Dopamine
| dopamine | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | 3-hydroxytyramine4-(2-aminoethyl)catechol4-(2-aminoethyl)pyrocatecholHydroxytyramin4-(2-Aminoethyl)-1,2-benzenediol4-(2-azanylethyl)benzene-1,2-diolDopastatalpha-(3,4-Dihydroxyphenyl)-beta-aminoethane3,4-DihydroxyphenylethylamineDynatraDophamine4-(2-Aminoethyl)-Pyrocatechola-(3,4-Dihydroxyphenyl)-b-aminoethaneDopaminumHydroxytyramineDopaminRevivanIntropinOxytyramineDeoxyepinephrine2-(3,4-dihydroxyphenyl)ethylamine3,4-Dihydroxyphenethylamine3-Hydroxytyramine4-(2-Aminoethyl)benzene-1,2-diolDopamine4-(2-aminoethyl)-1,2-benzenediolDopamina | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | GeneCards: [1]; OMA:- orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Dopamine is an organic chemical of the catecholamine and phenethylamine families that plays several important roles in the human brain and body, as well as elsewhere in biology. Its name derives from its chemical structure: it is an amine formed by removing a carboxyl group from a molecule of L-DOPA. In the brain, dopamine functions as a neurotransmitter—a chemical released by nerve cells to send signals to other nerve cells. The brain includes several distinct dopamine systems, one of which plays a major role in reward-motivated behavior. Most types of reward increase the level of dopamine in the brain, and a variety of addictive drugs increase dopamine neuronal activity. Other brain dopamine systems are involved in motor control and in controlling the release of various hormones.
Several important diseases of the nervous system are associated with dysfunctions of the dopamine system. Parkinson's disease, a degenerative condition causing tremor and motor impairment, is caused by loss of dopamine-secreting neurons in a midbrain area called the substantia nigra. There is evidence that schizophrenia involves altered levels of dopamine activity, and the antipsychotic drugs that are frequently used to treat it have a primary effect of attenuating dopamine activity.[1] Attention deficit hyperactivity disorder (ADHD) and restless legs syndrome are associated with decreased dopamine activity.[2]
Outside the nervous system, dopamine functions in several parts of the body as a local chemical messenger. In the blood vessels, it inhibits norepinephrine release and acts as a vasodilator (at normal concentrations); in the kidneys, it increases sodium excretion and urine output; in the pancreas, it reduces insulin production; in the digestive system, it reduces gastrointestinal motility and protects intestinal mucosa; and in the immune system, it reduces the activity of lymphocytes. With the exception of the blood vessels, dopamine in each of these peripheral systems has a "paracrine" function: it is synthesized locally and exerts its effects near the cells that release it.
A variety of important drugs work by altering the way the body makes or uses dopamine. Dopamine itself is available for intravenous injection: although it cannot reach the brain from the bloodstream, its peripheral effects make it useful in the treatment of heart failure or shock, especially in newborn babies. L-DOPA, the metabolic precursor of dopamine, does reach the brain and is the most widely used treatment for Parkinson's disease. Dopaminergic stimulants can be addictive in high doses, but some are used at lower doses to treat ADHD. Conversely, many antipsychotic drugs act by suppressing the effects of dopamine. Drugs that act against dopamine by a different mechanism are also some of the most effective anti-nausea agents.
Functions
Cellular effects
| Family | Receptor | Gene | Type | Mechanism |
|---|---|---|---|---|
| D1-like | D1 | DRD1 | Gs-coupled. | Increase intracellular levels of cAMP by activating adenylate cyclase. |
| D5 | DRD5 | |||
| D2-like | D2 | DRD2 | Gi/Go-coupled. | Decrease intracellular levels of cAMP by inhibiting adenylate cyclase. |
| D3 | DRD3 | |||
| D4 | DRD4 |
Like many other biologically active substances, dopamine exerts its effects by binding to and activating receptors located on the surface of cells.[3] In mammals, five subtypes of dopamine receptors have been identified, labeled D1 through D5.[3] All of them function as G protein-coupled receptors, meaning that they exert their effects via a complex second messenger system.[4] Dopamine receptors in mammals can be divided into two families, known as D1-like and D2-like.[3] For receptors located on neurons in the nervous system, the ultimate effect of D1-like activation (D1 and D5) can be excitation (via opening of sodium channels) or inhibition (via opening of potassium channels); the ultimate effect of D2-like activation (D2, D3, and D4) is usually inhibition of the target neuron.[4] Consequently, it is incorrect to describe dopamine itself as either excitatory or inhibitory: its effect on a target neuron depends on which types of receptors are present on the membrane of that neuron and on the internal responses of that neuron to cyclic AMP.[4] D1 receptors are the most numerous dopamine receptors in the human nervous system; D2 receptors are next; D3, D4, and D5 receptors are present at significantly lower levels.[4]
Storage, release, and reuptake
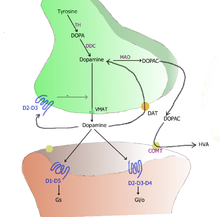
TH: tyrosine hydroxylase
DOPA: L-DOPA
DAT: dopamine transporter
DDC: DOPA decarboxylase
VMAT: vesicular monoamine transporter 2
MAO: Monoamine oxidase
COMT: Catechol-O-methyl transferase
HVA: Homovanillic acid
Inside the brain dopamine functions as a neurotransmitter, and is controlled by a set of mechanisms common to all monoamine neurotransmitters.[3] After synthesis, dopamine is transported from the cytosol into synaptic vesicles by the vesicular monoamine transporter 2 (VMAT2).[5] Dopamine is stored in these vesicles until it is ejected into the synaptic cleft, typically after an action potential causes the vesicles to release their contents directly into the synaptic cleft through a process called exocytosis, but also sometimes as a result of mechanisms that do not require action potentials.[6]
Once in the synapse, dopamine binds to and activates dopamine receptors, which can be located either on postsynaptic target cells or on the membrane of the presynaptic dopamine-releasing cell itself (i.e., D2 short autoreceptors).[3] After an action potential, the dopamine molecules quickly become unbound from their receptors. They are then absorbed back into the presynaptic cell, via reuptake mediated either by the high-affinity dopamine transporter or by the low-affinity plasma membrane monoamine transporter.[7] Once back in the cytosol, dopamine can either be broken down by monoamine oxidase or repackaged into vesicles by VMAT2, making it available for future release.[5]
In the brain the level of extracellular dopamine is modulated by two mechanisms: tonic and phasic transmission.[8] Tonic dopamine transmission occurs when small amounts of dopamine are released independently of neuronal activity, and is regulated by the activity of other neurons and neurotransmitter reuptake.[8] Phasic dopamine release results from the activity of the dopamine-containing cells themselves.[8]
Nervous system

Inside the brain, dopamine plays important roles in motor control, motivation, arousal, cognitive control, reinforcement, and reward, as well as basic lower-level functions including lactation, sexual gratification, and nausea.
