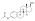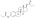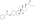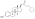Estrogen ester: Difference between revisions
Cite error - refname defined multiple times (relies on transcluded ref) |
|||
| Line 14: | Line 14: | ||
{{Parenteral potencies and durations of estrogens}} |
{{Parenteral potencies and durations of estrogens}} |
||
Polyestradiol phosphate is an atypical estradiol ester.<ref name="pmid3217277">{{cite journal | vauthors = Gunnarsson PO, Norlén BJ | title = Clinical pharmacology of polyestradiol phosphate | journal = Prostate | volume = 13 | issue = 4 | pages = 299–304 | year = 1988 | pmid = 3217277 | doi = 10.1002/pros.2990130405| url = }}</ref><ref name="pmid8610057" |
Polyestradiol phosphate is an atypical estradiol ester.<ref name="pmid3217277">{{cite journal | vauthors = Gunnarsson PO, Norlén BJ | title = Clinical pharmacology of polyestradiol phosphate | journal = Prostate | volume = 13 | issue = 4 | pages = 299–304 | year = 1988 | pmid = 3217277 | doi = 10.1002/pros.2990130405| url = }}</ref><ref name="pmid8610057"/> It is a [[phosphoric acid]] ester of estradiol in the form of a [[polymer]], with an average polymer chain length of approximately 13 [[repeat unit]]s of [[estradiol phosphate]].<ref name="pmid3217277" /> That is, each polyestradiol phosphate [[molecule]] is a polymer consisting on average of 13 estradiol phosphate molecules bonded together.<ref name="pmid3217277" /> Polyestradiol phosphate is slowly [[bond cleavage|cleaved]] into estradiol and phosphoric acid by [[phosphatase]]s.<ref name="pmid3217277" /> Compared to conventional estradiol esters, polyestradiol phosphate has an extremely long duration; its [[elimination half-life]] is approximately 70 days.<ref name="pmid8610057" /> Whereas conventional estradiol esters form a long-lasting depot in muscle and fat at the site of injection,<ref name="pmid16112947" /> this is not the case with polyestradiol phosphate.<ref name="Arzneistoff-Profile">{{cite book|title=Arzneistoff-Profile|editor1-last=Dinnendahl|editor1-first=V|editor2-last=Fricke|editor2-first=U|publisher=Govi Pharmazeutischer Verlag|location=Eschborn, Germany|year=2010|edition=23|volume=4|isbn=978-3-7741-98-46-3|language=German}}</ref> Instead, polyestradiol phosphate is taken up rapidly into the bloodstream following injection (by 90% within 24 hours), where it circulates, and is accumulated in the [[reticuloendothelial system]].<ref name="Arzneistoff-Profile" /> Unlike other estradiol esters, polyestradiol phosphate is resistant to hydrolysis, which may be because it is a [[phosphatase inhibitor]] and may inhibit its own [[metabolism]].<ref name="pmid3217277" /> |
||
Estrogen esters also occur naturally in the body, for instance [[estrogen conjugate]]s like [[estrone sulfate]] and [[estrone glucuronide]] and the very long-lived [[lipoidal estradiol]], which is constituted by ultra-long-chain esters like [[estradiol palmitate]] (ester of 16 carbons) and [[estradiol stearate]] (ester of 18 carbons).<ref name="pmid16112947" /><ref name="OettelSchillinger2012" /><ref name="pmid2197972">{{cite journal | vauthors = Hochberg RB, Pahuja SL, Larner JM, Zielinski JE | title = Estradiol-fatty acid esters. Endogenous long-lived estrogens | journal = Ann. N. Y. Acad. Sci. | volume = 595 | issue = | pages = 74–92 | year = 1990 | pmid = 2197972 | doi = 10.1111/j.1749-6632.1990.tb34284.x| url = }}</ref> |
