Levonorgestrel
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Plan B, others |
| Other names | LNG; d-Norgestrel; d(–)-Norgestrel; D-Norgestrel; WY-5104; SH-90999; NSC-744007; 18-Methylnorethisterone; 17α-Ethynyl-18-methyl-19-nortestosterone; 17α-Ethynyl-18-methylestr-4-en-17β-ol-3-one; 13β-Ethyl-17α-hydroxy-18,19-dinorpregn-4-en-20-yn-3-one |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a610021 |
| Pregnancy category |
|
| Routes of administration | By mouth, transdermal patch, intrauterine device, subcutaneous implant |
| Drug class | Progestin; Progestogen |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 95% (range 85–100%)[1][2] |
| Protein binding | 98% (50% to albumin, 48% to SHBG)[1] |
| Metabolism | Liver (reduction, hydroxylation, conjugation)[1][3] |
| Metabolites | • 5α-Dihydro-LNG[1] |
| Elimination half-life | 24–32 hours[1] |
| Excretion | Urine: 20–67% Feces: 21–34%[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.011.227 |
| Chemical and physical data | |
| Formula | C21H28O2 |
| Molar mass | 312.446 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Levonorgestrel is a hormonal medication which is used in a number of birth control methods.[4] In pill form, sold under the brand name Plan B among others, it is useful within 120 hours as emergency birth control.[4] It becomes less effective the longer after sex and only works before pregnancy has occurred.[4] It is also combined with an estrogen to make combined oral birth control pills.[5] Within an intrauterine device (IUD), sold as Mirena among others, it is effective for long-term prevention of pregnancy.[4] An implantable form of levonorgestrel is also available in some countries.[6]
Common side effects include nausea, breast tenderness, headaches, and increased, decreased, or irregular menstrual bleeding.[4] When used as a form of emergency contraception, if pregnancy occurs, there is no evidence its use harms the baby.[4] It is safe to use during breastfeeding.[4] Birth control that contains levonorgestrel will not change the risk of sexually transmitted infections.[4] It is a progestin and has effects similar to those of the hormone progesterone.[4] It works mostly by preventing ovulation and closing off the cervix to prevent the passage of sperm.[4]
Levonorgestrel was discovered in 1963 and was introduced for medical use together with ethinylestradiol in 1970.[7][8] It is on the World Health Organization's List of Essential Medicines, the most effective and safe medicines needed in a health system.[9] It is available as a generic medication.[10] The wholesale cost in the developing world costs between 0.23 and 1.65 USD for the dose required for emergency birth control.[11] In the United States it is over the counter for all ages.[12]
Medical uses
Birth control
At low doses, levonorgestrel is used in monophasic and triphasic formulations of combined oral contraceptive pills, with available monophasic doses ranging from 100–250 µg, and triphasic doses of 50 µg/75 µg/125 µg.[13] It is combined with the estrogen ethinylestradiol in these formulations.[13]
At very low daily dose of 30 µg, levonorgestrel is used in some progestogen-only pill formulations.[13]
Levonorgestrel is the active ingredient in a number of intrauterine devices including Mirena and Skyla.[13][14] It is also the active ingredient in the birth control implants Norplant and Jadelle.[13][14]
Emergency birth control
Levonorgestrel is used in emergency contraceptive pills (ECPs), both in a combined Yuzpe regimen which includes estrogen, and as a levonorgestrel-only method. The levonorgestrel-only method uses levonorgestrel 1.5 mg (as a single dose or as two 0.75 mg doses 12 hours apart) taken within 3 days of unprotected sex, with one study indicating that beginning as late as 120 hours (5 days) after intercourse could be effective.
The primary mechanism of action of levonorgestrel as a progestogen-only emergency contraceptive pill is, according to International Federation of Gynecology and Obstetrics (FIGO), to prevent fertilization by inhibition of ovulation and thickening of cervical mucus.[15][16][17][18] FIGO has stated that: "review of the evidence suggests that LNG [levonorgestreol] ECPs cannot prevent implantation of a fertilized egg. Language on implantation should not be included in LNG ECP product labeling."[19][20] In November 2013, the European Medicines Agency (EMA) approved a change to the label saying it cannot prevent implantation of a fertilized egg.[21]
Other studies still find the evidence to be unclear.[22] While it is unlikely that emergency contraception affects implantation it is impossible to completely exclude the possibility of post-fertilization effect.[23]
In November 2013, the EMA also approved a change to the label for HRA Pharma's NorLevo saying: "In clinical trials, contraceptive efficacy was reduced in women weighing 75 kg [165 pounds] or more, and levonorgestrel was not effective in women who weighed more than 80 kg [176 pounds]."[21][24][25] In November 2013 and January 2014, the FDA and the EMA said they were reviewing whether increased weight and body mass index (BMI) reduce the efficacy of emergency contraceptives.[21]
Hormone therapy
Levonorgestrel is used in combination with an estrogen in menopausal hormone therapy.[13][26] It is used under the brand name Klimonorm as a combined oral tablet with estradiol valerate and under the brand name Climara Pro as a combined transdermal patch with estradiol.[13][26]
Available forms
