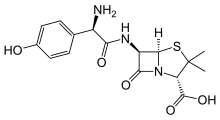
Amoxicillin
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /əˌmɒksɪˈsɪlɪn/ |
| Trade names | Amoxil, Trimox, others[1] |
| Other names | Amoxycillin, amox, Amoxycillin (AAN AU) |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a685001 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| Drug class | β-lactam antibiotic, aminopenicillin |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 95% by mouth |
| Metabolism | less than 30% biotransformed in liver |
| Elimination half-life | 61.3 minutes |
| Excretion | Kidneys |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.043.625 |
| Chemical and physical data | |
| Formula | C16H19N3O5S |
| Molar mass | 365.40 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.6±0.1 [7] g/cm3 |
| |
| |
| (verify) | |
Amoxicillin is an antibiotic medication belonging to the aminopenicillin class of the penicillin family. The drug is used to treat bacterial infections[8] such as middle ear infection, strep throat, pneumonia, skin infections, odontogenic infections, and urinary tract infections.[8] It is taken by mouth, or less commonly by injection.[8][9]
Common adverse effects include nausea and rash.[8] It may also increase the risk of yeast infections and, when used in combination with clavulanic acid, diarrhea.[10] It should not be used in those who are allergic to penicillin.[8] While usable in those with kidney problems, the dose may need to be decreased.[8] Its use in pregnancy and breastfeeding does not appear to be harmful.[8] Amoxicillin is in the beta-lactam family of antibiotics.[8]
Amoxicillin was discovered in 1958 and came into medical use in 1972.[11][12] Amoxil was approved for medical use in the United States in 1974,[4][5] and in the United Kingdom in 1977.[2] It is on the (WHO) World Health Organization's List of Essential Medicines.[13][14] It is one of the most commonly prescribed antibiotics in children.[15] Amoxicillin is available as a generic medication.[8] In 2020, it was the 40th most commonly prescribed medication in the United States, with more than 15 million prescriptions.[16][17]
Medical uses

Amoxicillin is used in the treatment of a number of infections, including acute otitis media, streptococcal pharyngitis, pneumonia, skin infections, urinary tract infections, Salmonella infections, Lyme disease, and chlamydia infections.[8][18]
Acute otitis media
Children with acute otitis media who are younger than six months of age are generally treated with amoxicillin or other antibiotics. Although most children with acute otitis media who are older than two years old do not benefit from treatment with amoxicillin or other antibiotics, such treatment may be helpful in children younger than two years old with acute otitis media that is bilateral or accompanied by ear drainage.[19] In the past, amoxicillin was dosed three times daily when used to treat acute otitis media, which resulted in missed doses in routine ambulatory practice. There is now evidence that two times daily dosing or once daily dosing has similar effectiveness.[20]
Respiratory infections
Amoxicillin and amoxicillin-clavulanate have been recommended by guidelines as the drug of choice for bacterial sinusitis and other respiratory infections.[18] Most sinusitis infections are caused by viruses, for which amoxicillin and amoxicillin-clavulanate are ineffective,[21] and the small benefit gained by amoxicillin may be overridden by the adverse effects.[22] Amoxicillin is recommended as the preferred first-line treatment for community-acquired pneumonia in adults by the National Institute for Health and Care Excellence, either alone (mild to moderate severity disease) or in combination with a macrolide.[23] The World Health Organization (WHO) recommends amoxicillin as first-line treatment for pneumonia that is not "severe".[24] Amoxicillin is used in post-exposure inhalation of anthrax to prevent disease progression and for prophylaxis.[18]
H. pylori
It is effective as one part of a multi-drug regimen for treatment of stomach infections of Helicobacter pylori. It is typically combined with a proton-pump inhibitor (such as omeprazole) and a macrolide antibiotic (such as clarithromycin); other drug combinations are also effective.[25]
Lyme borreliosis
Amoxicillin is effective for treatment of early cutaneous Lyme borreliosis; the effectiveness and safety of oral amoxicillin is neither better nor worse than common alternatively-used antibiotics.[26]
Odontogenic infections
Amoxicillin is used to treat odontogenic infections, infections of the tongue, lips, and other oral tissues. It may be prescribed following a tooth extraction, particularly in those with compromised immune systems.[27]
Skin infections
Amoxicillin is occasionally used for the treatment of skin infections,[18] such as acne vulgaris.[28] It is often an effective treatment for cases of acne vulgaris that have responded poorly to other antibiotics, such as doxycycline and minocycline.[29]