Dopaminergic neurons (i.e., neurons whose primary neurotransmitter is dopamine) are comparatively few in number—a total of around 400,000 in the human brain[9]—and their cell bodies are confined to a few relatively small brain areas, but they send projections to many other brain areas and exert powerful effects on their targets.[10] These dopaminergic cell groups were first mapped in 1964 by Annica Dahlström and Kjell Fuxe, who assigned them labels starting with the letter "A" (for "aminergic").[11] In their scheme, areas A1 through A7 contain the neurotransmitter norepinephrine, whereas A8 through A14 contain dopamine. Here is a list of the dopaminergic areas they identified:
- The substantia nigra, a small midbrain area that forms a component of the basal ganglia. The dopamine neurons are found mainly in a part of this structure called the pars compacta (cell group A8) and nearby (group A9).[10] In humans, the projection of dopamine neurons from the substantia nigra pars compacta to the dorsal striatum, termed the nigrostriatal pathway, plays a significant role in the control of motor function and in learning new motor programs.[12] The name substantia nigra is Latin for "dark substance", and refers to the fact that the dopaminergic neurons there are darkly pigmented. These neurons are especially vulnerable to damage, and when a large fraction of them die, the result is a Parkinsonian syndrome.[13]
- The ventral tegmental area (VTA), another midbrain area. The most prominent group of VTA dopamine neurons (cell group A10) project to the prefrontal cortex via the mesocortical pathway and another smaller group project to the nucleus accumbens via the mesolimbic pathway. Together, these two pathways are collectively termed the mesocorticolimbic projection.[10][12] The VTA also sends dopaminergic projections to the amygdala, cingulate gyrus, hippocampus, and olfactory bulb.[10][12] Mesocorticolimbic neurons play a central role in reward and other aspects of motivation.[12]
- The posterior hypothalamus. These dopaminergic cells (group A11) project to the spinal cord, but their function is not well established.[14] There is some evidence that pathology in this area plays a role in restless legs syndrome, a condition in which people have difficulty sleeping due to an overwhelming compulsion to constantly move parts of the body, especially the legs.[14]
- The arcuate nucleus (cell group A12) and periventricular nucleus (cell group A14) of the hypothalamus. An important projection from these dopaminergic neurons, termed the tuberoinfundibular pathway, goes to the pituitary gland, where it influences the secretion of the hormone prolactin.[15] Dopamine is the primary neuroendocrine inhibitor of the secretion of prolactin from the anterior pituitary gland.[15] Dopamine produced by neurons in the arcuate nucleus is secreted into the hypothalamo-hypophysial blood vessels of the median eminence, which supply the pituitary gland.[15] The lactotrope cells that produce prolactin, in the absence of dopamine, secrete prolactin continuously; dopamine inhibits this secretion.[15] In the context of regulating prolactin secretion, dopamine is occasionally called prolactin-inhibiting factor (PIF), prolactin-inhibiting hormone (PIH), or prolactostatin.[15]
- The zona incerta. These cells (group A13) project to several areas of the hypothalamus, and participate in the control of gonadotropin-releasing hormone, which is necessary to activate the development of reproductive systems that occurs following puberty, both in males and females.[15]
An additional group of dopamine-secreting neurons is found in the retina of the eye.[16] These neurons are amacrine cells, meaning that they have no axons.[16] They release dopamine into the extracellular medium, and are specifically active during daylight hours, becoming silent at night.[16] This retinal dopamine acts to enhance the activity of cone cells in the retina while suppressing rod cells—the result is to increase sensitivity to color and contrast during bright light conditions, at the cost of reduced sensitivity when the light is dim.[16]
Basal ganglia

The largest and most important sources of dopamine in the vertebrate brain are a pair of structures called the substantia nigra and ventral tegmental area.[10] These two structures are closely related to each other and functionally similar in many respects.[10] Both are components of the basal ganglia, a complex network of structures located mainly at the base of the forebrain.[10] The largest component of the basal ganglia is the striatum.[17] The substantia nigra sends a dopaminergic projection to the dorsal striatum, while the ventral tegmental area sends a similar type of dopaminergic projection to the ventral striatum.[10]
The neural circuitry of the basal ganglia is exceptionally complex, and progress in understanding their functions has been slow.[17] The most popular hypotheses, broadly stated, propose that the basal ganglia play a central role in action selection.[18] The action selection theory in its simplest form proposes that when a person or animal is in a situation where several behaviors are possible, activity in the basal ganglia determines which of them is executed, by releasing that response from inhibition while continuing to inhibit other motor systems that if activated would generate competing behaviors.[19] Thus the basal ganglia, in this concept, are responsible for initiating behaviors, but not for determining the details of how they are carried out. In other words, they essentially form a decision-making system.[19]
The basal ganglia can be divided into several sectors, and each is involved in controlling particular types of actions.[20] The ventral sector of the basal ganglia (containing the ventral striatum and ventral tegmental area) operates at the highest level of the hierarchy, selecting actions at the whole-organism level.[19] The dorsal sectors (containing the dorsal striatum and substantia nigra) operate at lower levels, selecting the specific muscles and movements that are used to implement a given behavior pattern.[20]
Dopamine contributes to the action selection process in at least two important ways. First, it sets the "threshold" for initiating actions.[18] The higher the level of dopamine activity, the lower the impetus required to evoke a given behavior.[18] As a consequence, high levels of dopamine lead to high levels of motor activity and impulsive behavior; low levels of dopamine lead to torpor and slowed reactions.[18] Parkinson's disease, in which dopamine levels in the substantia nigra circuit are greatly reduced, is characterized by stiffness and greatly reduced movement—however, when people with the disease are confronted with strong stimuli such as a serious threat, their reactions can be as vigorous as those of a healthy person.[21] In the opposite direction, drugs that increase the effects of dopamine, such as cocaine or amphetamine, produce heightened levels of activity, including at the highest levels psychomotor agitation and stereotyped movements.[22]
The second important effect of dopamine is as a "teaching" signal.[18] When an action is followed by an increase in dopamine activity, the basal ganglia circuit is altered in a way that makes the same response easier to evoke when similar situations arise in the future.[18] This is a form of operant conditioning, in which dopamine plays the role of a reward signal.[19]
Reward
The aspect of dopamine that has drawn the most interest is its connection with pleasure and reward. There is plenty of evidence for a strong relationship, including: (1) Drugs that act rapidly and directly to increase dopamine levels, such as cocaine and methamphetamine, are among the most addictive substances known.[23] (2) Many other addictive drugs that do not act directly on dopamine, such as heroin and nicotine, cause increases in dopamine levels by indirect mechanisms.[23] (3) Conversely, drugs that reduce dopamine levels, such as the neuroleptic drugs often used to treat psychosis, tend to cause anhedonia, that is, diminished ability to experience pleasure.(4) Many types of pleasurable experiences—such as sex, eating good food, or playing video games—increase dopamine release.[24] (5) Direct electrical stimulation of dopamine pathways, using electrodes implanted in the brain, is experienced as pleasurable, and many types of animals are willing to work to obtain it.[23] (6) Microelectrode recordings from the brains of animals have shown that dopamine neurons in the ventral tegmental area and substantia nigra are strongly activated by a wide variety of rewarding events.[25]
In spite of the overwhelming evidence showing a strong association between dopamine and reward, there has been a great deal of dispute about whether the function of dopamine should be described as reward per se, or as some more complex construct that relates strongly to reward. The difficulty arises from several observations, including: (1) in addition to being rewarding, dopamine is also arousing—it produces a general increase in movement of all sorts; (2) dopamine release can be caused by events that do not seem to have anything to do with reward, most notably pain; (3) some aspects of pleasure and reward do not rely on dopamine mechanisms.[26] One of the most popular alternatives to the reward theory is the "incentive salience" theory, which argues that the function of dopamine is to increase the effects of motivators of all sorts, both positive and negative.[26]
Until recently the "reward" and "incentive salience" theories competed, each favored by a group of investigators who argued that the evidence that seemed to support the other theory was misleading.[27] Currently, however, the most popular view is that dopamine neurons have a range of functions, with some responding to salience (including aversive stimuli), while others behave as reward neurons.[27] Reward-responsive neurons predominate in the ventral tegmental area, but can also be found in the substantia nigra.[27]
Even if the basic validity of the reward concept is accepted, detailed studies have shown that dopamine cannot simply be equated with pleasure.[28] Some aspects of pleasure and reward involve dopamine, others do not.[28] In an effort to explain the distinction in easily understandable terms, Kent Berridge suggested that reward-related behaviors can be divided into two types, which he called "wanting" and "liking".[28] "Wanting" occurs when an animal, given access to some stimulus such as food or a drug, executes some type of active behavior in order to acquire it.[28] (Some writers such as Jaak Panksepp prefer to use the term "seeking" rather than "wanting", as it better conveys the idea that active behavior is involved.[29]) "Liking" occurs when an animal, after having obtained access to something rewarding, continues to consume it until satiated, and shows expressions of pleasure.[28] There is considerable evidence that the dopamine system is crucial for wanting but not for liking.[30] Drugs that increase the effects of dopamine (most notably stimulants such as methamphetamine or cocaine) produce increases in "wanting" behaviors, but do not greatly alter expressions of pleasure or change levels of satiation.[30] Conversely, opiate drugs such as heroin or morphine produce increases in expressions of pleasure but do not greatly alter "wanting" behaviors.[30] Animals in which the ventral tegmental dopamine system has been rendered inactive do not seek food, and will starve to death if left to themselves, but if food is placed in their mouths they will consume it and show expressions indicative of pleasure.[31]
Even the relationship of dopamine to the "wanting" aspect of reward shows complexity. A substantial body of evidence indicates that dopamine encodes not reward itself, but rather reward prediction error, that is, the degree to which reward is unexpected.[25] According to this hypothesis, which derived initially from dopamine cell activity recordings made by Wolfram Schultz, rewards that are expected do not produce any activation of dopamine cells, but rewards that are unexpected, or greater than expected produce a short-lasting increase in dopamine, whereas the omission of an expected reward actually causes dopamine release to drop below its background level.[25] The "prediction error" hypothesis has drawn particular interest from computational neuroscientists, because an influential computational-learning method known as temporal difference learning makes heavy use of a signal that encodes prediction error.[25] This confluence of theory and data has led to a fertile interaction between neuroscientists and computer scientists interested in machine learning.[25]
Learning from positive and negative information
Studies show that the pharmacological increase of dopamine levels results in a reduction of the ability to learn from negative outcomes[32] and negative information[33]. This suggests dopamine plays a role in learning from undesirable outcomes and information.