Estrogen esters also occur naturally in the body, for instance [[estrogen conjugate]]s like [[estrone sulfate]] and [[estrone glucuronide]] and the very long-lived [[lipoidal estradiol]], which is constituted by ultra-long-chain esters like [[estradiol palmitate]] (ester of 16 carbons) and [[estradiol stearate]] (ester of 18 carbons).<ref name="pmid16112947" /><ref name="OettelSchillinger2012" /><ref name="pmid2197972">{{cite journal | vauthors = Hochberg RB, Pahuja SL, Larner JM, Zielinski JE | title = Estradiol-fatty acid esters. Endogenous long-lived estrogens | journal = Ann. N. Y. Acad. Sci. | volume = 595 | issue = | pages = 74–92 | year = 1990 | pmid = 2197972 | doi = 10.1111/j.1749-6632.1990.tb34284.x| url = }}</ref> |
||
Revision as of 10:58, 3 January 2019
An estrogen ester is an ester of an estrogen, most typically of estradiol but also of other estrogens such as estrone, estriol, and even nonsteroidal estrogens like diethylstilbestrol.[1][2][3] Esterification renders estradiol into a prodrug of estradiol with increased resistance to first-pass metabolism, slightly improving its oral bioavailability.[1][2][4] In addition, estrogen esters have increased lipophilicity, which results in a longer duration when given by intramuscular or subcutaneous injection due to the formation of a long-lasting local depot in muscle and fat.[1][2][3] Conversely, this is not the case with intravenous injection or oral administration.[1][5] Estrogen esters are rapidly hydrolyzed into their parent estrogen by esterases once they have been released from the depot.[1][2] Because estradiol esters are prodrugs of estradiol, they are considered to be natural and bioidentical forms of estrogen.[2][1][6]
Estrogen esters are used in hormone therapy, hormonal contraception, and high-dose estrogen therapy (e.g., for prostate cancer and breast cancer), among other indications.[1][2] The first estrogen ester to be marketed was estradiol benzoate in 1936,[7][8] which was followed by many more.[9][10] One of the most widely used estradiol esters is estradiol valerate, which was first introduced in 1954.[11] Other major estradiol esters that are or have been used in medicine include estradiol acetate, estradiol cypionate, estradiol dipropionate, estradiol enantate, estradiol undecylate, and polyestradiol phosphate (an estrogen ester polymer), as well as the nitrogen mustard alkylating antineoplastic agent estramustine phosphate (estradiol normustine phosphate).[2][12]
Pharmacology
Estrogen esters are essentially inactive themselves, with esters such as estradiol valerate and estradiol sulfate having about 2% of the affinity of estradiol for the estrogen receptor.[13] Likewise, the estrogen ether mestranol (ethinylestradiol 3-methyl ether) has about 1% of the affinity of estradiol for the estrogen receptor.[13] Estrone sulfate has less than 1% of the affinity of estradiol for the estrogen receptor.[14]
In general, the longer the fatty acid ester chain of an estrogen ester, the greater its lipophilicity, and the longer the duration of the estrogen ester with intramuscular injection.[1][12] It has been said that, via intramuscular injection, the duration of estradiol benzoate (with an ester of length 1 carbon plus a benzene ring) is 2 to 3 days, of estradiol dipropionate (with two esters each of length 2 carbons) is 1 to 2 weeks, of estradiol valerate (ester of 5 carbons) is 1 to 3 weeks, and of estradiol cypionate (ester of 3 carbons plus a cyclopentane ring) is 3 to 4 weeks.[15] Estradiol enantate (ester of 7 carbons) has a duration of at least 30 days.[2][16][17] Likewise, estradiol undecylate (ester of 10 carbons) has a very extended duration, which is longer than that of all of the aforementioned esters.[12][18][19]
| Estrogen | Dose | Cmax | Tmax | Duration |
|---|---|---|---|---|
| Estradiol benzoate | 5 mg | E2: 940 pg/mL E1: 343 pg/mL |
E2: 1.8 days E1: 2.4 days |
4–5 days |
| Estradiol valerate | 5 mg | E2: 667 pg/mL E1: 324 pg/mL |
E2: 2.2 days E1: 2.7 days |
7–8 days |
| Estradiol cypionate | 5 mg | E2: 338 pg/mL E1: 145 pg/mL |
E2: 3.9 days E1: 5.1 days |
11 days |
| Notes: All via i.m. injection of oil solution. Determinations via radioimmunoassay with chromatographic separation. Sources: See template. | ||||