Infections in infants in resource-limited settings
Amoxicillin is recommended by the World Health Organization for the treatment of infants with signs and symptoms of pneumonia in resource-limited situations when the parents are unable or unwilling to accept hospitalization of the child. Amoxicillin in combination with gentamicin is recommended for the treatment of infants with signs of other severe infections when hospitalization is not an option.[30]
Prevention of bacterial endocarditis
It is also used to prevent bacterial endocarditis and as a pain-reliever in high-risk people having dental work done, to prevent Streptococcus pneumoniae and other encapsulated bacterial infections in those without spleens, such as people with sickle-cell disease, and for both the prevention and the treatment of anthrax.[8] The United Kingdom recommends against its use for infectious endocarditis prophylaxis.[31] These recommendations do not appear to have changed the rates of infection for infectious endocarditis.[32]
Combination treatment
Amoxicillin is susceptible to degradation by β-lactamase-producing bacteria, which are resistant to most β-lactam antibiotics, such as penicillin. For this reason, it may be combined with clavulanic acid, a β-lactamase inhibitor. This drug combination is commonly called co-amoxiclav.[33]
Spectrum of activity
It is a moderate-spectrum, bacteriolytic, β-lactam antibiotic in the aminopenicillin family used to treat susceptible Gram-positive and Gram-negative bacteria. It is usually the drug of choice within the class because it is better-absorbed, following oral administration, than other β-lactam antibiotics. In general, Streptococcus, Bacillus subtilis, Enterococcus, Haemophilus, Helicobacter, and Moraxella are susceptible to amoxicillin, whereas Citrobacter, Klebsiella and Pseudomonas aeruginosa are resistant to it.[34] Some E. coli and most clinical strains of Staphylococcus aureus have developed resistance to amoxicillin to varying degrees.[35]
Adverse effects
Adverse effects are similar to those for other β-lactam antibiotics, including nausea, vomiting, rashes, and antibiotic-associated colitis. Loose bowel movements (diarrhea) may also occur. Rarer adverse effects include mental changes, lightheadedness, insomnia, confusion, anxiety, sensitivity to lights and sounds, and unclear thinking. Immediate medical care is required upon the first signs of these adverse effects.[8]
The onset of an allergic reaction to amoxicillin can be very sudden and intense; emergency medical attention must be sought as quickly as possible. The initial phase of such a reaction often starts with a change in mental state, skin rash with intense itching (often beginning in fingertips and around groin area and rapidly spreading), and sensations of fever, nausea, and vomiting. Any other symptoms that seem even remotely suspicious must be taken very seriously. However, more mild allergy symptoms, such as a rash, can occur at any time during treatment, even up to a week after treatment has ceased. For some people allergic to amoxicillin, the adverse effects can be fatal due to anaphylaxis.[8]
Use of the amoxicillin/clavulanic acid combination for more than one week has caused a drug-induced immunoallergic-type hepatitis in some patients. Young children having ingested acute overdoses of amoxicillin manifested lethargy, vomiting, and renal dysfunction.[36][37]
There is poor reporting of adverse effects of amoxicillin from clinical trials. For this reason, the severity and frequency of adverse effects from amoxicillin is probably higher than reported from clinical trials.[10]
Nonallergic rash
Between 3 and 10% of children taking amoxicillin (or ampicillin) show a late-developing (>72 hours after beginning medication and having never taken penicillin-like medication previously) rash, which is sometimes referred to as the "amoxicillin rash". The rash can also occur in adults and may rarely be a component of the DRESS syndrome.[38]
The rash is described as maculopapular or morbilliform (measles-like; therefore, in medical literature, it is called "amoxicillin-induced morbilliform rash".[39]). It starts on the trunk and can spread from there. This rash is unlikely to be a true allergic reaction and is not a contraindication for future amoxicillin usage, nor should the current regimen necessarily be stopped. However, this common amoxicillin rash and a dangerous allergic reaction cannot easily be distinguished by inexperienced persons, so a healthcare professional is often required to distinguish between the two.[40][41]
A nonallergic amoxicillin rash may also be an indicator of infectious mononucleosis. Some studies indicate about 80–90% of patients with acute Epstein–Barr virus infection treated with amoxicillin or ampicillin develop such a rash.[42]
-
Nonallergic amoxicillin rash eight days after first dose: This photo was taken 24 hours after the rash began.
-
Eight hours after the first photo, individual spots have grown and begun to merge.