Outside the nervous system
Dopamine does not cross the blood–brain barrier, so its synthesis and functions in peripheral areas are to a large degree independent of its synthesis and functions in the brain.[34] A substantial amount of dopamine circulates in the bloodstream, but its functions there are not entirely clear.[35] Dopamine is found in blood plasma at levels comparable to those of epinephrine, but in humans, over 95% of the dopamine in the plasma is in the form of dopamine sulphate, a conjugate produced by the enzyme Sulfotransferase 1A3/1A4 acting on free dopamine.[35] The bulk of this dopamine sulphate is produced in the mesenteric organs that surround parts of the digestive system.[35] The production of dopamine sulphate is thought to be a mechanism for detoxifying dopamine that is ingested as food or produced by the digestive process—plasma levels typically rise more than fifty-fold after a meal.[35] Dopamine sulphate has no known biological functions and is excreted in urine.[35]
The relatively small quantity of unconjugated dopamine in the bloodstream may be produced by the sympathetic nervous system, the digestive system, or possibly other organs.[35] It may act on dopamine receptors in peripheral tissues, or be metabolized, or be converted to norepinephrine by the enzyme dopamine beta hydroxylase, which is released into the bloodstream by the adrenal medulla.[35] Some dopamine receptors are located in the walls of arteries, where they act as a vasodilator and an inhibitor of norepinephrine release.[36] These responses might be activated by dopamine released from the carotid body under conditions of low oxygen, but whether arterial dopamine receptors perform other biologically useful functions is not known.[36]
Beyond its role in modulating blood flow, there are several peripheral systems in which dopamine circulates within a limited area and performs an exocrine or paracrine function.[35] The peripheral systems in which dopamine plays an important role include:
- The immune system. Dopamine acts upon receptors present on immune cells, especially lymphocytes.[37] Dopamine can also affect immune cells in the spleen, bone marrow, and circulatory system.[38] In addition, dopamine can be synthesized and released by immune cells themselves.[37] The main effect of dopamine on lymphocytes is to reduce their activation level. The functional significance of this system is unclear, but it affords a possible route for interactions between the nervous system and immune system, and may be relevant to some autoimmune disorders.[38]
- The kidneys. Multiple types of dopamine receptors are present in cells of the kidneys. Dopamine is also synthesized there, by tubule cells, and discharged into the tubular fluid. Its actions include increasing the blood supply to the kidneys, increasing filtration by the glomeruli, and increasing excretion of sodium in the urine. Defects in renal dopamine function can be produced by high blood pressure or by genetic problems, and can lead to reduced sodium excretion as well as hypertension.[39]
- The pancreas. The role of dopamine here is somewhat complex. The pancreas consists of two parts, known as exocrine and endocrine. The exocrine part synthesizes enzymes and other substances, and secretes them into the small intestine, where food is digested.[40] One of the substances synthesized and secreted by the exocrine pancreas is dopamine.[40] The function of this secreted dopamine after it enters the small intestine is not clearly established—the possibilities include protecting the intestinal mucosa from damage and reducing gastrointestinal motility (the rate at which food moves through the intestines).[40]
- The endocrine part of the pancreas, also known as the islets of Langerhans, synthesizes hormones including insulin, and secretes them into the bloodstream.[40] There is evidence that the beta cells that synthesize insulin contain dopamine receptors, and that dopamine acts to reduce the amount of insulin they release.[40] The source of their dopamine input is not clearly established—it may come from dopamine that circulates in the bloodstream and derives from the sympathetic nervous system, or it may be synthesized locally by other types of pancreatic cells.[40]
Medical uses

Dopamine, sold under the trade names Intropin, Dopastat, Revimine, among others, is widely used as a medication: it is on the World Health Organization's List of Essential Medicines.[41] It is most commonly used in the treatment of severe low blood pressure, slow heart rates, or cardiac arrest, especially in newborn infants.[42] It is given by injection.
Its effects, depending on dosage, include an increase in sodium excretion by the kidneys, an increase in urine output, an increase in heart rate, and an increase in blood pressure.[43] The half-life of dopamine in plasma is very short—approximately one minute in adults, two minutes in newborn infants, up to five minutes in preterm infants—so it is usually given in a continuous intravenous drip rather than a single injection.[43] At low doses it acts through the sympathetic nervous system to increase heart muscle contraction force and heart rate thereby increasing cardiac output and blood pressure.[44] Higher doses also causes vasoconstriction that further increases blood pressure.[44][45] Older literature also describes very low doses thought to improve kidney function without other consequences, but recent reviews have concluded that doses at such low levels are not effective and may sometimes be harmful.[46]
Side effects of dopamine include negative effects on kidney function and cardiac arrhythmias.[44] The LD50, or toxic dose which is expected to be lethal in 50% of the population, has been found to be: 59 mg/kg (mouse; administered intravenously); 950 mg/kg (mouse; administered intraperitoneally); 163 mg/kg (rat; administered intraperitoneally); 79 mg/kg (dog; administered intravenously).[47]
Pharmacology
Dopamine itself is a useful drug, in injectable form, but there are many other important drugs that exert their effects by acting on dopamine systems in various parts of the brain or body. Some are used for medical or recreational purposes, but neurochemists have also developed a variety of research drugs that bind with high affinity to specific types of dopamine receptors and agonize or antagonize their effects, plus a variety that affect other aspects of dopamine physiology.[48]
L-DOPA
L-DOPA or Levodopa—the two names are equivalent—is the metabolic precursor of dopamine: it is converted to dopamine in the brain and various parts of the body by the enzyme DOPA decarboxylase.[49] Because L-DOPA, unlike dopamine itself, is capable of crossing the blood-brain barrier, it is widely used to treat Parkinson's disease, as well as dopa-responsive dystonias.[34] It is often co-administered with an inhibitor of peripheral decarboxylation such as carbidopa or benserazide, to reduce the amount converted to dopamine in the periphery and thereby increase the amount of L-DOPA that enters the brain.[34] When L-DOPA is administered regularly over a long time period, a variety of unpleasant side effects such as dyskinesia often begin to appear; even so, it is considered the best available long-term treatment option for most cases of Parkinson's disease.[34]
Psychostimulants

Cocaine, substituted amphetamines (including methamphetamine), Adderall, methylphenidate (marketed as Ritalin or Concerta), MDMA (ecstasy) and other so-called psychostimulants exert their effects primarily or partly by increasing dopamine levels in the brain; however, they do so by a variety of mechanisms.[50] Cocaine and methylphenidate are dopamine transporter blockers: they non-competitively inhibit dopamine reuptake, resulting in increased dopamine concentrations in the synaptic cleft.[51][52] Like cocaine, substituted amphetamines increase the concentration of dopamine in the synaptic cleft, but by a different mechanism, involving complex intracellular effects.[6] MDMA also increases dopamine levels by a complex combination of mechanisms.[53]
The effects of psychostimulants include increases in heart rate, body temperature, and sweating; improvements in alertness, attention, and endurance; increases in pleasure produced by rewarding events; but at higher doses agitation, anxiety, or even loss of contact with reality.[50] Drugs in this group can have a high addiction potential, due to their activating effects on the dopamine-mediated reward system in the brain.[50] However some can also be useful, at lower doses, for treating attention deficit hyperactivity disorder (ADHD).[54] An important differentiating factor is the onset and duration of action.[50] Cocaine can take effect in seconds if it is injected or inhaled in free-base form; the effects last from 5 to 90 minutes.[55] This rapid and brief action gives it high addiction potential.[50] Methylphenidate taken in pill form, in contrast, can take two hours to reach peak levels in the bloodstream, and depending on formulation the effects can last for up to 12 hours.[54] These slow and sustained actions reduce the addiction potential and make it more useful for treating ADHD.[54]
Antipsychotic drugs
A range of drugs that reduce dopamine activity have been found useful in the treatment of schizophrenia and other disorders that produce psychosis.[56] These antipsychotic drugs are also sometimes known as neuroleptics or "major tranquilizers", in contrast to "minor tranquilizers" such as Valium that are used to treat anxiety or sleep disorders.[56] The most prominent effect of these drugs is to reduce the activity of dopamine systems, mainly by antagonizing D2 receptors.[57] Antipsychotic drugs have a broadly suppressive effect on most types of active behavior, and particularly reduce the delusional and agitated behavior characteristic of overt psychosis.[57] The introduction of the first widely used antipsychotic drug, chlorpromazine (Thorazine), in the 1950s, led to the release of many schizophrenia patients from institutions in the years that followed.[56]
Even so, the widespread use of antipsychotic drugs has long been controversial.[58] There are several reasons for this. First, antipsychotic drugs are perceived as very aversive by people who have to take them, because they produce a general dullness of thought and suppress the ability to experience pleasure.[59] Second, it is difficult to show that they act specifically against psychotic behaviors rather than merely suppressing all types of active behavior.[58] Third, they can produce a range of serious side effects, including weight gain, diabetes, fatigue, sexual dysfunction, hormonal changes, and a type of movement disorder known as tardive dyskinesia.[60] Some of these side effects may continue long after the cessation of drug use, or even permanently.[60]
The first drugs introduced specifically for the treatment of psychosis all had strong direct effects on dopamine receptors of the D2 class.[57] Drugs of this type are known as typical antipsychotics.[56] In the following decades other types of antipsychotic drugs with fewer serious side-effects were developed, known as atypical antipsychotics.[56] Many of these newer drugs do not act directly on dopamine receptors, but detailed investigation has shown that almost all of them produce alterations in dopamine activity indirectly.[61] There remains substantial dispute, however, about how much of an improvement in the patient experience these drugs produce.[58]
Diseases and disorders
The dopamine system plays a central role in important medical conditions including Parkinson's disease, attention deficit hyperactivity disorder, schizophrenia, and drug addiction.