| Estrogen | Form | Dose (mg) | Duration by dose (mg) | ||
|---|---|---|---|---|---|
| EPD | CICD | ||||
| Estradiol | Aq. soln. | ? | – | <1 d | |
| Oil soln. | 40–60 | – | 1–2 ≈ 1–2 d | ||
| Aq. susp. | ? | 3.5 | 0.5–2 ≈ 2–7 d; 3.5 ≈ >5 d | ||
| Microsph. | ? | – | 1 ≈ 30 d | ||
| Estradiol benzoate | Oil soln. | 25–35 | – | 1.66 ≈ 2–3 d; 5 ≈ 3–6 d | |
| Aq. susp. | 20 | – | 10 ≈ 16–21 d | ||
| Emulsion | ? | – | 10 ≈ 14–21 d | ||
| Estradiol dipropionate | Oil soln. | 25–30 | – | 5 ≈ 5–8 d | |
| Estradiol valerate | Oil soln. | 20–30 | 5 | 5 ≈ 7–8 d; 10 ≈ 10–14 d; 40 ≈ 14–21 d; 100 ≈ 21–28 d | |
| Estradiol benz. butyrate | Oil soln. | ? | 10 | 10 ≈ 21 d | |
| Estradiol cypionate | Oil soln. | 20–30 | – | 5 ≈ 11–14 d | |
| Aq. susp. | ? | 5 | 5 ≈ 14–24 d | ||
| Estradiol enanthate | Oil soln. | ? | 5–10 | 10 ≈ 20–30 d | |
| Estradiol dienanthate | Oil soln. | ? | – | 7.5 ≈ >40 d | |
| Estradiol undecylate | Oil soln. | ? | – | 10–20 ≈ 40–60 d; 25–50 ≈ 60–120 d | |
| Polyestradiol phosphate | Aq. soln. | 40–60 | – | 40 ≈ 30 d; 80 ≈ 60 d; 160 ≈ 120 d | |
| Estrone | Oil soln. | ? | – | 1–2 ≈ 2–3 d | |
| Aq. susp. | ? | – | 0.1–2 ≈ 2–7 d | ||
| Estriol | Oil soln. | ? | – | 1–2 ≈ 1–4 d | |
| Polyestriol phosphate | Aq. soln. | ? | – | 50 ≈ 30 d; 80 ≈ 60 d | |
Notes and sources
Notes: All aqueous suspensions are of microcrystalline particle size. Estradiol production during the menstrual cycle is 30–640 µg/d (6.4–8.6 mg total per month or cycle). The vaginal epithelium maturation dosage of estradiol benzoate or estradiol valerate has been reported as 5 to 7 mg/week. An effective ovulation-inhibiting dose of estradiol undecylate is 20–30 mg/month. Sources: See template. | |||||
Polyestradiol phosphate is an atypical estradiol ester.[20][21] It is a phosphoric acid ester of estradiol in the form of a polymer, with an average polymer chain length of approximately 13 repeat units of estradiol phosphate.[20] That is, each polyestradiol phosphate molecule is a polymer consisting on average of 13 estradiol phosphate molecules bonded together.[20] Polyestradiol phosphate is slowly cleaved into estradiol and phosphoric acid by phosphatases.[20] Compared to conventional estradiol esters, polyestradiol phosphate has an extremely long duration; its elimination half-life is approximately 70 days.[21] Whereas conventional estradiol esters form a long-lasting depot in muscle and fat at the site of injection,[1] this is not the case with polyestradiol phosphate.[22] Instead, polyestradiol phosphate is taken up rapidly into the bloodstream following injection (by 90% within 24 hours), where it circulates, and is accumulated in the reticuloendothelial system.[22] Unlike other estradiol esters, polyestradiol phosphate is resistant to hydrolysis, which may be because it is a phosphatase inhibitor and may inhibit its own metabolism.[20]
Estrogen esters also occur naturally in the body, for instance estrogen conjugates like estrone sulfate and estrone glucuronide and the very long-lived lipoidal estradiol, which is constituted by ultra-long-chain esters like estradiol palmitate (ester of 16 carbons) and estradiol stearate (ester of 18 carbons).[1][2][23]
Time–concentration data
-
Normal menstrual cycle, estradiol benzoate, estradiol valerate, estradiol cypionate, estradiol enantate, estradiol undecylate, and polyestradiol phosphate
-
Estradiol benzoate, estradiol valerate, and estradiol cypionate
-
Estradiol benzoate, estradiol valerate, estradiol cypionate, and estradiol enanthate
-
Estradiol valerate and estradiol undecylate (i.m.)
-
Estradiol (i.v.), estradiol benzoate, estradiol valerate, and estradiol undecylate (i.m.)
-
Estradiol cypionate
-
Polyestradiol phosphate (every 4 weeks)
-
Polyestradiol phosphate (320 mg i.m.)
