113423.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Salke RC, Rowland TW, Burke EJ (1985). "Left ventricular size and function in body builders using anabolic steroids". Medicine and science in sports and exercise. 17 (6): 701–4. doi:10.1249/00005768-198512000-00014. PMID 4079743.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Trenton AJ, Currier GW (2005). "Behavioural manifestations of anabolic steroid use". CNS Drugs. 19 (7): 571–95. doi:10.2165/00023210-200519070-00002. PMID 15984895.
- ^ a b Kanayama G, Hudson JI, Pope HG (2008). "Long-Term Psychiatric and Medical Consequences of Anabolic-Androgenic Steroid Abuse: A Looming Public Health Concern?". Drug Alcohol Depend. 98 (1–2): 1–12. doi:10.1016/j.drugalcdep.2008.05.004. PMC 2646607. PMID 18599224.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Brower KJ (2002). "Anabolic steroid abuse and dependence". Curr Psychiatry Rep. 4 (5): 377–87. doi:10.1007/s11920-002-0086-6. PMID 12230967.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Hartgens and Kuipers (2004), p. 514–515
- ^ Fudala P, Weinrieb R, Calarco J, Kampman K, Boardman C (2003). "An evaluation of anabolic-androgenic steroid abusers over a period of 1 year: seven case studies". Annals of clinical psychiatry : official journal of the American Academy of Clinical Psychiatrists. 15 (2): 121–30. PMID 12938869.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pagonis TA, Angelopoulos NV, Koukoulis GN, Hadjichristodoulou CS (2006). "Psychiatric side effects induced by supraphysiological doses of combinations of anabolic steroids correlate to the severity of abuse". Eur. Psychiatry. 21 (8): 551–62. doi:10.1016/j.eurpsy.2005.09.001. PMID 16356691.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Pat Lenehan, "Anabolic Steroids: And Other Performance-enhancing Drugs", CRC Press, 2003, ISBN 0-415-28030-3, page 23
- ^ Thiblin I, Petersson A (2005). "Pharmacoepidemiology of anabolic androgenic steroids: a review". Fundam Clin Pharmacol. 19 (1): 27–44. doi:10.1111/j.1472-8206.2004.00298.x. PMID 15660958.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Beaver KM, Vaughn MG, Delisi M, Wright JP (2008). "Anabolic-Androgenic Steroid Use and Involvement in Violent Behavior in a Nationally Representative Sample of Young Adult Males in the United States". Am J Public Health. 98 (12): 2185–7. doi:10.2105/AJPH.2008.137018. PMC 2636528. PMID 18923108.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Bahrke MS, Yesalis CE, Wright JE (1996). "Psychological and behavioural effects of endogenous testosterone and anabolic-androgenic steroids. An update". Sports medicine (Auckland, N.Z.). 22 (6): 367–90. doi:10.2165/00007256-199622060-00005. PMID 8969015.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Tricker R, Casaburi R, Storer T; et al. (1996). "The effects of supraphysiological doses of testosterone on angry behavior in healthy eugonadal men—a clinical research center study". J. Clin. Endocrinol. Metab. 81 (10): 3754–8. doi:10.1210/jc.81.10.3754. PMID 8855834.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Pope, Harrison G. (2000). "Effects of Supraphysiologic Doses of Testosterone on Mood and Aggression in Normal Men". Med Sci Sports Exerc. 57 (2). Arch Gen Psychiatry: 133–140. doi:10.1001/archpsyc.57.2.133. PMID 10665615. Archived from the original on 23 March 2007. Retrieved 2007-04-24.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help); Unknown parameter|month=ignored (help) - ^ Pagonis TA, Angelopoulos NV, Koukoulis GN, Hadjichristodoulou CS, Toli PN (2006). "Psychiatric and hostility factors related to use of anabolic steroids in monozygotic twins". Eur. Psychiatry. 21 (8): 563–9. doi:10.1016/j.eurpsy.2005.11.002. PMID 16529916.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Perry PJ, Kutscher EC, Lund BC, Yates WR, Holman TL, Demers L (2003). "Measures of aggression and mood changes in male weightlifters with and without androgenic anabolic steroid use". J. Forensic Sci. 48 (3): 646–51. PMID 12762541.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Teens & Steroids: A Dangerous Mix". CBS. CBS Broadcasting Inc. 2004-06-03. Archived from the original on 10 July 2007. Retrieved 2007-06-27.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Uzych L (1992). "Anabolic-androgenic steroids and psychiatric-related effects: a review". Canadian Journal of Psychiatry. 37 (1): 23–8. PMID 1551042.