Parkinson's disease
Parkinson's disease is an age-related disorder characterized by stiffness of the body, slowing of movement, and trembling of limbs when they are not in use.[21] In advanced stages it progresses to dementia and eventually death.[21] The main symptoms are caused by massive loss of dopamine-secreting cells in the substantia nigra.[62] These dopamine cells are especially vulnerable to damage, and a variety of insults, including encephalitis (as depicted in the book and movie "Awakenings"), repeated sports-related concussions, and some forms of chemical poisoning (ex. MPTP), can lead to substantial cell loss, producing a parkinsonian syndrome that is similar in its main features to Parkinson's disease.[63] Most cases of Parkinson's disease, however, are idiopathic, meaning that the cause of cell death cannot be identified.[63]
The most widely used treatment for parkinsonism is administration of L-DOPA, the metabolic precursor for dopamine.[34] This treatment cannot restore the dopamine cells that have been lost, but it causes the remaining cells to produce more dopamine, thereby compensating for the loss to at least some degree.[34] In advanced stages the treatment begins to fail because the cell loss is so severe that the remaining ones cannot produce enough dopamine regardless of L-DOPA levels.[34] As this stage is approached, the metabolic regulatory mechanisms in the dopamine cells, operating far above their normal level, become erratic, producing dopamine dysregulation syndrome, in which patients fluctuate unpredictably between states of hyperactivity and paralysis.[64] Other drugs that enhance dopamine function, such as bromocryptine and pergolide, are also sometimes used to treat Parkinsonism, but in most cases L-DOPA appears to give the best tradeoff between positive effects and negative side-effects.[34]
Attention deficit hyperactivity disorder
Altered dopamine neurotransmission is implicated in attention deficit hyperactivity disorder (ADHD), a condition associated with impaired cognitive control, in turn leading to problems with regulating attention (attentional control), inhibiting behaviors (inhibitory control), and forgetting things or missing details (working memory), among other problems.[65] There are genetic links between dopamine receptors, the dopamine transporter and ADHD, in addition to links to other neurotransmitter receptors and transporters.[66] The most important relationship between dopamine and ADHD involves the drugs that are used to treat ADHD.[30] Some of the most effective therapeutic agents for ADHD are psychostimulants such as methylphenidate (Ritalin, Concerta) and amphetamine (Adderall, Dexedrine), drugs that increase both dopamine and norepinephrine levels in brain.[30] In 2015, a systematic review and a meta-analysis of high quality clinical trials found that, when used at low (therapeutic) doses, stimulants produce unambiguous improvements in working memory, episodic memory, and inhibitory control in adults.[67][68] These clinical effects of psychostimulants are mediated through the indirect activation of dopamine and norepinephrine receptors, specifically dopamine receptor D1 and adrenoceptor A2, in the prefrontal cortex.[65]
Drug addiction

A variety of addictive drugs produce an increase in reward-related dopamine activity.[50] For some addictive drugs such as heroin, altered dopamine levels in the reward system may play only a minor role in addiction, but for other drugs, including nicotine and psychomotor stimulants such as cocaine and methamphetamine, increased postsynaptic dopamine receptor activation or increased levels of synaptic dopamine appear to be the primary factor.[69] When people addicted to stimulants go through withdrawal, they do not experience the physical suffering associated with withdrawal from alcohol or opiates; instead they experience "craving", an intense desire for the drug characterized by irritability, restlessness, and other arousal symptoms.[70]
The dopamine system plays a crucial role in several aspects of addiction. At the earliest stage, genetic differences that alter the expression of dopamine receptors in the brain can predict whether a person will find stimulants appealing or aversive.[71] Consumption of stimulants produces increases in brain dopamine levels that last from minutes to hours.[50] Finally, the chronic elevation in dopamine that comes with repetitive stimulant consumption triggers a wide-ranging set of structural changes in the brain, one of which is a reduction in responses to dopamine in the basal ganglia, with the consequence that every type of experience is perceived as less rewarding than before drug consumption began.[72] Treatment of stimulant addiction is very difficult, because even if consumption ceases, the "craving" that comes with psychological withdrawal does not.[70] Even when the craving seems to be extinct, it may re-emerge when a person experiences environmental stimuli (friends, locations, situations, etc.) that are associated with the drug.[70]
Pain
Dopamine plays a role in pain processing in multiple levels of the central nervous system including the spinal cord, periaqueductal gray (PAG), thalamus, basal ganglia, and cingulate cortex.[73] Decreased levels of dopamine have been associated with painful symptoms that frequently occur in Parkinson's disease.[73] Abnormalities in dopaminergic neurotransmission also occur in several painful clinical conditions, including burning mouth syndrome, fibromyalgia, and restless legs syndrome.[73]
Nausea
Nausea and vomiting are largely determined by activity in a brainstem area known as the chemoreceptor trigger zone.[74] This area contains a large population of type D2 dopamine receptors.[74] Consequently, drugs that activate D2 receptors have a high potential to cause nausea.[74] This group includes some medications that are administered for Parkinson's disease, as well as other dopamine agonists such as apomorphine.[75] In some cases, D2-receptor antagonists such as metoclopramide are useful as anti-nausea drugs.[74]
Psychosis
As outlined in the section above on antipsychotic drugs, psychiatrists in the early 1950s discovered that a class of drugs known as major tranquilizers were often effective at reducing the psychotic symptoms of schizophrenia.[56] By the 1970s researchers came to an understanding that the primary neural effect of these antipsychotic drugs is to reduce the activity of dopamine systems.[56] The realization that all then-known antipsychotic drugs reduce dopamine activity led researchers in the 1970s to propose the so-called dopamine hypothesis of schizophrenia, which postulates that schizophrenia is caused, at least in the majority of cases, by hyperactivity of brain dopamine systems.[76] The dopamine hypothesis drew additional support from the observation that psychotic symptoms were often intensified by dopamine-enhancing stimulants such as methamphetamine, and that these drugs could produce psychosis even in healthy people if taken in large doses.[76]
Other observations, however, have caused the dopamine hypothesis to lose popularity, at least in its simple original form.[76] For one thing, patients with schizophrenia do not typically show measurably increased levels of brain dopamine activity.[76] Also, a variety of drugs acting by mechanisms that don't appear to involve dopamine can produce psychotic symptoms, most notably ketamine and phencyclidine (PCP), whose direct effects are on glutamate NMDA receptors.[76] Perhaps most importantly, drugs that reduce dopamine activity are a very imperfect treatment for schizophrenia: they only reduce a subset of symptoms, while producing severe short-term and long-term side effects.[60] Even so, many psychiatrists and neuroscientists continue to believe that schizophrenia involves some sort of dopamine system dysfunction.[56] As the "dopamine hypothesis" has evolved over time, however, the sorts of dysfunctions it postulates have tended to become increasingly subtle and complex.[56]
Comparative biology and evolution
Microorganisms
There are no reports of dopamine in archaea, but it has been detected in some types of bacteria and in a type of protozoan called Tetrahymena.[77] Perhaps more importantly, there are types of bacteria that contain homologs of all the enzymes that animals use to synthesize dopamine.[78] It has been proposed that animals derived their dopamine-synthesizing machinery from bacteria, via horizontal gene transfer that may have occurred relatively late in evolutionary time, perhaps as a result of the symbiotic incorporation of bacteria into eukaryotic cells that gave rise to mitochondria.[78]
Animals
Dopamine is used as an intercellular messenger in virtually all multicellular animals.[79] In sponges only a single report exists of the presence of dopamine, with no indication of its function;[80] however, dopamine has been reported in the nervous systems of numerous radially symmetric species, including cnidaria (jellyfish, hydra, corals, etc.).[81] This dates the emergence of dopamine as a neurotransmitter back to the earliest appearance of the nervous system, over 500 million years ago in the Cambrian era. Among existing species, dopamine functions as a neurotransmitter in vertebrates, echinoderms, arthropods, molluscs, and several types of worms.[82][83]
In every type of animal that has been examined, dopamine acts to modify motor behavior.[79] In the much-studied nematode worm Caenorhabditis elegans, it reduces locomotion and increases food-exploratory movements; in planarian worms it produces "screw-like" movements; in leeches it inhibits swimming and promotes crawling; etc. Across a wide range of vertebrates, dopamine has an "activating" effect on behavior-switching and response selection, comparable to its effect in mammals.[79]
Dopamine also consistently plays a role in reward learning, in all animal groups that have been examined except arthropods, although recent evidence suggests that dopamine at least mediates reward learning in fruit flies.[84] In nematodes, planarians, molluscs, D. melanogaster, and vertebrates, animals can be trained to repeat an action if it is consistently followed by an increase in dopamine levels.[79] Arthropods have long been believed to be an exception, though. In these species—insects, crustaceans, etc.—dopamine was thought to have an aversive effect, with reward instead mediated by octopamine, a neurotransmitter that is not found in vertebrates but is thought to be closely related to norepinephrine.[84] More recent studies, however, have found that the rewarding effect of octopamine comes from its activation of a set of dopaminergic neurons that had not been accessed in previous efforts.[84] A separate dopamine-producing population of cells appears to increase aversion learning of olfactory cues, much like in mammals.[84]