Chemistry


| Estrogen | Structure | Ester(s) | Relative mol. weight |
Relative E2 contentb |
log Pc | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position(s) | Moiet(ies) | Type | Lengtha | ||||||
| Estradiol | – | – | – | – | 1.00 | 1.00 | 4.0 | ||
| Estradiol acetate | C3 | Ethanoic acid | Straight-chain fatty acid | 2 | 1.15 | 0.87 | 4.2 | ||
| Estradiol benzoate | C3 | Benzoic acid | Aromatic fatty acid | – (~4–5) | 1.38 | 0.72 | 4.7 | ||
| Estradiol dipropionate | C3, C17β | Propanoic acid (×2) | Straight-chain fatty acid | 3 (×2) | 1.41 | 0.71 | 4.9 | ||
| Estradiol valerate | C17β | Pentanoic acid | Straight-chain fatty acid | 5 | 1.31 | 0.76 | 5.6–6.3 | ||
| Estradiol benzoate butyrate | C3, C17β | Benzoic acid, butyric acid | Mixed fatty acid | – (~6, 2) | 1.64 | 0.61 | 6.3 | ||
| Estradiol cypionate | C17β | Cyclopentylpropanoic acid | Cyclic fatty acid | – (~6) | 1.46 | 0.69 | 6.9 | ||
| Estradiol enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | 6.7–7.3 | ||
| Estradiol dienanthate | C3, C17β | Heptanoic acid (×2) | Straight-chain fatty acid | 7 (×2) | 1.82 | 0.55 | 8.1–10.4 | ||
| Estradiol undecylate | C17β | Undecanoic acid | Straight-chain fatty acid | 11 | 1.62 | 0.62 | 9.2–9.8 | ||
| Estradiol stearate | C17β | Octadecanoic acid | Straight-chain fatty acid | 18 | 1.98 | 0.51 | 12.2–12.4 | ||
| Estradiol distearate | C3, C17β | Octadecanoic acid (×2) | Straight-chain fatty acid | 18 (×2) | 2.96 | 0.34 | 20.2 | ||
| Estradiol sulfate | C3 | Sulfuric acid | Water-soluble conjugate | – | 1.29 | 0.77 | 0.3–3.8 | ||
| Estradiol glucuronide | C17β | Glucuronic acid | Water-soluble conjugate | – | 1.65 | 0.61 | 2.1–2.7 | ||
| Estramustine phosphated | C3, C17β | Normustine, phosphoric acid | Water-soluble conjugate | – | 1.91 | 0.52 | 2.9–5.0 | ||
| Polyestradiol phosphatee | C3–C17β | Phosphoric acid | Water-soluble conjugate | – | 1.23f | 0.81f | 2.9g | ||
| Footnotes: a = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic or cyclic fatty acids. b = Relative estradiol content by weight (i.e., relative estrogenic exposure). c = Experimental or predicted octanol/water partition coefficient (i.e., lipophilicity/hydrophobicity). Retrieved from PubChem, ChemSpider, and DrugBank. d = Also known as estradiol normustine phosphate. e = Polymer of estradiol phosphate (~13 repeat units). f = Relative molecular weight or estradiol content per repeat unit. g = log P of repeat unit (i.e., estradiol phosphate). Sources: See individual articles. | |||||||||
See also
- Pharmacokinetics of estradiol
- List of estrogen esters
- List of estrogens
- Steroid ester
- Progestogen ester
- Androgen ester
References
- ^ a b c d e f g h i j Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947.
- ^ a b c d e f g h i Michael Oettel; Ekkehard Schillinger (6 December 2012). Estrogens and Antiestrogens II: Pharmacology and Clinical Application of Estrogens and Antiestrogen. Springer Science & Business Media. pp. 235–237, 261, 271. ISBN 978-3-642-60107-1.
Natural estrogens considered here include: [...] Esters of 17β-estradiol, such as estradiol valerate, estradiol benzoate and estradiol cypionate. Esterification aims at either better absorption after oral administration or a sustained release from the depot after intramuscular administration. During absorption, the esters are cleaved by endogenous esterases and the pharmacologically active 17β-estradiol is released; therefore, the esters are considered as natural estrogens.
- ^ a b R. S. Satoskar; S. D. Bhandarkar &nirmala N. Rege (1969). Pharmacology And Pharmacotherapeutics (New Revised 21 St Ed.). Popular Prakashan. p. 24. ISBN 978-81-7991-527-1. Retrieved 29 May 2012.