-
At 23 hours after the first photo, the color appears to be fading, and much of rash has spread to confluence.
Interactions
Amoxicillin may interact with these drugs:
- Anticoagulants (dabigatran, warfarin).[18][43][44][45]
- Methotrexate (chemotherapy and immunosuppressant).[44][45]
- Typhoid, Cholera and BCG vaccines.[44][45]
- Probenecid reduces renal excretion and increases blood levels of amoxicillin.[44][45]
- Oral contraceptives potentially become less effective.[46]
- Allopurinol (gout treatment).[44][45][47]
- Mycophenolate (immunosuppressant)[45]
Pharmacology
Amoxicillin (α-amino-p-hydroxybenzyl penicillin) is a semisynthetic derivative of penicillin with a structure similar to ampicillin but with better absorption when taken by mouth, thus yielding higher concentrations in blood and in urine.[48] Amoxicillin diffuses easily into tissues and body fluids. It will cross the placenta and is excreted into breastmilk in small quantities. It is metabolized by the liver and excreted into the urine. It has an onset of 30 minutes and a half-life of 3.7 hours in newborns and 1.4 hours in adults.[18]
Amoxicillin attaches to the cell wall of susceptible bacteria and results in their death. It also is a bactericidal compound. It is effective against streptococci, pneumococci, enterococci, Haemophilus influenzae, Escherichia coli, Proteus mirabilis, Neisseria meningitidis, Neisseria gonorrhoeae, Shigella, Chlamydia trachomatis, Salmonella, Borrelia burgdorferi, and Helicobacter pylori.[18] As a derivative of ampicillin, amoxicillin is a member of the penicillin family and, like penicillins, is a β-lactam antibiotic.[49] It inhibits cross-linkage between the linear peptidoglycan polymer chains that make up a major component of the bacterial cell wall. It has two ionizable groups in the physiological range (the amino group in alpha-position to the amide carbonyl group and the carboxyl group).[50]
History
Amoxicillin was one of several semisynthetic derivatives of 6-aminopenicillanic acid (6-APA) developed by the Beecham Group in the 1960s. It was invented by Anthony Alfred Walter Long and John Herbert Charles Nayler, two British scientists.[51][52] It became available in 1972 and was the second aminopenicillin to reach the market (after ampicillin in 1961).[53][54][55] Co-amoxiclav became available in 1981.[54]
Society and culture
Economics
Amoxicillin is relatively inexpensive.[56] In 2022, a survey of 8 generic antibiotics commonly prescribed in the United States found their average cost to be about $42.67, while amoxicillin was sold for $12.14 on average.[57]
Modes of delivery
Pharmaceutical manufacturers make amoxicillin in trihydrate form, for oral use available as capsules, regular, chewable and dispersible tablets, syrup and pediatric suspension for oral use, and as the sodium salt for intravenous administration.[medical citation needed]
An extended-release is available.[6][58] The intravenous form of amoxicillin is not sold in the United States.[59] When an intravenous aminopenicillin is required in the United States, ampicillin is typically used. When there is an adequate response to ampicillin, the course of antibiotic therapy may often be completed with oral amoxicillin.[60]
Research with mice indicated successful delivery using intraperitoneally injected amoxicillin-bearing microparticles.[61]
Names
"Amoxicillin" is the International Nonproprietary Name (INN), British Approved Name (BAN), and United States Adopted Name (USAN), while "amoxycillin" is the Australian Approved Name (AAN).[citation needed]
Amoxicillin is one of the semisynthetic penicillins discovered by former pharmaceutical company Beecham Group. The patent for amoxicillin has expired, thus amoxicillin and co-amoxiclav preparations are marketed under various brand names across the world.[1]
Veterinary uses
Amoxicillin is also sometimes used as an antibiotic for animals. The use of amoxicillin for animals intended for human consumption (chickens, cattle, and swine for example) has been approved.[62]
References
- ^ a b "International brand names for amoxicillin". Drugs.com. Archived from the original on 29 May 2016. Retrieved 15 November 2016.
- ^ a b "Amoxil Vials for Injection 500mg - Summary of Product Characteristics (SmPC)". (emc). 4 November 2021. Retrieved 8 October 2022.
- ^ "Amoxil (amoxicillin) Capsules, Tablets, Chewable Tablets, and Powder for Oral Suspension". DailyMed. Retrieved 8 October 2022.
- ^ a b "Amoxil: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 8 October 2022.