Levonorgestrel is available alone in emergency contraceptive pills and progestogen-only pills, in combination with ethinylestradiol in birth control pills, in combination with estradiol valerate in oral tablets for use in menopausal hormone therapy, in combination with estradiol in a transdermal patch for use in menopausal hormone therapy, alone as an intrauterine device for use in hormonal birth control, and alone as a subcutaneous birth control implant.[13][27][28]
Contraindications
Known or suspected pregnancy is a contraindication of levonorgestrel as an emergency contraceptive.[29]
Side effects
After intake of 1.5 mg levonorgestrel in clinical trials, very common side effects (reported by 10% or more) included: hives, dizziness, headache, nausea, abdominal pain, uterine pain, delayed menstruation, heavy menstruation, uterine bleeding, and fatigue; common side effects (reported by 1% to 10%) included diarrhea, vomiting, and painful menstruation; these side effects usually disappeared within 48 hours.[30][31]
Overdose
Overdose of levonorgestrel as an emergency contraceptive has not been described.[29] Nausea and vomiting might be expected.[29]
Interactions
If taken together with drugs that induce the CYP3A4 cytochrome P450 liver enzyme, levonorgestrel may be metabolized faster and may have lower effectiveness.[32]
Pharmacology
Pharmacodynamics
Levonorgestrel is a progestogen; that is, an agonist of the progesterone receptor (PR), the main biological target of the progestogen sex hormone progesterone.[1] It is also a weak agonist of the androgen receptor (AR), the main biological target of the androgen sex hormone testosterone.[1] Levonorgestrel has no other important hormonal activity, including no estrogenic, glucocorticoid, or antimineralocorticoid activity.[1] The lack of significant mineralocorticoid or antimineralocorticoid activity with levonorgestrel is in spite of a relatively high affinity for the mineralocorticoid receptor of as much as 75% of that of aldosterone.[1] Due to its progestogenic activity, levonorgestrel has antigonadotropic effects, and is able to suppress fertility and gonadal sex hormone production in both women and men.[1][33]
| Compound | PR | AR | ER | GR | MR | SHBG | CBG | |
|---|---|---|---|---|---|---|---|---|
| Levonorgestrel | 150–162 | 34a, 45 | 0 | 1–8 | 17–75 | 50 | 0 | |
| 5α-Dihydrolevonorgestrel | 50 | 38a | 0 | ? | ? | ? | ? | |
| 3α,5α-Tetrahydrolevonorgestrel | ? | ? | 0.4 | ? | ? | ? | ? | |
| 3β,5α-Tetrahydrolevonorgestrel | ? | ? | 2.4 | ? | ? | ? | ? | |
| Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone (a = mibolerone) for the AR, E2 for the ER, DEXA for the GR, aldosterone for the MR, DHT for SHBG, and cortisol for CBG. Sources:[1][34][35][36] | ||||||||
Androgenic activity
Levonorgestrel is a weakly androgenic progestin and in women may cause androgenic side effects such as decreased sex hormone-binding globulin (SHBG) levels, decreased HDL cholesterol levels, weight gain, and acne.[1][37] In combination with a potent estrogen like ethinylestradiol however, all contraceptives containing androgenic progestins are negligibly androgenic in clinical practice and can in fact be used to treat androgen-dependent conditions like acne and hirsutism in women.[37] This is because ethinylestradiol causes a marked increase in SHBG levels and thereby decreases levels of free and hence bioactive testosterone, acting as a functional antiandrogen.[37] Nonetheless, contraceptives containing progestins that are less androgenic increase SHBG levels to a greater relative extent and may be more effective for such indications.[37] Levonorgestrel is currently the most androgenic progestin that remains used in contraceptives, and contraceptives containing levonorgestrel may be less effective for androgen-dependent conditions relative to those containing other progestins that are less androgenic.[38][39][40]
Antigonadotropic effects
In men, levonorgestrel causes marked suppression of circulating testosterone levels secondary to its antigonadotropic effects.[41] Because of this, and due to its androgenic activity being only weak and hence insufficient for purposes of androgen replacement in males, levonorgestrel has potent functional antiandrogenic effects in men, and is able to produce associated adverse effects like decreased libido and erectile dysfunction among others.[41] In relation to this, levonorgestrel has been combined with an androgen like testosterone or dihydrotestosterone when it has been studied as a hormonal contraceptive in men.[33][41]
Pharmacokinetics
The bioavailability of levonorgestrel is approximately 95% (range 85 to 100%).[1][2] The plasma protein binding of levonorgestrel is about 98%.[1] It is bound 50% to albumin and 48% to SHBG.[1] Levonorgestrel is metabolized in the liver, via reduction, hydroxylation, and conjugation (specifically glucuronidation and sulfation).[1][3] Oxidation occurs primarily at the C2α and C16β positions, while reduction occurs in the A ring.[3] 5α-Dihydrolevonorgestrel is produced as an active metabolite of levonorgestrel by 5α-reductase.[1] The elimination half-life of levonorgestrel is 24 to 32 hours, although values as short as 8 hours and as great as 45 hours have been reported.[1][3] About 20 to 67% of a single oral dose of levonorgestrel is eliminated in urine and 21 to 34% in feces.[3]
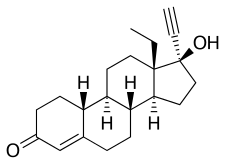
Chemistry