- ^ Wood RI (2004). "Reinforcing aspects of androgens". Physiol. Behav. 83 (2): 279–89. doi:10.1016/j.physbeh.2004.08.012. PMID 15488545.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Basaria S, Wahlstrom JT, Dobs AS (2001). "Clinical review 138: Anabolic-androgenic steroid therapy in the treatment of chronic diseases". J. Clin. Endocrinol. Metab. 86 (11): 5108–17. doi:10.1210/jc.86.11.5108. PMID 11701661.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ranke MB, Bierich JR (1986). "Treatment of growth hormone deficiency". Clinics in endocrinology and metabolism. 15 (3): 495–510. doi:10.1016/S0300-595X(86)80008-1. PMID 2429792.
- ^ Grunfeld C, Kotler D, Dobs A, Glesby M, Bhasin S (2006). "Oxandrolone in the treatment of HIV-associated weight loss in men: a randomized, double-blind, placebo-controlled study". J. Acquir. Immune Defic. Syndr. 41 (3): 304–14. doi:10.1097/01.qai.0000197546.56131.40. PMID 16540931.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Berger JR, Pall L, Hall CD, Simpson DM, Berry PS, Dudley R (1996). "Oxandrolone in AIDS-wasting myopathy". AIDS. 10 (14): 1657–62. PMID 8970686.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Arslanian S, Suprasongsin C (1997). "Testosterone treatment in adolescents with delayed puberty: changes in body composition, protein, fat, and glucose metabolism". J. Clin. Endocrinol. Metab. 82 (10): 3213–20. doi:10.1210/jc.82.10.3213. PMID 9329341.
- ^ Aribarg A, Sukcharoen N, Chanprasit Y, Ngeamvijawat J, Kriangsinyos R (1996). "Suppression of spermatogenesis by testosterone enanthate in Thai men". Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 79 (10): 624–9. PMID 8996996.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kenny AM, Prestwood KM, Gruman CA, Marcello KM, Raisz LG (2001). "Effects of transdermal testosterone on bone and muscle in older men with low bioavailable testosterone levels". J. Gerontol. A Biol. Sci. Med. Sci. 56 (5): M266–72. doi:10.1093/gerona/56.5.M266. PMID 11320105.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Baum NH, Crespi CA (2007). "Testosterone replacement in elderly men". Geriatrics. 62 (9): 14–8. PMID 17824721.
- ^ Francis RM (2001). "Androgen replacement in aging men". Calcif. Tissue Int. 69 (4): 235–8. doi:10.1007/s00223-001-1051-9. PMID 11730258.
- ^ Nair KS, Rizza RA, O'Brien P; et al. (2006). "DHEA in elderly women and DHEA or testosterone in elderly men". N. Engl. J. Med. 355 (16): 1647–59. doi:10.1056/NEJMoa054629. PMID 17050889.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Shah K, Montoya C, Persons R (2007). "Do testosterone injections increase libido for elderly hypogonadal patients?". The Journal of family practice. 56 (4): 301–5. PMID 17403329.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Yassin A, Saad F (2007). "Improvement of sexual function in men with late-onset hypogonadism treated with testosterone only". The journal of sexual medicine. 4 (2): 497–501. doi:10.1111/j.1743-6109.2007.00442.x. PMID 17367445.
- ^ Arver S, Dobs A, Meikle A; et al. (1997). "Long-term efficacy and safety of a permeation-enhanced testosterone transdermal system in hypogonadal men". Clin. Endocrinol. (Oxf). 47 (6): 727–37. doi:10.1046/j.1365-2265.1997.3071113.x. PMID 9497881.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Nieschlag E, Büchter D, Von Eckardstein S; et al. (1999). "Repeated intramuscular injections of testosterone undecanoate for substitution therapy in hypogonadal men". Clin. Endocrinol. (Oxf). 51 (6): 757–63. doi:10.1046/j.1365-2265.1999.00881.x. PMID 10619981.
{{cite journal}}: Explicit use of et al. in:|author=(help)CS1 maint: multiple names: authors list (link) - ^ Moore E, Wisniewski A, Dobs A (2003). "Endocrine treatment of transsexual people: a review of treatment regimens, outcomes, and adverse effects". J. Clin. Endocrinol. Metab. 88 (8): 3467–73. doi:10.1210/jc.2002-021967. PMID 12915619.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sjöqvist F, Garle M, Rane A (2008). "Use of doping agents, in particular anabolic steroids, in sports and society". Lancet. 371 (9627): 1872–82. doi:10.1016/S0140-6736(08)60801-6. PMID 18514731.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Yesalis CE, Kennedy NJ, Kopstein AN, Bahrke MS (1993). "Anabolic-androgenic steroid use in the United States". JAMA. 270 (10): 1217–21. doi:10.1001/jama.270.10.1217. PMID 8355384.