- ^ a b "Trimox: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 8 October 2022.
- ^ a b "Moxatag (amoxicillin extended-release) Tablets Initial U.S. Approval: 1974". DailyMed. Retrieved 8 October 2022.
- ^ "Amoxicillin". www.chemsrc.com.
- ^ a b c d e f g h i j k l m "Amoxicillin". Drugs.com, The American Society of Health-System Pharmacists. 3 January 2022. Retrieved 24 November 2022.
- ^ "Amoxicillin Sodium for Injection". EMC. 10 February 2016. Archived from the original on 27 October 2016. Retrieved 26 October 2016.
- ^ a b Gillies M, Ranakusuma A, Hoffmann T, Thorning S, McGuire T, Glasziou P, Del Mar C (January 2016). "Common harms from amoxicillin: a systematic review and meta-analysis of randomized placebo-controlled trials for any indication". CMAJ. 187 (1): E21–E31. doi:10.1503/cmaj.140848. PMC 4284189. PMID 25404399.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 490. ISBN 978-3-527-60749-5. Archived from the original on 8 September 2017.
- ^ Roy J (2012). An introduction to pharmaceutical sciences production, chemistry, techniques and technology. Cambridge: Woodhead Pub. p. 239. ISBN 978-1-908818-04-1. Archived from the original on 8 September 2017.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ World Health Organization (2021). World Health Organization model list of essential medicines: 22nd list (2021). Geneva: World Health Organization. hdl:10665/345533. WHO/MHP/HPS/EML/2021.02.
- ^ Kelly D (2008). Diseases of the liver and biliary system in children (3 ed.). Chichester, UK: Wiley-Blackwell. p. 217. ISBN 978-1-4443-0054-3. Archived from the original on 8 September 2017.
- ^ "The Top 300 of 2020". ClinCalc. Retrieved 7 October 2022.
- ^ "Amoxicillin - Drug Usage Statistics". ClinCalc. Retrieved 7 October 2022.
- ^ a b c d e f g "Amoxicillin" (PDF). Davis. 2017. Archived (PDF) from the original on 8 September 2017. Retrieved 22 March 2017.
- ^ Venekamp RP, Sanders SL, Glasziou PP, Del Mar CB, Rovers MM (June 2015). "Antibiotics for acute otitis media in children". The Cochrane Database of Systematic Reviews. 2015 (6): CD000219. doi:10.1002/14651858.CD000219.pub4. PMC 7043305. PMID 26099233.
- ^ Thanaviratananich S, Laopaiboon M, Vatanasapt P (December 2013). "Once or twice daily versus three times daily amoxicillin with or without clavulanate for the treatment of acute otitis media". The Cochrane Database of Systematic Reviews (12): CD004975. doi:10.1002/14651858.CD004975.pub3. PMID 24338106.
- ^ "Five Things Physicians and Patients Should Question" (PDF). Choosing Wisely: An Initiative of the ABIM Foundation. American Academy of Allergy, Asthma, and Immunology. Archived (PDF) from the original on 3 November 2012. Retrieved 14 August 2012.
- ^ Ahovuo-Saloranta A, Rautakorpi UM, Borisenko OV, Liira H, Williams JW, Mäkelä M (February 2014). Ahovuo-Saloranta A (ed.). "Antibiotics for acute maxillary sinusitis in adults". The Cochrane Database of Systematic Reviews (2): CD000243. doi:10.1002/14651858.CD000243.pub3. PMID 24515610. (Retracted, see doi:10.1002/14651858.cd000243.pub4)
- ^ National Clinical Guideline Centre (UK) (2014). Pneumonia. National Institute for Health and Care Excellence: Clinical Guidelines. National Institute for Health and Care Excellence (UK). PMID 25520986. Archived from the original on 8 September 2017 – via U.S. National Library of Medicine.
{{cite book}}:|work=ignored (help) - ^ Revised WHO Classification and Treatment of Pneumonia in Children at Health Facilities - NCBI Bookshelf. WHO Guidelines Approved by the Guidelines Review Committee. World Health Organization. 2014. ISBN 978-92-4-150781-3. PMID 25535631. Archived from the original on 8 September 2017.
- ^ Chey WD, Leontiadis GI, Howden CW, Moss SF (February 2017). "ACG Clinical Guideline: Treatment of Helicobacter pylori Infection". The American Journal of Gastroenterology. 112 (2): 212–239. doi:10.1038/ajg.2016.563. PMID 28071659. S2CID 9390953.