Plants

Many plants synthesize dopamine to varying degrees, including a variety of food plants.[85] The highest concentrations have been observed in bananas—the fruit pulp of red and yellow bananas contains dopamine at levels of 40 to 50 parts per million by weight.[85] Potatoes, avocados, broccoli, and Brussels sprouts may also contain dopamine at levels of 1 part per million or more; oranges, tomatoes, spinach, beans, and other plants contain measurable concentrations less than 1 part per million.[85] The dopamine in plants is synthesized from the amino acid tyrosine, by biochemical mechanisms similar to those that animals use.[85] It can be metabolized in a variety of ways, producing melanin and a variety of alkaloids as byproducts.[85] The functions of plant catecholamines have not been clearly established, but there is evidence that they play a role in the response to stressors such as bacterial infection, act as growth-promoting factors in some situations, and modify the way that sugars are metabolized. The receptors that mediate these actions have not yet been identified, nor have the intracellular mechanisms that they activate.[85]
Dopamine consumed in food cannot act on the brain, because it cannot cross the blood–brain barrier.[34] However, there are also a variety of plants that contain L-DOPA, the metabolic precursor of dopamine.[86] The highest concentrations are found in the leaves and bean pods of plants of the genus Mucuna, especially in Mucuna pruriens (velvet beans), which have been used as a source for L-DOPA as a drug.[87] Another plant containing substantial amounts of L-DOPA is Vicia faba, the plant that produces fava beans (also known as "broad beans"). The level of L-DOPA in the beans, however, is much lower than in the pod shells and other parts of the plant.[88] The seeds of Cassia and Bauhinia trees also contain substantial amounts of L-DOPA.[86]
In the marine green alga Ulvaria obscura, which is a major component of some algal blooms, dopamine is present in very high concentrations, estimated at 4.4% of dry weight. There is evidence that this dopamine functions as an anti-herbivore defense, reducing consumption by snails and isopods.[89]
As a precursor for melanin
Melanins are a family of dark-pigmented substances found in a wide range of organisms.[90] Chemically they are closely related to dopamine, and there is a type of melanin, known as dopamine-melanin, that can be synthesized by oxidation of dopamine via the enzyme tyrosinase.[90] The melanin that darkens human skin is not of this type: it is synthesized by a pathway that uses L-DOPA as a precursor but not dopamine.[90] However, there is substantial evidence that the "neuromelanin" that gives a dark color to the brain's substantia nigra is at least in part dopamine-melanin.[91]
Dopamine-derived melanin probably appears in at least some other biological systems as well. Some of the dopamine in plants is likely to be used as a precursor for dopamine-melanin.[92] The complex patterns that appear on butterfly wings, as well as black-and-white stripes on the bodies of insect larvae, are also thought to be caused by spatially structured accumulations of dopamine-melanin.[93]
Biochemical mechanisms
In humans, dopamine is synthesized in brain cells and adrenal cells from the precursor chemical L-DOPA. In brain cells, it is transported to synaptic sites and packaged into vesicles for release, which occurs during synaptic transmission. After release, free dopamine is either reabsorbed into the presynaptic terminal for reuse, or broken down by the enzymes monoamine oxidase or COMT, producing a variety of degradation metabolites, whose end products are ultimately excreted in the urine.
Biosynthesis

Dopamine is synthesized in a restricted set of cell types, mainly neurons and cells in the medulla of the adrenal glands.[3] The metabolic pathway is:
- L-Phenylalanine → L-Tyrosine → L-DOPA → Dopamine
Thus the direct precursor of dopamine is L-DOPA, which can be synthesized indirectly from the essential amino acid phenylalanine or directly from the non-essential amino acid tyrosine.[49] These amino acids are found in nearly every protein and, as such, are provided by ingestion of protein-containing food, with tyrosine being the most common. Although dopamine is also found in many types of food, it is incapable of crossing the blood–brain barrier that surrounds and protects the brain.[34] It must therefore be synthesized inside the brain in order to perform its neural actions.[34]
L-Phenylalanine is converted into L-tyrosine by the enzyme phenylalanine hydroxylase (PAH), with molecular oxygen (O2) and tetrahydrobiopterin (THB) as cofactors. L-Tyrosine is converted into L-DOPA by the enzyme tyrosine hydroxylase (TH), with tetrahydrobiopterin (THB), O2, and probably ferrous iron (Fe2+) as cofactors.[49] L-DOPA is converted into dopamine by the enzyme aromatic L-amino acid decarboxylase (AADC; also known as DOPA decarboxylase (DDC)), with pyridoxal phosphate (PLP) as the cofactor.[49]
Dopamine itself is used as precursor in the synthesis of the neurotransmitter norepinephrine and the hormone epinephrine.[49] Dopamine is converted into norepinephrine by the enzyme dopamine β-hydroxylase (DBH), with O2 and L-ascorbic acid as cofactors.[49] Norepinephrine is converted into epinephrine by the enzyme phenylethanolamine N-methyltransferase (PNMT) with S-adenosyl-L-methionine (SAM) as the cofactor.[49]
It should be noted that some of the cofactors also require their own synthesis.[49] Deficiency in any required amino acid or cofactor can impair the synthesis of dopamine, norepinephrine, and epinephrine.[49]
Degradation

MAO: Monoamine oxidase
COMT: catechol-O-methyltransferase
HVA: Homovanillic acid
Dopamine is broken down into inactive metabolites by a set of enzymes, monoamine oxidase (MAO), catechol-O-methyl transferase (COMT), and aldehyde dehydrogenase (ALDH), acting in sequence.[35] Both isoforms of monoamine oxidase, MAO-A and MAO-B, are effective.[49] A variety of breakdown pathways are possible, but regardless of which pathway is followed, the primary final product is homovanillic acid (also known as Vanillylmandelic acid), which has no known biological activity.[35] From the bloodstream, homovanillic acid is filtered out by the kidneys and then excreted in the urine.[35] Homovanillic acid levels in the bloodstream have sometimes been used as a measure of brain dopamine activity, but the validity of this approach has been questioned due to the difficulty of compensating for peripheral dopamine metabolism.[94]
Chemistry
Chemically, a dopamine molecule consists of a catechol structure (a benzene ring with two hydroxyl side groups) with one amine group attached via an ethyl chain.[95] As such, dopamine is the simplest possible catecholamine, a family that also includes the neurotransmitters norepinephrine and epinephrine.[96] The presence of a benzene ring with this amine attachment makes it a phenethylamine, a family that includes numerous psychoactive drugs.[97]
Dopamine, like most amines, is an organic base.[98] In acidic environments, it is generally protonated.[98] The protonated form is highly water-soluble and relatively stable, though it is capable of becoming oxidized if exposed to oxygen or other oxidants.[98] In basic environments, dopamine is not protonated.[98] In this free base form, it is less water-soluble and also more highly reactive.[98] Because of the increased stability and water-solubility of the protonated form, dopamine is supplied for chemical or pharmaceutical use as dopamine hydochloride, that is, the hydrochloride salt that is created when dopamine is combined with hydrochloric acid.[98] In dry form, dopamine hydrochloride is a fine colorless powder.[98]
Oxidation
Dopamine in the body is normally broken down by oxidation catalyzed by the enzyme monoamine oxidase. However, dopamine is also capable of autoxidation, that is, direct reaction with oxygen, yielding quinones plus various free radicals as products.[99] The rate of autoxidation can be increased by the presence of ferrous iron or other factors. The ability of dopamine autoxidation to produce quinones and free radicals makes it a potent cell toxin, and there is evidence that this mechanism may contribute to cell loss that occurs in Parkinson's disease or other conditions.[100]
Polydopamine
Research motivated by mussel adhesive proteins led to the discovery in 2007 that a wide variety of materials, if placed in a solution of dopamine at slightly basic pH, will become coated with a layer of polymerized dopamine, often referred to as polydopamine.[101][102] This polymerized dopamine forms by a spontaneous oxidation reaction, and is formally a type of melanin.[103] Synthesis usually involves reaction of dopamine hydrochloride with Tris as a base in water. The structure of polydopamine is unknown.[102]
Polydopamine coatings can form on objects ranging in size from nanoparticles to large surfaces.[103] Polydopamine layers have chemical properties that have the potential to be extremely useful, and numerous studies have examined their possible applications.[103] At the simplest level, they can be used for protection against damage by light, or to form capsules for drug delivery.[103] At a more sophisticated level, their adhesive properties may make them useful as substrates for biosensors or other biologically active macromolecules.[103]
History
Dopamine was first synthesized in 1910 by George Barger and James Ewens at Wellcome Laboratories in London, England[104] and first identified in the human brain by Kathleen Montagu in 1957. It was named dopamine because it is a monoamine whose precursor in the Barger-Ewens synthesis is 3,4-dihydroxyphenylalanine (levodopamine or L-DOPA). Dopamine's function as a neurotransmitter was first recognized in 1958 by Arvid Carlsson and Nils-Åke Hillarp at the Laboratory for Chemical Pharmacology of the National Heart Institute of Sweden.[105] Carlsson was awarded the 2000 Nobel Prize in Physiology or Medicine for showing that dopamine is not only a precursor of norepinephrine (noradrenaline) and epinephrine (adrenaline), but also itself a neurotransmitter.[106]
See also
References
- ^ Moncrieff J (2008). The myth of the chemical cure. A critique of psychiatric drug treatment. Basingstoke, UK: Palgrave MacMillan. ISBN 0-230-57432-7.