{{cite journal}}: CS1 maint: multiple names: authors list (link)" - ^ Andrew, Parkinson (2006). "Anabolic-Androgenic Steroids: A Survey of 500 Users". Medicine & Science in Sports & Exercise. 38 (4). American College of Sports Medicine: 644–651. doi:10.1249/01.mss.0000210194.56834.5d. PMID 16679978. Archived from the original on 4 May 2007. Retrieved 2007-04-24.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Copeland J, Peters R, Dillon P (1998). "A study of 100 anabolic-androgenic steroid users". Med. J. Aust. 168 (6): 311–2. PMID 9549549.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ Eastley, Tony (January 18, 2006). "Steroid study debunks user stereotypes". abc.net.au. Retrieved 2007-04-24.
- ^ Pope HG, Kanayama G, Ionescu-Pioggia M, Hudson JI (2004). "Anabolic steroid users' attitudes towards physicians". Addiction. 99 (9): 1189–94. doi:10.1111/j.1360-0443.2004.00781.x. PMID 15317640.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Kanayama G, Barry S, Hudson JI, Pope HG (2006). "Body image and attitudes toward male roles in anabolic-androgenic steroid users". The American Journal of Psychiatry. 163 (4): 697–703. doi:10.1176/appi.ajp.163.4.697. PMID 16585446.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Hickson R, Czerwinski S, Falduto M, Young A (1990). "Glucocorticoid antagonism by exercise and androgenic-anabolic steroids". Medicine and science in sports and exercise. 22 (3): 331–40. PMID 2199753.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "Title 21 United States Code (USC) Controlled Substances Act". US Department of Justice. Archived from the original on 1 August 2009. Retrieved 2009-09-07.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Controlled Drugs and Substances Act". Canada Department of Justice. Retrieved 2007-04-25.
- ^ Deacon, James (2). "Biceps in a bottle". Maclean's: 52.
{{cite journal}}: Check date values in:|date=and|year=/|date=mismatch (help); Unknown parameter|month=ignored (help) - ^ "Steroids". Australian Institute of Criminology. 2006. Archived from the original on 2007-04-05. Retrieved 2007-05-06.
- ^ "Library of congress search". Library of congress. Retrieved 2007-05-06.
- ^ H.R. 4658
- ^ "News from DEA, Congressional Testimony, 03/16/04". Retrieved 2007-04-24.
- ^ http://es.fifa.com/mm/document/afdeveloping/medical/50/29/56/fifadocregulations_09.01.09_e.pdf
- ^ "Olympic movement anti-doping code" (PDF). International Olympic Committee. 1999. Retrieved 2007-05-06.
- ^ "The nba and nbpa anti-drug program". NBA Policy. findlaw.com. 1999. Retrieved 2007-05-06.
- ^ "NHL/NHLPA performance-enhancing substances program summary". nhlpa.com. Archived from the original on 2 June 2007. Retrieved 2007-05-06.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "List of Prohibited Substances" (PDF). nflpa.com. 2006. Retrieved 2007-05-06.
- ^ "World anti-doping code" (PDF). WADA. 2003. Archived from the original (PDF) on 7 August 2007. Retrieved 2007-07-10.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Prohibited list of 2005" (PDF). WADA. 2005. Retrieved 2007-05-06.
- ^ "Spain's senate passes anti-doping law". Associated press. Herald Tribune. October 5, 2006. Retrieved 2007-05-06.
- ^ Johnson, Kevin (2006-02-20). "Italian anti-doping laws could mean 3 years in jail". USA Today. Retrieved 2007-05-06.
- ^ "Act on promotion of doping-free sport" (PDF). kum.dk. 2004. Retrieved 2007-05-06. [dead link]
- ^ "Protection of health of athletes and the fight against doping" (PDF). WADA. 2006. Retrieved 2007-05-06.
- ^ "Anti-doping legislation in the netherlands" (PDF). WADA. 2006. Retrieved 2007-05-06.
- ^ "The Swedish Act prohibiting certain doping substances (1991:1969)" (PDF). WADA. 1991. Retrieved 2007-05-06.