- ^ Torbahn G, Hofmann H, Rücker G, Bischoff K, Freitag MH, Dersch R, et al. (November 2018). "Efficacy and Safety of Antibiotic Therapy in Early Cutaneous Lyme Borreliosis: A Network Meta-analysis". JAMA Dermatology. 154 (11): 1292–1303. doi:10.1001/jamadermatol.2018.3186. PMC 6248135. PMID 30285069.
- ^ Tancawan AL, Pato MN, Abidin KZ, Asari AS, Thong TX, Kochhar P, et al. (2015). "Amoxicillin/Clavulanic Acid for the Treatment of Odontogenic Infections: A Randomised Study Comparing Efficacy and Tolerability versus Clindamycin". International Journal of Dentistry. 2015: 472470. doi:10.1155/2015/472470. PMC 4537712. PMID 26300919.
- ^ "Adolescent Acne: Management". Archived from the original on 22 December 2010.
- ^ "Amoxicillin and Acne Vulgaris". scienceofacne.com. 5 September 2012. Archived from the original on 21 July 2012. Retrieved 17 August 2012.
- ^ Guideline: Managing Possible Serious Bacterial Infection in Young Infants When Referral Is Not Feasible - NCBI Bookshelf. WHO Guidelines Approved by the Guidelines Review Committee. World Health Organization. 2015. ISBN 978-92-4-150926-8. PMID 26447263. Archived from the original on 8 September 2017.
- ^ "CG64 Prophylaxis against infective endocarditis: Full guidance" (PDF). NICE. Archived from the original (PDF) on 12 November 2011. Retrieved 8 June 2011.
- ^ Thornhill MH, Dayer MJ, Forde JM, Corey GR, Chu VH, Couper DJ, Lockhart PB (May 2011). "Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study". BMJ. 342: d2392. doi:10.1136/bmj.d2392. PMC 3086390. PMID 21540258.
- ^ "Amoxicillin Susceptibility and Resistance Data" (PDF). Archived from the original (PDF) on 13 July 2019. Retrieved 20 July 2013.
- ^ "Amoxicillin spectrum of bacterial susceptibility and Resistance" (PDF). Archived from the original (PDF) on 22 December 2018. Retrieved 8 April 2012.
- ^ Tadesse BT, Ashley EA, Ongarello S, Havumaki J, Wijegoonewardena M, González IJ, Dittrich S (September 2017). "Antimicrobial resistance in Africa: a systematic review". BMC Infectious Diseases. 17 (1): 616. doi:10.1186/s12879-017-2713-1. PMC 5594539. PMID 28893183.
- ^ Cundiff J, Joe S (January 2007). "Amoxicillin-clavulanic acid-induced hepatitis". American Journal of Otolaryngology. 28 (1): 28–30. doi:10.1016/j.amjoto.2006.06.007. PMID 17162128.
- ^ Baselt R (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 81–83.
- ^ "Amoxicillin Rash". Tufts Medical Center. Tufts Medicine MelroseWakefield Hospital. Retrieved 24 June 2023.
- ^ Barbaud AM, Béné MC, Schmutz JL, Ehlinger A, Weber M, Faure GC (April 1997). "Role of delayed cellular hypersensitivity and adhesion molecules in amoxicillin-induced morbilliform rashes". Archives of Dermatology. 133 (4): 481–6. doi:10.1001/archderm.1997.03890400081011. PMID 9126012. INIST 2654598.
- ^ Pichichero ME (April 2005). "A review of evidence supporting the American Academy of Pediatrics recommendation for prescribing cephalosporin antibiotics for penicillin-allergic patients". Pediatrics. 115 (4): 1048–57. doi:10.1542/peds.2004-1276. PMID 15805383. S2CID 21246804.
- ^ Schmitt BD (2005). Your child's health: the parents' one-stop reference guide to symptoms, emergencies, common illnesses, behavior problems, healthy development (2nd ed.). New York: Bantam Books. ISBN 978-0-553-38369-0.
- ^ Kagan BM (April 1977). "Ampicillin rash". The Western Journal of Medicine. 126 (4): 333–5. PMC 1237570. PMID 855325.
- ^ British National Formulary 57 March 2009
- ^ a b c d e Arcangelo VP, Peterson AM, Wilbur V, Reinhold JA (17 August 2016). Pharmacotherapeutics for Advanced Practice: A Practical Approach. LWW. ISBN 978-1-496-31996-8.