- ^ Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM (2009). "Evaluating dopamine reward pathway in ADHD: clinical implications". JAMA. 302 (10): 1084–91. doi:10.1001/jama.2009.1308. PMID 19738093.
- ^ a b c d e f Seeman P (2009). "Chapter 1: Historical overview: Introduction to the dopamine receptors". In Neve K (ed.). The Dopamine Receptors. Springer. pp. 1–22. ISBN 1-60327-333-6.
- ^ a b c d Romanelli RJ, Williams JT, Neve KA (2009). "Chapter 6: Dopamine receptor signalling: intracellular pathways to behavior". In Neve KA (ed.). The Dopamine Receptors. Springer. pp. 137–174. ISBN 1-60327-333-6.
- ^ a b Eiden LE, Schäfer MK, Weihe E, Schütz B (2004). "The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine". Pflugers Arch. 447 (5): 636–40. doi:10.1007/s00424-003-1100-5. PMID 12827358.
- ^ a b Miller GM (2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry. 116 (2): 164–76. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ^ Torres GE, Gainetdinov RR, Caron MG (2003). "Plasma membrane monoamine transporters: structure, regulation and function". Nature Reviews Neuroscience. 4 (1): 13–25. doi:10.1038/nrn1008. PMID 12511858.
- ^ a b c Rice ME, Patel JC, Cragg SJ (December 2011). "Dopamine release in the basal ganglia". Neuroscience. 198: 112–37. doi:10.1016/j.neuroscience.2011.08.066. PMC 3357127. PMID 21939738.
- ^ Schultz W (2007). "Multiple dopamine functions at different time courses". Annual Review of Neuroscience. 30: 259–88. doi:10.1146/annurev.neuro.28.061604.135722. PMID 17600522.
- ^ a b c d e f g h Björklund A, Dunnett SB (2007). "Dopamine neuron systems in the brain: an update". Trends in Neurosciences. 30 (5): 194–202. doi:10.1016/j.tins.2007.03.006. PMID 17408759.
- ^ Dahlstroem A, Fuxe K (1964). "Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons". Acta Physiologica Scandinavica. Supplementum. 232: SUPPL 232:1–55. PMID 14229500.
- ^ a b c d Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 147–148, 154–157. ISBN 0-07-148127-3.
- ^ Christine CW, Aminoff MJ (2004). "Clinical differentiation of parkinsonian syndromes: prognostic and therapeutic relevance". The American Journal of Medicine. 117 (6): 412–9. doi:10.1016/j.amjmed.2004.03.032. PMID 15380498.
- ^ a b Paulus W, Schomburg ED (2006). "Dopamine and the spinal cord in restless legs syndrome: does spinal cord physiology reveal a basis for augmentation?". Sleep Medicine Reviews. 10 (3): 185–96. doi:10.1016/j.smrv.2006.01.004. PMID 16762808.
- ^ a b c d e f Ben-Jonathan N, Hnasko R (2001). "Dopamine as a prolactin (PRL) inhibitor". Endocrine Reviews. 22 (6): 724–63. doi:10.1210/er.22.6.724. PMID 11739329.
- ^ a b c d Witkovsky P (2004). "Dopamine and retinal function". Documenta Ophthalmologica. Advances in Ophthalmology. 108 (1): 17–40. doi:10.1023/B:DOOP.0000019487.88486.0a. PMID 15104164.
- ^ a b Fix JD (2008). "Basal Ganglia and the Striatal Motor System". Neuroanatomy (Board Review Series) (4th ed.). Baltimore: Wulters Kluwer & Lippincott Wiliams & Wilkins. pp. 274–281. ISBN 0-7817-7245-1.
- ^ a b c d e f Chakravarthy VS, Joseph D, Bapi RS (2010). "What do the basal ganglia do? A modeling perspective" (PDF). Biological Cybernetics. 103 (3): 237–53. doi:10.1007/s00422-010-0401-y. PMID 20644953. Retrieved 24 September 2015.
- ^ a b c d Floresco SB (2015). "The nucleus accumbens: an interface between cognition, emotion, and action" (PDF). Annual Review of Psychology. 66: 25–52. doi:10.1146/annurev-psych-010213-115159. PMID 25251489. Retrieved 24 September 2015.
- ^ a b Balleine BW, Dezfouli A, Ito M, Doya K (2015). "Hierarchical control of goal-directed action in the cortical–basal ganglia network". Current Opinion in Behavioral Sciences. 5: 1–7. doi:10.1016/j.cobeha.2015.06.001.
- ^ a b c Jankovic J (2008). "Parkinson's disease: clinical features and diagnosis". Journal of Neurology, Neurosurgery, and Psychiatry. 79 (4): 368–376. doi:10.1136/jnnp.2007.131045. PMID 18344392. Retrieved 24 September 2015.
- ^ Pattij T, Vanderschuren LJ (2008). "The neuropharmacology of impulsive behaviour" (PDF). Trends in Pharmacological Sciences. 29 (4): 192–9. doi:10.1016/j.tips.2008.01.002. PMID 18304658. Retrieved 24 September 2015.
- ^ a b c Wise RA (1996). "Addictive drugs and brain stimulation reward". Annual Review of Neuroscience. 19: 319–40. doi:10.1146/annurev.ne.19.030196.001535. PMID 8833446.
- ^ Arias-Carrión O, Pöppel E (2007). "Dopamine, learning and reward-seeking behavior". Act Neurobiol Exp. 67 (4): 481–488.
- ^ a b c d e Schultz W (2015). "Neuronal reward and decision signals: from theories to data" (PDF). Physiological Reviews. 95 (3): 853–951. Retrieved 24 September 2015.
- ^ a b Berridge KC, Robinson TE (1998). "What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience?". Brain Research. Brain Research Reviews. 28 (3): 309–69. PMID 9858756.
- ^ a b c Bromberg-Martin ES, Matsumoto M, Hikosaka O (2010). "Dopamine in motivational control: rewarding, aversive, and alerting". Neuron. 68 (5): 815–34. doi:10.1016/j.neuron.2010.11.022. PMC 3032992. PMID 21144997.
- ^ a b c d e Robinson TE, Berridge KC (1993). "The neural basis of drug craving: an incentive-sensitization theory of addiction". Brain Research. Brain Research Reviews. 18 (3): 247–91. PMID 8401595.