- ^ Mareck U, Geyer H, Opfermann G, Thevis M, Schänzer W. Factors influencing the steroid profile in doping control analysis. J. Mass Spectrom. 43: 877-891, 2008.
- ^ Fragkaki AG, Angelis YS, Tsantili-Kakoulidou A, Koupparis M, Georgakopoulos C. Schemes of metabolic patterns of anabolic androgenic steroids for the estimation of metabolites of designer steroids in human urine. J. Steroid Biochem. Mol. Biol. 115: 44-61, 2009.
- ^ Blackledge RD. Bad science: the instrumental data in the Floyd Landis case. Clin Chim. Acta. 406: 8-13, 2009.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 8th edition, Biomedical Publications, Foster City, CA, 2008, pp. 95, 393, 403, 649, 695, 952, 962, 1078, 1156, 1170, 1442, 1501, 1581.
- ^ Assael, Shaun (2007-09-24). "'Raw Deal' busts labs across U.S., many supplied by China". ESPN The Magazine. Archived from the original on 14 October 2007. Retrieved 2007-09-24.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Yesalis, C (2000). "Source of Anabolic Steroids". Anabolic Steroids in Sport and Exercise. Champaign, Ill.: Human Kinetics. ISBN 978-0-88011-786-9.
- ^ Black, Terry (1996). "Does the Ban on Drugs in Sport Improve Societal Welfare?". Faculty of Business, Queensland University of Technology. Retrieved 2007-04-24.
- ^ Richard W. Pound. (2006). "Organized Crime". Inside dope : how drugs are the biggest threat to sports, why you should care, and what can be done about them. Mississaug, Ontario: Wiley. p. 175. ISBN 978-0-470-83733-7.
- ^ Kanayama G, Hudson JI, Pope HG (2008). "Long-Term Psychiatric and Medical Consequences of Anabolic-Androgenic Steroid Abuse: A Looming Public Health Concern?". Drug Alcohol Depend. 98 (1–2): 1–12. doi:10.1016/j.drugalcdep.2008.05.004. PMC 2646607. PMID 18599224.
{{cite journal}}: Unknown parameter|month=ignored (help)CS1 maint: multiple names: authors list (link) - ^ "Steroids". National Institute on Drug Abuse. GDCADA. Archived from the original on 2007-09-11. Retrieved 2007-09-13.
- ^ "The Drug Enforcement Administration's International Operations (Redacted)". Office of the Inspector General. USDOJ. Retrieved 2007-09-13.
Tygart, Travis T. "Steroids, the Media, and Youth." Prevention Researcher Integrated Research Services, Inc., Vol. 16 Supplement. December 2009: 7-9. SIRS Researcher. Web. 25 Oct 2010.
Eisenhauer, Lisa. "Do I Look OK?." St. Louis Post-Dispatch (St. Louis, MO). Nov. 7 2005: HF1+. SIRS Researcher. Web. 25 Oct 2010.
Further reading
- D. Kochakian, Charles (2000). Anabolic Steroids in Sport and Exercise. Human Kinetics. ISBN 0-88011-786-9.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - Daniels, R. C. (February 1, 2003). The Anabolic Steroid Handbook. Richard C Daniels. p. 80. ISBN 0-9548227-0-6.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - Gallaway, Steve (January 15, 1997). The Steroid Bible. Belle Intl; 3rd Sprl edition. p. 125. ISBN 1-890342-00-9.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - Llewellyn, William (January 28, 2007). ANABOLICS 2007 : Anabolic Steroid Reference Manual (6th Ed.). Body of Science. p. 988. ISBN 978-0-9679304-6-6.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help) - Roberts, Anthony (2006). Anabolic Steroids: Ultimate Research Guide. Anabolic Books, LLC. p. 394. ISBN 1-59975-100-3.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); Unknown parameter|month=ignored (help) - Yesalis, Charles E. (2000). Anabolic Steroids in Sport and Exercise. Human Kinetics Publishers; 2nd edition. p. 493. ISBN 0-88011-786-9.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help); Unknown parameter|month=ignored (help) - Sigmarsson, Victor (January 15, 2007). Inside Secrets: The Bodybuilder’s Guide To Buying Steroids On The Internet!. SA LABS LLC ; 2nd edition. p. 7. ISBN 978-1-4507-5402-6.
{{cite book}}: Cite has empty unknown parameter:|coauthors=(help)
Tygart, Travis T. "Steroids, the Media, and Youth." Prevention Researcher Integrated Research Services, Inc., Vol. 16 Supplement. December 2009: 7-9. SIRS Researcher. Web. 25 Oct 2010.