Levonorgestrel, also known as 17α-ethynyl-18-methyl-19-nortestosterone or as 17α-ethynyl-18-methylestr-4-en-17β-ol-3-one, is a synthetic estrane steroid and a derivative of testosterone.[42][43] It is the C13β or levorotatory stereoisomer and enantiopure form of norgestrel, the C13α or dextrorotatory isomer being inactive.[44][45] Levonorgestrel is more specifically a derivative of norethisterone (17α-ethynyl-19-nortestosterone) and is the parent compound of the gonane (18-methylestrane) subgroup of the 19-nortestosterone family of progestins.[46] Levonorgestrel acetate and levonorgestrel butanoate are C17β esters of levonorgestrel.[47][48]
History
Norgestrel (rac-13-ethyl-17α-ethynyl-19-nortestosterone), the racemic mixture containing levonorgestrel and dextronorgestrel, was discovered by Hughes and colleagues at Wyeth in 1963 via structural modification of norethisterone (17α-ethynyl-19-nortestosterone).[7][49][50][51] It was the first progestogen to be manufactured via total chemical synthesis.[50][51] Norgestrel was introduced for medical use as a combined birth control pill with ethinylestradiol under the brand name Eugynon in Germany in 1966 and under the brand name Ovral in the United States 1968, and as a progestogen-only pill under the brand name Ovrette in the United States in 1973.[51][52][53][54] Following its discovery, norgestrel had been licensed by Wyeth to Schering AG, which separated the racemic mixture into its two optical isomers and identified levonorgestrel (13β-ethyl-17α-ethynyl-19-nortestosterone) as the active component of the mixture.[8][50][51] Levonorgestrel was first studied in humans by 1970, and was introduced for medical use in Germany as a combined birth control pill with ethinylestradiol under the brand name Neogynon in August 1970.[8][52][53][55][56][57] A more widely used formulation, containing lower doses of ethinylestradiol and levonorgestrel, was introduced under the brand name Microgynon by 1973.[13][58][59] In addition to combined formulations, levonorgestrel was introduced as a progestogen-only pill under the brand names Microlut by 1972 and Microval by 1974.[60][61] Many other formulations and brand names of levonorgestrel-containing birth control pills have also been marketed.[13]
Levonorgestrel, taken alone in a single high dose, was first evaluated as a form of emergency contraception in 1973.[62] It was the second progestin to be evaluated for such purposes, following a study of quingestanol acetate in 1970.[62][63] In 1974, the Yuzpe regimen, which consisted of high doses of a combined birth control pill containing ethinylestradiol and norgestrel, was described as a method of emergency contraception by A. Albert Yuzpe and colleagues, and saw widespread interest.[64][65] Levonorgestrel-only emergency contraception was introduced under the brand name Postinor by 1978.[66] Ho and Kwan published the first study comparing levonorgestrel only and the Yuzpe regimen as methods of emergency contraception in 1993 and found that they had similar effectiveness but that levonorgestrel alone was better-tolerated.[67][68] In relation to this, the Yuzpe regimen has largely been replaced as a method of emergency contraception by levonorgrestrel-only preparations.[69] Levonorgestrel-only emergency contraception was approved in the United States under the brand name Plan B in 1999, and has also been marketed widely elsewhere throughout the world under other brand names such as Levonelle and NorLevo in addition to Postinor.[13][70] In 2013, the Food and Drug Administration approved Plan B One-Step for sale over-the-counter in the United States without a prescription or age restriction.[71]
Levonorgestrel has also been introduced for use as a progestogen-only intrauterine device under the brand names Mirena and Skyla among others, as a progestogen-only birth control implant under the brand names Norplant and Jadelle, as a combined oral tablet with estradiol valerate for menopausal hormone therapy under the brand name Klimonorm, and as a combined transdermal patch with estradiol for menopausal hormone therapy under the brand name Climara Pro.[13][14][26] Ester prodrugs of levonorgestrel such as levonorgestrel acetate and levonorgestrel butanoate have been developed and studied as other forms of birth control such as long-acting progestogen-only injectable contraceptives and contraceptive vaginal rings, but have not been marketed for medical use.[47][48]
Society and culture
Generic names
Levonorgestrel is the generic name of the drug and its INN, USAN, USP, BAN, DCIT, and JAN, while lévonorgestrel is its DCF.[13][42][43] It is also known as d-norgestrel, d(–)-norgestrel, or D-norgestrel, as well as by its developmental code names WY-5104 (Wyeth) and SH-90999 (Schering AG).[13][42][43][60]
Brand names
Levonorgestrel is marketed alone or in combination with an estrogen (specifically ethinylestradiol, estradiol, or estradiol valerate) under a multitude of brand names throughout the world, including Alesse, Altavera, Alysena, Amethia, Amethyst, Ashlyna, Aviane, Camrese, Chateal, Climara Pro, Daysee, Emerres, Enpresse, Erlibelle, Escapelle, Falmina, Introvale, Isteranda, Jadelle, Jaydess, Jolessa, Klimonorm, Kurvelo, Kyleena, Lessina, Levlen, Levodonna, Levonelle, Levonest, Levosert, Levora, Liletta, Loette, Logynon, LoSeasonique, Lutera, Lybrel, Marlissa, Microgynon, Microlut, Min-Ovral, Miranova, Mirena, My Way, Myzilra, Next Choice, Nordette, Norgeston, NorLevo, Norplant, Option 2, Orsythia, Ovima, Ovranette, Plan B, Plan B One-Step, Portia, Postinor, Postinor-2, Preventeza, Ramonna, Rigevidon, Quartette, Quasense, Seasonale, Seasonique, Skyla, Sronyx, Tri-Levlen, Trinordiol, Triphasil, Triquilar, Tri-Regol, Trivora, and Upostelle, among many others.[13][43][72] These formulations are used as emergency contraceptives, normal contraceptives, or in menopausal hormone therapy for the treatment of menopausal symptoms.