- ^ a b c d e f Reis W, Gaio J, Trang T, Reis H, Tang J, Juma H, Ramos F, Santos HD, Reis C (2020), Prabhakar H, Mahajan C, Kapoor I (eds.), "Antibiotics", Pharmacology in Clinical Neurosciences: A Quick Guide, Singapore: Springer, pp. 265–497, doi:10.1007/978-981-15-3591-8_10, ISBN 978-981-15-3591-8, retrieved 9 April 2023
- ^ Zhanel GG, Siemens S, Slayter K, Mandell L (November 1999). "Antibiotic and oral contraceptive drug interactions: Is there a need for concern?". The Canadian Journal of Infectious Diseases. 10 (6): 429–433. doi:10.1155/1999/539376. PMC 3250726. PMID 22346401.
- ^ Comeau D, Heaton K, Gordon A (January 2012), Rakel RE, Rakel DP (eds.), "Chapter 32 - Rheumatology and Musculoskeletal Problems", Textbook of Family Medicine (Eighth ed.), Philadelphia: W.B. Saunders, pp. 648–689, doi:10.1016/b978-1-4377-1160-8.10032-6, ISBN 978-1-4377-1160-8
- ^ Handsfield HH, Clark H, Wallace JF, Holmes KK, Turck M (February 1973). "Amoxicillin, a new penicillin antibiotic". Antimicrobial Agents and Chemotherapy. 3 (2): 262–5. doi:10.1128/AAC.3.2.262. PMC 444397. PMID 4208282.
- ^ Alcamo IE (2003). Microbes and Society: An Introduction to Microbiology. Jones & Bartlett Learning. p. 198. ISBN 978-0-7637-1430-7.
- ^ Sezer AD, ed. (2016). Application of Nanotechnology in Drug Delivery. INTECH. p. 423. ISBN 978-953-51-1628-8. Retrieved 24 July 2019.
- ^ GB patent 978178, John Herbert Charles Nayler & Harry Smith, "Penicillins", published 1964-12-16, assigned to Beecham Research Laboratories Ltd
- ^ GB patent 1241844, Long AA, Nayler JH, "Penicillins", published 1971-08-04, assigned to Beecham Group LTD
- ^ Geddes AM, Klugman KP, Rolinson GN (December 2007). "Introduction: historical perspective and development of amoxicillin/clavulanate". International Journal of Antimicrobial Agents. 30 (Suppl 2): S109-12. doi:10.1016/j.ijantimicag.2007.07.015. PMID 17900874.
- ^ a b Raviña E (2014). The Evolution of Drug Discovery. Weinheim: Wiley-VCH. p. 262. ISBN 978-3-527-32669-3.
- ^ Bruggink A (2001). Synthesis of β-lactam antibiotics. Springer. p. 17. ISBN 978-0-7923-7060-4.
- ^ Hanno PM, Guzzo TJ, Malkowicz SB, Wein AJ (2014). Penn Clinical Manual of Urology E-Book. Elsevier Health Sciences. p. 122. ISBN 978-0-323-24466-4.
- ^ "How Much Do Antibiotics Cost Without Insurance in 2021?".
- ^ "Drug Approval Package: Moxatag (amoxicillin extended-release) NDA #050813".
- ^ Marek CL, Timmons SR (2018). Nowak A (ed.). Pediatric Dentistry: Infancy Through Adolescence. Saunders. ISBN 978-0-323-60826-8.
- ^ "A Quick Guide to Switch : Antibiotics: IV to Oral" (PDF). Safetyandquality.gov.au. Archived (PDF) from the original on 9 October 2022. Retrieved 1 March 2022.
- ^ Farazuddin M, Chauhan A, Khan RM, Owais M (August 2011). "Amoxicillin-bearing microparticles: potential in the treatment of Listeria monocytogenes infection in Swiss albino mice". Bioscience Reports. 31 (4): 265–72. doi:10.1042/BSR20100027. PMID 20687896.
- ^ Ramos F, Boison J, Friedlander LG. "Amoxicillin" (PDF). fao.org. Food and Agriculture Organization of the United Nations. Archived (PDF) from the original on 9 October 2022. Retrieved 8 November 2019.
Further reading
- Neal MJ (2002). Medical Pharmacology at a Glance (4th ed.). Oxford: Blackwell Science. ISBN 978-0-632-05244-8.
External links
- "Amoxicillin". Drug Information Portal. U.S. National Library of Medicine.



