From Wikipedia, the free encyclopedia
Kynurenic acid
Names
IUPAC name
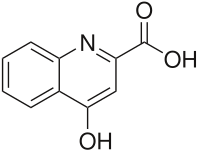
4-hydroxyquinoline-2-carboxylic acid
Other names
Kinurenic acid, kynuronic acid, quinurenic acid, transtorine
Identifiers
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.007.047
KEGG
InChI=1S/C10H7NO3/c12-9-5-8(10(13)14)11-7-4-2-1-3-6(7)9/h1-5H,(H,11,12)(H,13,14)
Y Key: HCZHHEIFKROPDY-UHFFFAOYSA-N
Y InChI=1/C10H7NO3/c12-9-5-8(10(13)14)11-7-4-2-1-3-6(7)9/h1-5H,(H,11,12)(H,13,14)
Key: HCZHHEIFKROPDY-UHFFFAOYAN
O=C\2c1c(cccc1)NC(=C/2)/C(=O)O
Properties
C10 H7 N1 O3
Molar mass
189.168 g/mol
Melting point
282.5°C
Except where otherwise noted, data are given for materials in their
standard state (at 25 °C [77 °F], 100 kPa).
Chemical compound
Kynurenic acid (KYNA ) is a product of the normal metabolism of amino acid L-tryptophan . It has been shown that kynurenic acid possesses neuroactive activity. It acts as an antiexcitotoxic and anticonvulsant , most likely through acting as an antagonist at excitatory amino acid receptors. Because of this activity, it may influence important neurophysiologic and neuropathologic processes. As a result, kynurenic acid has been considered for use in therapy in certain neurobiological disorders. Conversely, increased levels of kynurenic acid have also been linked to certain pathological conditions.
Kynurenic acid was discovered in 1853 by the German chemist Justus von Liebig in dog urine, which it was apparently named after.[1]
It is formed from L-kynurenine in a reaction catalyzed by the enzyme kynurenine—oxoglutarate transaminase .
Mechanism of action KYNA has been found to act on three receptors:
Role in disease High levels of kynurenic acid have been identified in patients suffering from tick-borne encephalitis , schizophrenia and HIV -related illnesses. In all these situations increased levels were associated with confusion and psychotic symptoms. Kynurenic acid acts in the brain as a glycine-site NMDAr antagonist, key in glutamatergic neurotransmission system, which is thought to be involved in the pathophysiology and pathogenesis of schizophrenia.
A kynurenic acid hypothesis of schizophrenia has been proposed in 2007,[5] [6] dopamine hypothesis of schizophrenia with the glutamate hypothesis of the disease.
High levels of kynurenic acid have been identified in human urine in certain metabolic disorders, such as marked pyridoxine deficiency and deficiency/absence of kynureninase .
When researchers decreased the levels of kynurenic acid in the brains of mice, the cognition was shown to improve markedly. [7]
See also References
^ Liebig, J., Uber Kynurensäure, Justus Liebigs Ann. Chem. , 86: 125-126, 1853.