As an emergency contraceptive, levonorgestrel is often referred to colloquially as the "morning-after pill".[73][74]
Availability
Levonorgestrel is very widely marketed throughout the world and is available in almost every country.[13][43]
Accessibility
Levonorgestrel-containing emergency contraception is available over-the-counter in some countries, such as the United States.[71]
A policy update in 2015 required all Indian Health Services-run pharmacies, clinics, and emergency departments to have Plan B One-Step in stock, to distribute it to any woman (or her representative) who asked for it without a prescription, age verification, registration or any other requirement, to provide orientation training to all staff regarding the medication, to provide unbiased and medically accurate information about emergency contraception, and to make someone available at all times to distribute the pill in case the primary staffer objected to providing it on religious or moral grounds.[75]
Research
Levonorgestrel has been studied in combination with androgens such as testosterone and dihydrotestosterone as a hormonal contraceptive for men.[33][41]
References
- ^ a b c d e f g h i j k l m n o p q r Kuhl H (2005). "Pharmacology of estrogens and progestogens: influence of different routes of administration" (PDF). Climacteric. 8 Suppl 1: 3–63. doi:10.1080/13697130500148875. PMID 16112947. Archived from the original (PDF) on 2016-08-22.
{{cite journal}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Fotherby K (August 1996). "Bioavailability of orally administered sex steroids used in oral contraception and hormone replacement therapy". Contraception. 54 (2): 59–69. doi:10.1016/0010-7824(96)00136-9. PMID 8842581.
- ^ a b c d e f Donna Shoupe; Florence P. Haseltine (6 December 2012). Contraception. Springer Science & Business Media. pp. 22–. ISBN 978-1-4612-2730-4.
- ^ a b c d e f g h i j "Progestins (Etonogestrel, Levonorgestrel, Norethindrone)". The American Society of Health-System Pharmacists. Archived from the original on 2015-09-07. Retrieved Aug 21, 2015.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Postgraduate Gynecology. Jaypee Brothers Medical Pub. 2011. p. 159. ISBN 9789350250822. Archived from the original on 2015-09-26.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Chapter 1". Research on reproductive health at WHO : biennial report 2000-2001. Geneva: World health organization. 2002. ISBN 9789241562089. Archived from the original on 2015-09-26.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b A. Wayne Meikle (1 June 1999). Hormone Replacement Therapy. Springer Science & Business Media. pp. 383–. ISBN 978-1-59259-700-0.
The gonanes share the structural modifications found in the estranes and also possess [an ethyl] group at the position 13 and a keto group at position 3. Norgestrel was synthesized in 1963 and is a racemic mixture of dextro and levorotatory forms. The levorotatory form, levonorgestrel, provides the biological activity.
- ^ a b c Klaus Roth (2014). Chemische Leckerbissen. John Wiley & Sons. pp. 77–. ISBN 978-3-527-33739-2.
[Levonorgestrel (24): The product generated by Smith's norgestrel total synthesis was a racemate, so half of each consisted of the left- and the right-handed enantiomer. Chemists at Schering discovered that only the levorotatory enantiomer was effective [49] and developed a biotechnological process for the preparation of the pure levorotatory enantiomer. This was the active ingredient levonorgestrel born. With the single-acting enantiomer, the dose and thus the liver burden could be halved again. The resulting Neogynon® contained 0.25 mg levonorgestrel and 0.05 mg ethinylestradiol and was introduced in 1970.]
- ^ "WHO Model List of Essential Medicines (19th List)" (PDF). World Health Organization. April 2015. Archived from the original (PDF) on 13 December 2016. Retrieved 8 December 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Hamilton, Richard J. (2014). Tarascon pocket pharmacopoeia : 2014 deluxe lab-pocket edition (15th ed.). Sudbury: Jones & Bartlett Learning. pp. 310–312. ISBN 9781284053999. Archived from the original on 2015-09-26.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ "Levonorgestrel". International Drug Price Indicator Guide. Archived from the original on 22 January 2018. Retrieved 21 August 2015.
{{cite web}}: Unknown parameter|dead-url=ignored (|url-status=suggested) (help) - ^ "FDA approves Plan B One-Step emergency contraceptive for use without a prescription for all women of child-bearing potential". June 20, 2013. Archived from the original on 14 January 2016. Retrieved 2 February 2016.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d e f g h i j k l m n o p "Archived copy". Archived from the original on 2017-08-03. Retrieved 2017-08-03.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link) - ^ a b c Friend DR (October 2016). "Development of controlled release systems over the past 50 years in the area of contraception". J Control Release. 240: 235–241. doi:10.1016/j.jconrel.2015.12.043. PMID 26732558.
- ^ Trussell, James; Schwarz, Eleanor Bimla (2011). "Emergency contraception". In Hatcher, Robert A.; Trussell, James; Nelson, Anita L.; Cates, Willard Jr.; Kowal, Deborah; Policar, Michael S. (eds.). Contraceptive technology (20th revised ed.). New York: Ardent Media. pp. 113–145. ISBN 978-1-59708-004-0. ISSN 0091-9721. OCLC 781956734. p. 121:
Mechanism of action
Copper-releasing IUCs
When used as a regular or emergency method of contraception, copper-releasing IUCs act primarily to prevent fertilization. Emergency insertion of a copper IUC is significantly more effective than the use of ECPs, reducing the risk of pregnancy following unprotected intercourse by more than 99%.2,3 This very high level of effectiveness implies that emergency insertion of a copper IUC must prevent some pregnancies after fertilization.
Emergency contraceptive pills
To make an informed choice, women must know that ECPs—like the birth control pill, patch, ring, shot, and implant,76 and even like breastfeeding77—prevent pregnancy primarily by delaying or inhibiting ovulation and inhibiting fertilization, but may at times inhibit implantation of a fertilized egg in the endometrium. However, women should also be informed that the best available evidence indicates that ECPs prevent pregnancy by mechanisms that do not involve interference with post-fertilization events.