- ^ Gordon L. Amidon; Ping I. Lee; Elizabeth M. Topp (2000). Transport Processes in Pharmaceutical Systems. CRC Press. pp. 188–189. ISBN 978-0-8247-6610-8. Retrieved 29 May 2012.
- ^ Parkes AS (February 1938). "Effective Absorption of Hormones" (PDF). Br Med J. 1 (4024): 371–3. PMC 2085798. PMID 20781252.
- ^ Düsterberg B, Nishino Y (December 1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- ^ Enrique Raviña; Hugo Kubinyi (16 May 2011). The Evolution of Drug Discovery: From Traditional Medicines to Modern Drugs. John Wiley & Sons. p. 175. ISBN 978-3-527-32669-3. Retrieved 20 May 2012.
- ^ Folley SJ (December 1936). "The effect of oestrogenic hormones on lactation and on the phosphatase of the blood and milk of the lactating cow" (PDF). The Biochemical Journal. 30 (12): 2262–72. PMC 1263335. PMID 16746289.
- ^ J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 897–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. pp. 404–406. ISBN 978-3-88763-075-1. Retrieved 13 September 2012.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1477–. ISBN 978-0-8155-1856-3.
- ^ a b c Cite error: The named reference
pmid7389356was invoked but never defined (see the help page). - ^ a b Gudermann, T. (2005). "Endokrinpharmakologie": 187–220. doi:10.1007/3-540-26406-X_10.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA (March 1997). "Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta". Endocrinology. 138 (3): 863–70. doi:10.1210/endo.138.3.4979. PMID 9048584.
- ^ H.J. Buchsbaum (6 December 2012). The Menopause. Springer Science & Business Media. pp. 62–. ISBN 978-1-4612-5525-3.
- ^ Recio R, Garza-Flores J, Schiavon R, Reyes A, Diaz-Sanchez V, Valles V, Luz de la Cruz D, Oropeza G, Perez-Palacios G (1986). "Pharmacodynamic assessment of dihydroxyprogesterone acetophenide plus estradiol enanthate as a monthly injectable contraceptive". Contraception. 33 (6): 579–89. doi:10.1016/0010-7824(86)90046-6. PMID 3769482.
- ^ Wiemeyer JC, Fernandez M, Moguilevsky JA, Sagasta CL (1986). "Pharmacokinetic studies of estradiol enantate in menopausic women". Arzneimittelforschung. 36 (11): 1674–7. PMID 3814225.
- ^ Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- ^ R. S. Satoskar; S. D. Bhandarkar &nirmala N. Rege (1973). Pharmacology and Pharmacotherapeutics. Popular Prakashan. pp. 934–. ISBN 978-81-7991-527-1.
- ^ a b c d e Gunnarsson PO, Norlén BJ (1988). "Clinical pharmacology of polyestradiol phosphate". Prostate. 13 (4): 299–304. doi:10.1002/pros.2990130405. PMID 3217277.
- ^ a b Cite error: The named reference
pmid8610057was invoked but never defined (see the help page). - ^ a b Dinnendahl, V; Fricke, U, eds. (2010). Arzneistoff-Profile (in German). Vol. 4 (23 ed.). Eschborn, Germany: Govi Pharmazeutischer Verlag. ISBN 978-3-7741-98-46-3.
- ^ Hochberg RB, Pahuja SL, Larner JM, Zielinski JE (1990). "Estradiol-fatty acid esters. Endogenous long-lived estrogens". Ann. N. Y. Acad. Sci. 595: 74–92. doi:10.1111/j.1749-6632.1990.tb34284.x. PMID 2197972.
- ^ Shellenberger, T. E. (1986). "Pharmacology of estrogens": 393–410. doi:10.1007/978-94-009-4145-8_36.
{{cite journal}}: Cite journal requires|journal=(help)
Further reading
- Vermeulen A (1975). "Longacting steroid preparations". Acta Clin Belg. 30 (1): 48–55. doi:10.1080/17843286.1975.11716973. PMID 1231448.
- Oriowo MA, Landgren BM, Stenström B, Diczfalusy E (1980). "A comparison of the pharmacokinetic properties of three estradiol esters". Contraception. 21 (4): 415–24. doi:10.1016/s0010-7824(80)80018-7. PMID 7389356.
- Düsterberg B, Nishino Y (1982). "Pharmacokinetic and pharmacological features of oestradiol valerate". Maturitas. 4 (4): 315–24. doi:10.1016/0378-5122(82)90064-0. PMID 7169965.
- Sang GW (1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.