- ^ Wright JS, Panksepp J (2012). "An evolutionary framework to understand foraging, wanting, and desire: the neuropsychology of the SEEKING system" (PDF). Neuropsychoanalysis. 14 (1): 5–39. doi:10.1080/15294145.2012.10773683. Retrieved 24 September 2015.
- ^ a b c d e Berridge KC, Robinson TE, Aldridge JW (2009). "Dissecting components of reward: 'liking', 'wanting', and learning". Current Opinion in Pharmacology. 9 (1): 65–73. doi:10.1016/j.coph.2008.12.014. PMC 2756052. PMID 19162544. Cite error: The named reference "Berridge2" was defined multiple times with different content (see the help page).
- ^ Salamone JD, Correa M, Mingote S, Weber SM (2003). "Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse". The Journal of Pharmacology and Experimental Therapeutics. 305 (1): 1–8. doi:10.1124/jpet.102.035063. PMID 12649346. Retrieved 24 September 2015.
- ^ Frank, MJ., Seeberger LC., O'reilly, RC. (2004) By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science, Vol 306 (5703) PMID: 15528409
- ^ Sharot T., Guitart-Masip M., Korn C. W., Chowdhury R., Dolan R.J. (2012) How Dopamine Enhances an Optimism Bias in Humans. Current Biology, Vol 22 (16) doi:10.1016/j.cub.2012.05.053
- ^ a b c d e f g h i j k The National Collaborating Centre for Chronic Conditions, ed. (2006). "Symptomatic pharmacological therapy in Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 59–100. ISBN 1-86016-283-5. Retrieved 24 September 2015. Cite error: The named reference "Nice-pharma" was defined multiple times with different content (see the help page).
- ^ a b c d e f g h i j k Eisenhofer G, Kopin IJ, Goldstein DS (2004). "Catecholamine metabolism: a contemporary view with implications for physiology and medicine". Pharmacological Reviews. 56 (3): 331–49. doi:10.1124/pr.56.3.1. PMID 15317907.
- ^ a b Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (1998). "Dopamine receptors: from structure to function". Physiological Reviews. 78 (1): 189–225. PMID 9457173.
- ^ a b Buttarelli FR, Fanciulli A, Pellicano C, Pontieri FE (2011). "The dopaminergic system in peripheral blood lymphocytes: from physiology to pharmacology and potential applications to neuropsychiatric disorders". Current Neuropharmacology. 9 (2): 278–88. doi:10.2174/157015911795596612. PMC 3131719. PMID 22131937.
- ^ a b Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S (2010). "The immunoregulatory role of dopamine: an update". Brain, Behavior, and Immunity. 24 (4): 525–8. doi:10.1016/j.bbi.2009.10.015. PMC 2856781. PMID 19896530.
- ^ Carey RM (2001). "Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure". Hypertension. 38 (3): 297–302. doi:10.1161/hy0901.096422. PMID 11566894.
- ^ a b c d e f Rubí B, Maechler P (2010). "Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let's seek the balance". Endocrinology. 151 (12): 5570–81. doi:10.1210/en.2010-0745. PMID 21047943. Retrieved 24 September 2015.
- ^ "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. Retrieved 24 September 2015.
- ^ Noori S, Friedlich P, Seri I (2003). "Pharmacology Review Developmentally Regulated Cardiovascular, Renal, and Neuroendocrine Effects of Dopamine" (PDF). NeoReviews. 4 (10): e283–e288. Retrieved 24 September 2015.
- ^ a b Bhatt-Mehta V, Nahata MC (1989). "Dopamine and dobutamine in pediatric therapy". Pharmacotherapy. 9 (5): 303–14. PMID 2682552.
- ^ a b c Bronwen JB, Knights KM (2009). Pharmacology for Health Professionals (2nd ed.). Elsevier Australia. p. 192. ISBN 0-7295-3929-6.
- ^ De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL (2010). "Comparison of dopamine and norepinephrine in the treatment of shock". The New England Journal of Medicine. 362 (9): 779–89. doi:10.1056/NEJMoa0907118. PMID 20200382.
- ^ Karthik S, Lisbon A (2006). "Low-dose dopamine in the intensive care unit". Seminars in Dialysis. 19 (6): 465–71. doi:10.1111/j.1525-139X.2006.00208.x. PMID 17150046.
- ^ Lewis RJ (2004). Sax's Dangerous Properties of Industrial Materials, 11th Ed. Hoboken, NJ.: Wiley & Sons. p. 1552. ISBN 0-471-47662-5.
- ^ Standaert DG, Walsh RR (2011). "Pharmacology of dopaminergic neurotransmission". In Tashjian AH, Armstrong EJ, Golan DE (eds.). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. pp. 186–206. ISBN 1-4511-1805-8.
- ^ a b c d e f g h i j Musacchio JM (2013). "Chapter 1: Enzymes involved in the biosynthesis and degradation of catecholamines". In Iverson L (ed.). Biochemistry of Biogenic Amines. Springer. pp. 1–35. ISBN 1-4684-3171-4.
- ^ a b c d e f g Ghodse H (2010). Ghodse's Drugs and Addictive Behaviour: A Guide to Treatment (4 ed.). Cambridge University Press. pp. 87–92. ISBN 1-139-48567-9.
- ^ Heal DJ, Pierce DM (2006). "Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system". CNS Drugs. 20 (9): 713–38. PMID 16953648.
- ^ Freye E (2009). Pharmacology and abuse of cocaine, amphetamines, ecstasy and related designer drugs a comprehensive review on their mode of action, treatment of abuse and intoxication. Dordrecht: Springer. pp. 54–58. ISBN 90-481-2448-4.
- ^ Freye E (2009). Pharmacology and abuse of cocaine, amphetamines, ecstasy and related designer drugs a comprehensive review on their mode of action, treatment of abuse and intoxication. Dordrecht: Springer. pp. 147–150. ISBN 90-481-2448-4.
- ^ a b c Kimko HC, Cross JT, Abernethy DR (1999). "Pharmacokinetics and clinical effectiveness of methylphenidate". Clinical pharmacokinetics. 37 (6): 457–70. PMID 10628897.
- ^ Zimmerman JL (2012). "Cocaine intoxication". Critical care clinics. 28 (4): 517–26. PMID 22998988.
- ^ a b c d e f g h i Healy D (2004). The Creation of Psychopharmacology. Harvard University Press. pp. 37–73. ISBN 0-674-01599-1.
- ^ a b c Brunton L, Chabner B, Knollman B (2010). Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). McGraw Hill Professional. pp. 417–455.
- ^ a b c James, Adam (2 March 2008). "Myth of the antipsychotic". The Guardian. Guardian News and Media Limited. Retrieved 24 September 2015.
- ^ Lambert M, Schimmelmann BG, Karow A, Naber D (2003). "Subjective well-being and initial dysphoric reaction under antipsychotic drugs - concepts, measurement and clinical relevance". Pharmacopsychiatry. 36 Suppl 3 (Suppl 3): S181-90. doi:10.1055/s-2003-45128. PMID 14677077.
- ^ a b c Muench J, Hamer AM (2010). "Adverse effects of antipsychotic medications". American Family Physician. 81 (5): 617–22. PMID 20187598.
- ^ Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, Höschl C (2006). "Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia". CNS Drugs. 20 (5): 389–409. PMID 16696579.
- ^ Dickson DV (2007). "Neuropathology of movement disorders". Parkinson's disease and movement disorders. Hagerstown, MD: Lippincott Williams & Wilkins. pp. 271–83. ISBN 0-7817-7881-6.
{{cite book}}: Unknown parameter|editors=ignored (|editor=suggested) (help) - ^ a b Tuite PJ, Krawczewski K (2007). "Parkinsonism: a review-of-systems approach to diagnosis". Seminars in neurology. 27 (2): 113–22. doi:10.1055/s-2007-971174. PMID 17390256.
- ^ Merims D, Giladi N (2008). "Dopamine dysregulation syndrome, addiction and behavioral changes in Parkinson's disease". Parkinsonism & Related Disorders. 14 (4): 273–80. doi:10.1016/j.parkreldis.2007.09.007. PMID 17988927.
- ^ a b Malenka RC, Nestler EJ, Hyman SE (2009). "Chapters 10 and 13". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 266, 318–323. ISBN 0-07-148127-3.
- ^ Wu J, Xiao H, Sun H, Zou L, Zhu LQ (2012). "Role of dopamine receptors in ADHD: a systematic meta-analysis". Molecular Neurobiology. 45 (3): 605–20. doi:10.1007/s12035-012-8278-5. PMID 22610946.