ECPs do not cause abortion78 or harm an established pregnancy. Pregnancy begins with implantation according to medical authorities such as the US FDA, the National Institutes of Health79 and the American College of Obstetricians and Gynecologists (ACOG).80
Ulipristal acetate (UPA). One study has demonstrated that UP can delay ovulation.81... Another study found that UPA altered the endometrium, but whether this change would inhibit implantation is unknown.82
p. 122:
Progestin-only emergency contraceptive pills. Early treatment with ECPs containing only the progestin levonorgestrel has been show to impair the ovulatory process and luteal function.83–87
p. 123:
Combined emergency contraceptive pills. Several clinical studies have shown that combined ECPs containing ethinyl estradiol and levonorgestrel can inhibit or delay ovulation.107–110 - ^ RCOG Faculty of Sexual; Reproductive Healthcare; Clinical Effectiveness Unit (January 2012). Clinical guidance: emergency contraception (PDF). London: Royal College of Obstetricians and Gynaecologists. ISSN 1755-103X. Archived from the original (PDF) on 2012-05-26. Retrieved 2012-04-30.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) p.3:How does EC work?
In 2002, a judicial review ruled that pregnancy begins at implantation, not fertilisation.8 The possible mechanisms of action should be explained to the patient as some methods may not be acceptable, depending on individual beliefs about the onset of pregnancy and abortion.
Copper-bearing intrauterine device (Cu-IUD). Copper is toxic to the ovum and sperm and thus the copper-bearing intrauterine device (Cu-IUD) is effective immediately after insertion and works primarily by inhibiting fertilisation.9–11 A systematic review on mechanisms of action of IUDs showed that both pre- and postfertilisation effects contribute to efficacy.11 If fertilisation has already occurred, it is accepted that there is an anti-implantation effect,12,13
Levonorgestrel (LNG). The precise mode of action of levonorgestrel (LNG) is incompletely understood but it is thought to work primarily by inhibition of ovulation.16,17
Ulipristal acetate (UPA). UPA’s primary mechanism of action is thought to be inhibition or delay of ovulation.2 - ^ UNDP/UNFPA/WHO/World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP) (March 25, 2010). "Fact sheet on the safety of levonorgestrel-alone emergency contraceptive pills (LNG ECPs)" (PDF). Geneva: World Health Organization. Archived from the original (PDF) on March 16, 2012.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)Can LNG ECPs cause an abortion?
LNG ECPs do not interrupt an established pregnancy or harm a developing embryo.15 The evidence available to date shows that LNG ECP use does not prevent a fertilized egg from attaching to the uterine lining. The primary mechanism of action is to stop or disrupt ovulation; LNG ECP use may also prevent the sperm and egg from meeting.16 - ^ Speroff, Leon; Darney, Philip D. (2011). "Special uses of oral contraception: emergency contraception, the progestin-only minipill". A clinical guide for contraception (5th ed.). Philadelphia: Lippincott Williams & Wilkins. pp. 153–166. ISBN 978-1-60831-610-6. p. 155:
Emergency postcoital contraception
Levonorgestrel
Mechanism and efficacy - ^ Belluck, Pam (June 6, 2012). "No abortion role seen for morning-after pill". The New York Times. p. A1. Archived from the original on February 27, 2017.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)
Belluck, Pam (June 6, 2012). "Drug's nickname may have aided politicization". The New York Times. p. A14. - ^ International Federation of Gynecology and Obstetrics (FIGO) and International Consortium for Emergency Contraception (ICEC) (April 4, 2011). "Mechanism of action: How do levonorgestrel-only emergency contraceptive pills (LNG ECPs) prevent pregnancy?" (PDF). London: International Federation of Gynecology and Obstetrics. Archived from the original (PDF) on December 29, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)Levonorgestrel-only emergency contraceptive pills:
• Interfere with the process of ovulation;
• May possibly prevent the sperm and the egg from meeting.
Implications of the research:
• Inhibition or delay of ovulation is LNG ECPs principal and possibly only mechanism of action.
• Review of the evidence suggests that LNG-ECs cannot prevent implantation of a fertilized egg. Language on implantation should not be included in LNG ECP product labeling.
• The fact that LNG-ECs have no demonstrated effect on implantation explains why they are not 100% effective in preventing pregnancy, and are less effective the later they are taken. Women should be given a clear message that LNG-ECs are more effective the sooner they are taken.
• LNG ECPs do not interrupt a pregnancy (by any definition of the beginning of pregnancy). However, LNG ECPs can prevent abortions by reducing unwanted pregnancies. - ^ a b c Belluck, Pam (November 26, 2013). "New birth control label counters lawsuit claim; European authorities found that a drug like Plan B One-Step cannot prevent fertilized eggs from implanting in the womb". The New York Times. Archived from the original on March 4, 2014. Retrieved March 5, 2014.
{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)
HRA Pharma (November 2013). "NorLevo 1.5 mg tablet Patient Information Leaflet (PIL)" (PDF). Dublin: Irish Medicines Board. Archived from the original (PDF) on March 5, 2014. Retrieved March 5, 2014.NorLevo works by stopping your ovaries from releasing an egg. It cannot stop a fertilized egg from attaching to the womb.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)
HRA Pharma (November 2013). "NorLevo 1.5 mg tablet Summary of Product Characteristics (SPC)". Dublin: Irish Pharmaceutical Healthcare Association. Retrieved March 5, 2014.