^ Dobelis P., Varnell A., and Cooper, D.C. (2011). "Nicotinic α7 acetylcholine receptor-mediated currents are not modulated by the tryptophan metabolite kynurenic acid in adult hippocampal interneurons" . Nature Precedings . doi :10.1038/npre.2011.6277.1 . CS1 maint: multiple names: authors list (link ) >^ Grilli M, Raiteri L, Patti L, Parodi M, Robino F, Raiteri M, Marchi M (2006). "Modulation of the function of presynaptic α7 and non-α7 nicotinic receptors by the tryptophan metabolites, 5-hydroxyindole and kynurenate in mouse brain" . Br. J. Pharmacol . 149 (6): 724–32. doi :10.1038/sj.bjp.0706914 . PMC 2014664 PMID 17016503 . {{cite journal }}: CS1 maint: multiple names: authors list (link )^ Wang J, Simonavicius N, Wu X, Swaminath G, Reagan J, Tian H, Ling L (2006). "Kynurenic acid as a ligand for orphan G protein-coupled receptor GPR35". J. Biol. Chem . 281 (31): 22021–8. doi :10.1074/jbc.M603503200 . PMID 16754668 . {{cite journal }}: CS1 maint: multiple names: authors list (link ) CS1 maint: unflagged free DOI (link )^ Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G (2007). "The kynurenic acid hypothesis of schizophrenia". Physiol. Behav . 92 (1–2): 203–9. doi :10.1016/j.physbeh.2007.05.025 . PMID 17573079 . {{cite journal }}: CS1 maint: multiple names: authors list (link )^ Erhardt S, Schwieler L, Engberg G (2003). "Kynurenic acid and schizophrenia". Adv. Exp. Med. Biol . 527 : 155–65. PMID 15206728 . {{cite journal }}: CS1 maint: multiple names: authors list (link )^ Robert Schwarcz; Elmer, Greg I; Bergeron, Richard; Albuquerque, Edson X; Guidetti, Paolo; Wu, Hui-Qiu; Schwarcz, Robert (2010). "Reduction of Endogenous Kynurenic Acid Formation Enhances Extracellular Glutamate, Hippocampal Plasticity, and Cognitive Behavior" . Neuropsychopharmacology . 35 (8): 1734–1742. doi :10.1038/npp.2010.39 . PMC 3055476 PMID 20336058 .
External links
mAChRs Tooltip Muscarinic acetylcholine receptors
Agonists Antagonists
3-Quinuclidinyl benzilate 4-DAMP Aclidinium bromide (+formoterol )Abediterol AF-DX 250 AF-DX 384 Ambutonium bromide Anisodamine Anisodine Antihistamines (first-generation) (e.g., brompheniramine , buclizine , captodiame , chlorphenamine (chlorpheniramine) , cinnarizine , clemastine , cyproheptadine , dimenhydrinate , dimetindene , diphenhydramine , doxylamine , meclizine , mequitazine , perlapine , phenindamine , pheniramine , phenyltoloxamine , promethazine , propiomazine , triprolidine )AQ-RA 741 Atropine Atropine methonitrate Atypical antipsychotics (e.g., clozapine , fluperlapine , olanzapine (+fluoxetine ), rilapine , quetiapine , tenilapine , zotepine )Benactyzine Benzatropine (benztropine) Benzilone Benzilylcholine mustard Benzydamine BIBN 99 Biperiden Bornaprine Camylofin CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,368 CAR-302,537 CAR-302,668 Caramiphen Cimetropium bromide Clidinium bromide Cloperastine CS-27349 Cyclobenzaprine Cyclopentolate Darifenacin DAU-5884 Desfesoterodine Dexetimide DIBD Dicycloverine (dicyclomine) Dihexyverine Difemerine Diphemanil metilsulfate Ditran Drofenine EA-3167 EA-3443 EA-3580 EA-3834 Emepronium bromide Etanautine Etybenzatropine (ethybenztropine) Fenpiverinium Fentonium bromide Fesoterodine Flavoxate Glycopyrronium bromide (+beclometasone/formoterol , +indacaterol , +neostigmine )Hexahydrodifenidol Hexahydrosiladifenidol Hexbutinol Hexocyclium Himbacine HL-031,120 Homatropine Imidafenacin Ipratropium bromide (+salbutamol )Isopropamide J-104,129 Hyoscyamine Mamba toxin 3 Mamba toxin 7 Mazaticol Mebeverine