- ^ Spencer RC, Devilbiss DM, Berridge CW (June 2015). "The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex". Biological Psychiatry. 77 (11): 940–50. doi:10.1016/j.biopsych.2014.09.013. PMID 25499957.
{{cite journal}}: CS1 maint: year (link) - ^ Ilieva IP, Hook CJ, Farah MJ (2015). "Prescription Stimulants' Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis". Journal of Cognitive Neuroscience. 27 (6): 1069–89. doi:10.1162/jocn_a_00776. PMID 25591060.
- ^ "The dopamine theory of addiction: 40 years of highs and lows". Nature Reviews Neuroscience. 16 (5): 305–312. 2015. PMID 25873042.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c Sinha R (2013). "The clinical neurobiology of drug craving". Current Opinion in Neurobiology. 23 (4): 649–54. PMC 3735834. PMID 23764204.
- ^ "Addiction science: Uncovering neurobiological complexity". Neuropharmacology. 76 Pt B: 235–49. 2014. PMC 3818510. PMID 23688927.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Nestler EJ (2012). "Transcriptional mechanisms of drug addiction". Clinical Psychopharmacology and Neuroscience. 10 (3): 136–43. doi:10.9758/cpn.2012.10.3.136. PMC 3569166. PMID 23430970.
- ^ a b c Wood PB (2008). "Role of central dopamine in pain and analgesia". Expert Review of Neurotherapeutics. 8 (5): 781–97. doi:10.1586/14737175.8.5.781. PMID 18457535.
- ^ a b c d "Practical selection of antiemetics". American Family Physician. 69 (5): 1169–1174. 2004. PMID 15023018. Retrieved 24 September 2015.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ "Pharmacological treatment of Parkinson disease: a review". Journal of the American Medical Association. 311 (16): 1670–1683. 2014. PMID 24756517.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b c d e Howes OD, Kapur S (2009). "The dopamine hypothesis of schizophrenia: version III--the final common pathway". Schizophrenia Bulletin. 35 (3): 549–62. doi:10.1093/schbul/sbp006. PMC 2669582. PMID 19325164.
- ^ Roshchina VV (2010). "Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells". In Lyte M, Primrose PE (eds.). Microbial Endocrinology. New York: Springer. pp. 17–52. ISBN 1-4419-5576-3.
- ^ a b Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV (2004). "Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role?". Trends in Genetics. 20 (7): 292–9. doi:10.1016/j.tig.2004.05.007. PMID 15219393.
- ^ a b c d Barron AB, Søvik E, Cornish JL (2010). "The roles of dopamine and related compounds in reward-seeking behavior across animal phyla". Frontiers in Behavioral Neuroscience. 4: 163. doi:10.3389/fnbeh.2010.00163. PMC 2967375. PMID 21048897.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Liu H, Mishima Y, Fujiwara T, Nagai H, Kitazawa A, Mine Y, et al. (2004). "Isolation of Araguspongine M, a new stereoisomer of an Araguspongine/Xestospongin alkaloid, and dopamine from the marine sponge Neopetrosia exigua collected in Palau". Marine Drugs. 2 (4): 154–163. doi:10.3390/md204154.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Kass-Simon G, Pierobon P (2007). "Cnidarian chemical neurotransmission, an updated overview". Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 146 (1): 9–25. doi:10.1016/j.cbpa.2006.09.008. PMID 17101286.
- ^ Cottrell GA (1967). "Occurrence of dopamine and noradrenaline in the nervous tissue of some invertebrate species". British Journal of Pharmacology and Chemotherapy. 29 (1): 63–9. doi:10.1111/j.1476-5381.1967.tb01939.x. PMC 1557178. PMID 19108240.
- ^ Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR (2007). "Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans". Neuron. 55 (4): 662–76. doi:10.1016/j.neuron.2007.07.023. PMID 17698017.
- ^ a b c d Waddell S (2013). "Reinforcement signalling in Drosophila; dopamine does it all after all". Current Opinion in Neurobiology. 23 (3): 324–9. doi:10.1016/j.conb.2013.01.005. PMC 3887340. PMID 23391527.
- ^ a b c d e f Kulma A, Szopa J (2007). "Catecholamines are active compounds in plants". Plant Science. 172 (3): 433–440. doi:10.1016/j.plantsci.2006.10.013.
- ^ a b Ingle PK (2003). "L-DOPA bearing plants" (PDF). Natural Product Radiance. 2: 126–133. Retrieved 24 September 2015.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Wichers HJ, Visser JF, Huizing HJ, Pras N (1993). "Occurrence of L-DOPA and dopamine in plants and cell cultures of Mucuna pruriens and effects of 2, 4-d and NaCl on these compounds". Plant Cell, Tissue and Organ Culture. 33 (3): 259–264. doi:10.1007/BF02319010.
- ^ Longo R, Castellani A, Sberze P, Tibolla M (1974). "Distribution of l-dopa and related amino acids in Vicia". Phytochemistry. 13 (1): 167–171. doi:10.1016/S0031-9422(00)91287-1.
- ^ Van Alstyne KL, Nelson AV, Vyvyan JR, Cancilla DA (2006). "Dopamine functions as an antiherbivore defense in the temperate green alga Ulvaria obscura". Oecologia. 148 (2): 304–11. doi:10.1007/s00442-006-0378-3. PMID 16489461.
- ^ a b c Simon JD, Peles D, Wakamatsu K, Ito S (2009). "Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function". Pigment Cell & Melanoma Research. 22 (5): 563–79. doi:10.1111/j.1755-148X.2009.00610.x. PMID 19627559.
- ^ Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P, Double KL (2005). "Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease". Progress in Neurobiology. 75 (2): 109–24. doi:10.1016/j.pneurobio.2005.02.001. PMID 15784302.
- ^ Andrews RS, Pridham JB (1967). "Melanins from DOPA-containing plants". Phytochemistry. 6 (1): 13–18. doi:10.1016/0031-9422(67)85002-7.
- ^ Beldade P, Brakefield PM (2002). "The genetics and evo-devo of butterfly wing patterns". Nature Reviews Genetics. 3 (6): 442–52. doi:10.1038/nrg818. PMID 12042771.
- ^ Amin F, Davidson M, Davis KL (1992). "Homovanillic acid measurement in clinical research: a review of methodology". Schizophrenia Bulletin. 18 (1): 123–48. PMID 1553492. Retrieved 24 September 2015.
- ^ "Dopamine". PubChem. Retrieved 21 September 2015.
- ^ "Catecholamine". Brittanica. Retrieved 21 September 2015.
- ^ "Phenylethylamine". ChemicalLand21.com. Retrieved 21 September 2015.
- ^ a b c d e f g "Dopamine Hydrochloride". Analytical Profiles of Drug Substances. 11: 257–272. 1982.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ Sulzer D, Zecca L (2000). "Intraneuronal dopamine-quinone synthesis: a review". Neurotoxicity Research. 1 (3): 181–95. doi:10.1007/BF03033289. PMID 12835101.
- ^ Miyazaki I, Asanuma M (2008). "Dopaminergic neuron-specific oxidative stress caused by dopamine itself" (PDF). Acta Medica Okayama. 62 (3): 141–50. PMID 18596830. Retrieved 24 September 2015.
- ^ "Mussel-Inspired Surface Chemistry for Multifunctional Coatings". Science. 318: 426–430. 2007. Bibcode:2007Sci...318..426L. doi:10.1126/science.1147241.
{{cite journal}}: Unknown parameter|authors=ignored (help) - ^ a b Dreyer DR, Miller DJ, Freeman BD, Paul DR, Bielawski CW (2013). "Perspectives on poly(dopamine)". Chemical Sciences. 4: 3796. doi:10.1039/C3SC51501J.
- ^ a b c d e Lynge ME, van der Westen R, Postma A, Städler B (2011). "Polydopamine—a nature-inspired polymer coating for biomedical science" (PDF). Nanoscale. 3 (12): 4916–28. Bibcode:2011Nanos...3.4916L. doi:10.1039/c1nr10969c. PMID 22024699. Retrieved 24 September 2015.
- ^ Fahn S (2008). "The history of dopamine and levodopa in the treatment of Parkinson's disease". Movement Disorders. 23 Suppl 3: S497–508. doi:10.1002/mds.22028. PMID 18781671.
- ^ Benes FM (2001). "Carlsson and the discovery of dopamine". Trends in Pharmacological Sciences. 22 (1): 46–7. doi:10.1016/S0165-6147(00)01607-2. PMID 11165672.
- ^ Barondes, Samuel H. (2003). Better Than Prozac. New York: Oxford University Press. pp. 21–22, 39–40. ISBN 0-19-515130-5.
{{cite book}}: Unknown parameter|name-list-format=ignored (|name-list-style=suggested) (help)