European Medicines Agency (January 24, 2014). "Review of emergency contraceptives started". London: European Medicines Agency. Archived from the original on March 27, 2014. Retrieved March 5, 2014.{{cite news}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Mozzanega, B; Cosmi, E (June 2011). "How do levonorgestrel-only emergency contraceptive pills prevent pregnancy? Some considerations". Gynecological Endocrinology. 27 (6): 439–42. doi:10.3109/09513590.2010.501885. PMID 20670097.
- ^ Leung, VW; Levine, M; Soon, JA (February 2010). "Mechanisms of action of hormonal emergency contraceptives". Pharmacotherapy. 30 (2): 158–68. doi:10.1592/phco.30.2.158. PMID 20099990.
- ^ Glasier, Anna; Cameron, Sharon T.; Blithe, Diana; Scherrer, Bruno; Mathe, Henri; Levy, Delphine; Gainer, Erin; Ulmann, Andre (October 2011). "Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel". Contraception. 84 (4): 363–367. doi:10.1016/j.contraception.2011.02.009. PMID 21920190.
- ^ Trussell, James; Raymond, Elizabeth G.; Cleland, Kelly (February 2014). "Emergency contraception: a last chance to prevent unintended pregnancy" (PDF). Princeton: Office of Population Research at Princeton University, Association of Reproductive Health Professionals. Archived from the original (PDF) on September 23, 2010. Retrieved April 9, 2014.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c Kubíková, D. (2014). [Menopausal symptoms and hormone replacement therapy]. Praktické lékárenství, 10(2), 68-73. https://www.praktickelekarenstvi.cz/pdfs/lek/2014/02/05.pdf
- ^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Retrieved 6 September 2018.
- ^ Marilyn Winterton Edmunds; Maren Stewart Mayhew (16 September 2008). Pharmacology for the Primary Care Provider - E-Book. Elsevier Health Sciences. pp. 584–585. ISBN 0-323-06316-0.
- ^ a b c https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021998lbl.pdf
- ^ HRA Pharma (November 2013). "NorLevo 1.5 mg tablet Summary of Product Characteristics (SPC)". Dublin: Irish Pharmaceutical Healthcare Association. Retrieved April 9, 2014.
- ^ Chen X, Wu X, Zhu H. "Acute urticaria as a side effect of the Mirena® (levonorgestrel-releasing intrauterine system): a case report". BMC Res Notes. 7: 209. doi:10.1186/1756-0500-7-209. PMC 3992142. PMID 24708811.
{{cite journal}}: CS1 maint: multiple names: authors list (link) CS1 maint: unflagged free DOI (link) - ^ Medicines and Healthcare products Regulatory Agency (15 September 2016). "Levonorgestrel-containing emergency hormonal contraception: advice on interactions with hepatic enzyme inducers and contraceptive efficacy". GOV.UK. Archived from the original on 21 January 2017. Retrieved 6 June 2017.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c Nieschlag E (November 2010). "Clinical trials in male hormonal contraception". Contraception. 82 (5): 457–70. doi:10.1016/j.contraception.2010.03.020. PMID 20933120.
- ^ Philibert D, Bouchoux F, Degryse M, Lecaque D, Petit F, Gaillard M (October 1999). "The pharmacological profile of a novel norpregnance progestin (trimegestone)". Gynecol. Endocrinol. 13 (5): 316–26. doi:10.3109/09513599909167574. PMID 10599548.
- ^ Santillán R, Pérez-Palacios G, Reyes M, Damián-Matsumura P, García GA, Grillasca I, Lemus AE (September 2001). "Assessment of the oestrogenic activity of the contraceptive progestin levonorgestrel and its non-phenolic metabolites". Eur. J. Pharmacol. 427 (2): 167–74. doi:10.1016/S0014-2999(01)01263-8. PMID 11557270.
- ^ Cabeza M, Vilchis F, Lemus AE, Díaz de León L, Pérez-Palacios G (September 1995). "Molecular interactions of levonorgestrel and its 5 alpha-reduced derivative with androgen receptors in hamster flanking organs". Steroids. 60 (9): 630–5. doi:10.1016/0039-128X(95)00075-2. PMID 8545853.
- ^ a b c d Darney PD (1995). "The androgenicity of progestins". Am. J. Med. 98 (1A): 104S–110S. doi:10.1016/s0002-9343(99)80067-9. PMID 7825629.
- ^ John V. Knaus; John H. Isaacs (6 December 2012). Office Gynecology: Advanced Management Concepts. Springer Science & Business Media. pp. 151–. ISBN 978-1-4612-4340-3. Archived from the original on 8 September 2017.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ David E. Golan; Armen H. Tashjian; Ehrin J. Armstrong (15 December 2011). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. pp. 516–. ISBN 978-1-60831-270-2. Archived from the original on 8 September 2017.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Committee on the Relationship Between Oral Contraceptives and BreastCancer (1 January 1991). Oral Contraceptives and Breast Cancer. National Academies. pp. 147–. NAP:13774. Archived from the original on 8 September 2017.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d Meriggiola MC, Farley TM, Mbizvo MT (2003). "A review of androgen-progestin regimens for male contraception". J. Androl. 24 (4): 466–83. doi:10.1002/j.1939-4640.2003.tb02695.x. PMID 12826683.