Meladrazine Mepenzolate Methantheline Methoctramine Methylatropine Methylhomatropine Methylscopolamine Metixene Muscarinic toxin 7 N-Ethyl-3-piperidyl benzilate N-Methyl-3-piperidyl benzilate Nefopam Octatropine methylbromide (anisotropine methylbromide) Orphenadrine Otenzepad (AF-DX 116) Otilonium bromide Oxapium iodide Oxitropium bromide Oxybutynin Oxyphencyclimine Oxyphenonium bromide PBID PD-102,807 PD-0298029 Penthienate Pethidine pFHHSiD Phenglutarimide Phenyltoloxamine Pipenzolate bromide Piperidolate Pirenzepine Piroheptine Pizotifen Poldine Pridinol Prifinium bromide Procyclidine Profenamine (ethopropazine) Propantheline bromide Propiverine Quinidine 3-Quinuclidinyl thiochromane-4-carboxylate Revefenacin Rociverine RU-47,213 SCH-57,790 SCH-72,788 SCH-217,443 Scopolamine (hyoscine) Scopolamine butylbromide (hyoscine butylbromide) Silahexacyclium Sofpironium bromide Solifenacin SSRIs Tooltip Selective serotonin reuptake inhibitors (e.g., femoxetine , paroxetine )Telenzepine Terodiline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin , mirtazapine )Tiemonium iodide Timepidium bromide Tiotropium bromide Tiquizium bromide Tofenacin Tolterodine Tricyclic antidepressants (e.g., amitriptyline (+perphenazine ), amitriptylinoxide , butriptyline , cidoxepin , clomipramine , desipramine , desmethyldesipramine , dibenzepin , dosulepin (dothiepin) , doxepin , imipramine , lofepramine , nitroxazepine , northiaden (desmethyldosulepin) , nortriptyline , protriptyline , quinupramine , trimipramine )Tridihexethyl Trihexyphenidyl Trimebutine Tripitamine (tripitramine) Tropacine Tropatepine Tropicamide Trospium chloride Typical antipsychotics (e.g., chlorpromazine , chlorprothixene , cyamemazine (cyamepromazine) , loxapine , mesoridazine , thioridazine )Umeclidinium bromide (+vilanterol )WIN-2299 Xanomeline Zamifenacin
Precursors (and prodrugs )
nAChRs Tooltip Nicotinic acetylcholine receptors
Agonists PAMs Tooltip positive allosteric modulators )
5-HIAA 6-Chloronicotine A-84,543 A-366,833 A-582,941 A-867,744 ABT-202 ABT-418 ABT-560 ABT-894 Acetylcholine Altinicline Anabasine Anatabine Anatoxin-a AR-R17779 Bephenium hydroxynaphthoate Butinoline Butyrylcholine Carbachol Choline Cotinine Cytisine Decamethonium Desformylflustrabromine Dianicline Dimethylphenylpiperazinium Epibatidine Epiboxidine Ethanol (alcohol) Ethoxysebacylcholine EVP-4473 EVP-6124 Galantamine GTS-21 Ispronicline Ivermectin JNJ-39393406 Levamisole Lobeline MEM-63,908 (RG-3487) Morantel Nicotine (tobacco )NS-1738 PHA-543,613 PHA-709,829 PNU-120,596 PNU-282,987 Pozanicline Pyrantel Rivanicline RJR-2429 Sazetidine A SB-206553 Sebacylcholine SIB-1508Y SIB-1553A SSR-180,711 Suberyldicholine Suxamethonium (succinylcholine) Suxethonium (succinyldicholine) TC-1698 TC-1734 TC-1827 TC-2216 TC-5214 TC-5619 TC-6683 Tebanicline Tribendimidine Tropisetron UB-165 Varenicline WAY-317,538 XY-4083 Antagonists NAMs Tooltip negative allosteric modulators )
Precursors (and prodrugs )
AMPAR Tooltip α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor KAR Tooltip Kainate receptor NMDAR Tooltip N-Methyl-D-aspartate receptor
Group I
mGluR1 Tooltip Metabotropic glutamate receptor 1 mGluR5 Tooltip Metabotropic glutamate receptor 5
Group II
mGluR2 Tooltip Metabotropic glutamate receptor 2 mGluR3 Tooltip Metabotropic glutamate receptor 3
Group III
mGluR4 Tooltip Metabotropic glutamate receptor 4 mGluR6 Tooltip Metabotropic glutamate receptor 6 mGluR7 Tooltip Metabotropic glutamate receptor 7 mGluR8 Tooltip Metabotropic glutamate receptor 8