Based on animal studies and clinical studies in women, 19‐norderived progestins are known to be potent in terms of gonadotropin suppression (Couzinet et al, 1996). Among this class of steroidal compounds are norethisterone (NET), norethynodrel, and its dextrorotatory isomer LNG (ie, the biologically active form of this progestin). The progestins of this class are known to be potent suppressors of gonadotropin secretion, and when administered to men these compounds induced a profound suppression of sperm production (Frick, 1973). However, a decrease of libido and sexual potency was also reported, presumably due to the suppression of T production secondary to gonadotropin suppression (Kamischke et al, 2000b). Therefore, like other progestins available thus far, nor‐progestins should not be administered alone for male contraception because their residual androgenic activity is not sufficient to maintain androgen‐dependent physiological functions like libido or sexual potency (Kamischke et al, 2000a).
- ^ a b c J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 887–. ISBN 978-1-4757-2085-3. Archived from the original on 8 September 2017.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b c d e Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 605–. ISBN 978-3-88763-075-1. Archived from the original on 2017-09-08.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Brian K. Alldredge; Robin L. Corelli; Michael E. Ernst (1 February 2012). Koda-Kimble and Young's Applied Therapeutics: The Clinical Use of Drugs. Lippincott Williams & Wilkins. pp. 1072–. ISBN 978-1-60913-713-7. Archived from the original on 8 September 2017.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ J.P. Lavery; J.S. Sanfilippo (6 December 2012). Pediatric and Adolescent Obstetrics and Gynecology. Springer Science & Business Media. pp. 248–. ISBN 978-1-4612-5064-7. Archived from the original on 8 September 2017.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Stefan Offermanns; W. Rosenthal (14 August 2008). Encyclopedia of Molecular Pharmacology. Springer Science & Business Media. pp. 390–. ISBN 978-3-540-38916-3. Archived from the original on 8 September 2017.
{{cite book}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ a b Population Reports: Injectables and implants. Department of Medical and Public Affairs, George Washington University. 1987.
The Population Council also plans to test vaginal rings with two other progestins, ST-1435 and levonorgestrel acetate, alone and combined with ethinyl estradiol (168).
- ^ a b Crabbé P, Archer S, Benagiano G, Diczfalusy E, Djerassi C, Fried J, Higuchi T (1983). "Long-acting contraceptive agents: design of the WHO Chemical Synthesis Programme". Steroids. 41 (3): 243–53. doi:10.1016/0039-128X(83)90095-8. PMID 6658872.
- ^ J. Szejtli; L. Szente (6 December 2012). Proceedings of the Eighth International Symposium on Cyclodextrins: Budapest, Hungary, March 31–April 2, 1996. Springer Science & Business Media. pp. 317–. ISBN 978-94-011-5448-2.
[Norgestrel] was discovered by Hughes et al. (1963).
- ^ a b c Marcus Filshie; John Guillebaud (22 October 2013). Contraception: Science and Practice. Elsevier Science. pp. 12–. ISBN 978-1-4831-6366-6.
Norgestrel, developed by Wyeth and patented in 1964, was the first progestogen to be manufactured by total chemical synthesis. It was subsequently licensed to Schering AG, who separated the racemic mixture into an inactive structural isomer l-norgestrel and the active d-norgestrel -- more usually known as dextronorgestrel and levonorgestrel respectively, because of the optical isomerism that each displays.
- ^ a b c d Lara Marks (2010). Sexual Chemistry: A History of the Contraceptive Pill. Yale University Press. pp. 73, 76. ISBN 978-0-300-16791-7.
In 1964 the pharmaceutical company Wyeth developed norgestrel, the first progestogen to be made from a total chemical synthesis. Subsequently licensed to Schering AG, norgestrel was used to develop levonorgestrel, another active progestogen later used for oral contraception.
- ^ a b W. Gerhard Pohl (2004). Die wissenschaftliche Welt von gestern: die Preisträger des Ignaz L. Lieben-Preises 1865-1937 und des Richard Lieben-Preises 1912-1928 : ein Kapitel österreichischer Wissenschaftsgeschichte in Kurzbiografien. Böhlau Verlag Wien. pp. 150–. ISBN 978-3-205-77303-0.
[The contraceptive EUGYNON is launched in 1966. NEOGYNON follows in 1970.]
- ^ a b Teresa Ortiz-Gómez; María Jesús Santesmases (22 April 2016). Gendered Drugs and Medicine: Historical and Socio-Cultural Perspectives. Taylor & Francis. pp. 175–. ISBN 978-1-317-12981-3.
The 1966 marketing campaign for Schering's second contraceptive, Eugynon, [...] (Schering AG Berline 1966, 11). [...] In 1970 [Schering] had already conducted an opinion poll among doctors in the run up to the marketing campaign for the newly introduced Neogynon. [...]
- ^ Andrea Tone; Elizabeth Siegel Watkins (8 January 2007). Medicating Modern America: Prescription Drugs in History. NYU Press. pp. 118–119. ISBN 978-0-8147-8300-9.
- ^ Horst Albach (1993). Culture and Technical Innovation: A Cross-Cultural Analysis and Policy Recommendations. Walter de Gruyter. p. 952. ISBN 978-3-11-013947-1.
[Since the safety of ovulation inhibition by levonorgestrel was also proven in the clinical studies, the cycle was extremely stable and the side effects were low, the drug was on August 1, 1970 introduced as Neogynon 21 and Neogynon 28 in Germany on the market.] [...] After the OC market had risen sharply in 1968 and 1969, the launch of Neogynon / Schering and Stediril-d / Wyeth in August 1970 gave the market a fresh boost.]
- ^ Apelo, Ruben; Veloso, Irma (1970). "Results of a controlled study employing d-norgestrel and ethinyl estradiol". Contraception. 2 (6): 391–400. doi:10.1016/S0010-7824(70)80002-6. ISSN 0010-7824.
The results obtained in these series clinically confirmed the findings in animal work on the potency of d-norgestrel, i.e., that the biological activity of norgestrel resides largely in the d-enantlomer (5,6).
- ^ Brosens I, Van Assche A, Wijnants P (1971). "Comparative clinical and morphological studies on 2 oral contraceptives which contain DL-norgestrel and D-norgestrel respectively". Geburtshilfe und Frauenheilkunde. 31 (3): 251–257.
Comparison of the effects of Eugynon and Neogynon (.05 mg ethinyl estradiol with .5 mg norgestrel or with .25 mg d-norgestrel, respectively) in 272 women is reported. The 2 preparations were comparable as regards effectiveness (100%), cycle control, and endometrial and cervical morphology. No clinical or biological complications occurred, and the incidence of minor side effects was very small. The d-norgestrel preparation (Neogynon) may be preferable for metabolic reasons because of its lower steroid dose.
- ^ Schneider, W.; Spona, J.; Matt, K. (1974). "Inhibition of ovulation by means of a combined preparation with reduced amounts of active substance". Contraception. 9 (1): 81–92. doi:10.1016/0010-7824(74)90096-1. ISSN 0010-7824.
- ^ Brat T (1974). "Clinical trial with a new low oestrogen combined oral contraceptive". Curr Med Res Opin. 2 (8): 465–70. doi:10.1185/03007997409115244. PMID 4614952.
- ^ a b Scharff, H. J. (1972). Clinical experience with Microlut. Medical News Schering No. 1 1972. p. 2-9. https://www.popline.org/node/488633
- ^ Lambotte R, Werbrouck-Navette J (March 1974). "[Minipill as the new contraceptive method]". Rev Med Liege (in French). 29 (6): 157–9. PMID 4817042.
- ^ a b Kesserü, Esteban; Larrañaga, Alfredo; Parada, Julio (1973). "Postcoital contraception with D-norgestrel". Contraception. 7 (5): 367–379. doi:10.1016/0010-7824(73)90139-X. ISSN 0010-7824.
- ^ Rubio, Boris; Berman, Edel; Larranaga, Alfredo; Guiloff, Enrique (1970). "A new postcoital oral contraceptive". Contraception. 1 (5): 303–314. doi:10.1016/0010-7824(70)90016-8. ISSN 0010-7824.
- ^ Balaji (19 November 2009). Textbook of Oral and Maxillofacial Surgery. Elsevier India. p. 569. ISBN 978-81-312-0300-2.
There are two main methods involving oral emergency pills, commonly misleadingly described as the 'morning-after pill'. The first older method, developed in the mid-1970s, involves two high-dose combined pills containing oestrogen (50 ug ethinyloestradiol) and progesterone (0.25 mg levonorgestrel): the Yuzpe regime (Schering PC4 or Ovran). The second involves progesterone only (0.75 mg levonorgestrel), and therefore, has a lower incidence of side effects, in particular vomiting (6%).
- ^ Yuzpe AA, Thurlow HJ, Ramzy I, Leyshon JI (August 1974). "Post coital contraception--A pilot study". J Reprod Med. 13 (2): 53–8. PMID 4844513.
- ^ Farkas, M. (1978). Post-coital contraception with Postinor, a preparation containing 0.75 mg d-norgestrel. Magyar Nöorvosok Lapja, 41, 474. https://scholar.google.com/scholar?cluster=8190066278284376785
- ^ Paolo Giovanni Artini; Andrea R. Genazzani; Felice Petraglia (11 December 2001). Advances in Gynecological Endocrinology. CRC Press. pp. 96–. ISBN 978-1-84214-071-0.
- ^ Ho PC, Kwan MS (March 1993). "A prospective randomized comparison of levonorgestrel with the Yuzpe regimen in post-coital contraception". Hum. Reprod. 8 (3): 389–92. doi:10.1093/oxfordjournals.humrep.a138057. PMID 8473453.
- ^ King (21 October 2013). Varney's Midwifery. Jones & Bartlett Publishers. pp. 493–. ISBN 978-1-284-02542-2.
- ^ Mira Harrison-Woolrych (28 January 2015). Medicines For Women. Springer. pp. 205–. ISBN 978-3-319-12406-3.
- ^ a b "Archived copy". Archived from the original on 2017-08-03. Retrieved 2017-08-03.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)CS1 maint: archived copy as title (link) - ^ Trussell, James; Cleland, Kelly (2007-04-10). "Emergency Contraceptive Pills Worldwide". Princeton University. Archived from the original on 2007-05-22. Retrieved 2007-05-28.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help) - ^ Jeremy J N Oats; Suzanne Abraham (17 November 2011). Llewellyn-Jones Fundamentals of Obstetrics and Gynaecology E-Book. Elsevier Health Sciences. pp. 247–. ISBN 0-7234-3719-X.
- ^ Karen J. Carlson; Stephanie A. Eisenstat; Terra Diane Ziporyn (2004). The New Harvard Guide to Women's Health. Harvard University Press. pp. 285–. ISBN 978-0-674-01282-0.
- ^ Rankin, Kenrya. "This Policy Gives Native Women Equal Access to Emergency Contraception". Colorlines. Archived from the original on 2015-10-26. Retrieved 2015-10-24.
{{cite web}}: Unknown parameter|deadurl=ignored (|url-status=suggested) (help)
External links
- Levonorgestrel at the Encyclopædia Britannica
- Levonelle manufacturer's product information from Schering
- Monograph for levonorgestrel - Uk Medicines Information
- U.S. National Library of Medicine: Drug Information Portal - Levonorgestrel
