BCG vaccine
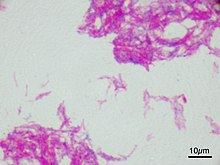 Microscopic image of the Calmette–Guérin bacillus, Ziehl–Neelsen stain, magnification: 1,000nn | |
| Vaccine description | |
|---|---|
| Target | Mycobacterium tuberculosis |
| Vaccine type | Attenuated |
| Clinical data | |
| Trade names | BCG Vaccine, BCG Vaccine AJV |
| AHFS/Drugs.com | Professional Drug Facts |
| License data |
|
| Routes of administration | Percutaneous, intravesical, intradermal |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
Bacillus Calmette–Guérin (BCG) vaccine is a vaccine primarily used against tuberculosis (TB).[6] It is named after its inventors Albert Calmette and Camille Guérin.[7][8] In countries where tuberculosis or leprosy is common, one dose is recommended in healthy babies as soon after birth as possible.[6] In areas where tuberculosis is not common, only children at high risk are typically immunized, while suspected cases of tuberculosis are individually tested for and treated.[6] Adults who do not have tuberculosis and have not been previously immunized but are frequently exposed may be immunized as well.[6] BCG also has some effectiveness against Buruli ulcer infection and other nontuberculous mycobacteria infections.[6] Additionally it is sometimes used as part of the treatment of bladder cancer.[9][10]
Rates of protection against tuberculosis infection vary widely and protection lasts up to twenty years.[6] Among children it prevents about 20% from getting infected and among those who do get infected it protects half from developing disease.[11] The vaccine is given by injection into the skin.[6] There is no evidence that additional doses are beneficial.[6]
Serious side effects are rare. Often there is redness, swelling, and mild pain at the site of injection.[6] A small ulcer may also form with some scarring after healing.[6] Side effects are more common and potentially more severe in those with poor immune function.[6] It is not safe for use during pregnancy.[6] The vaccine was originally developed from Mycobacterium bovis, which is commonly found in cows.[6] While it has been weakened, it is still live.[6]
The BCG vaccine was first used medically in 1921.[6] It is on the World Health Organization's List of Essential Medicines.[12] As of 2004[update], the vaccine is given to about 100 million children per year globally.[13]
Medical uses
Tuberculosis
The main use of BCG is for vaccination against tuberculosis. BCG vaccine can be administered after birth intradermally.[4] BCG vaccination can cause a false positive Mantoux test, although a very high-grade reading is usually due to active disease.
The most controversial aspect of BCG is the variable efficacy found in different clinical trials, which appears to depend on geography. Trials conducted in the UK have consistently shown a protective effect of 60 to 80%, but those conducted elsewhere have shown no protective effect, and efficacy appears to fall the closer one gets to the equator.[14][15]
A 1994 systematic review found that BCG reduces the risk of getting tuberculosis by about 50%.[14] There are differences in effectiveness, depending on region, due to factors such as genetic differences in the populations, changes in environment, exposure to other bacterial infections, and conditions in the lab where the vaccine is grown, including genetic differences between the strains being cultured and the choice of growth medium.[16][15]
A systematic review and meta analysis conducted in 2014, demonstrated that the BCG vaccine reduced infections by 19–27% and reduced progression to active tuberculosis by 71%.[11] The studies included in this review were limited to those that used interferon gamma release assay.
The duration of protection of BCG is not clearly known. In those studies showing a protective effect, the data are inconsistent. The MRC study showed protection waned to 59% after 15 years and to zero after 20 years; however, a study looking at Native Americans immunized in the 1930s found evidence of protection even 60 years after immunization, with only a slight waning in efficacy.[17]
BCG seems to have its greatest effect in preventing miliary tuberculosis or tuberculosis meningitis, so it is still extensively used even in countries where efficacy against pulmonary tuberculosis is negligible.[18]
The 100th anniversary of BCG was in 2021. It remains the only vaccine licensed against tuberculosis, which is an ongoing pandemic. Tuberculosis elimination is a goal of the World Health Organization, although the development of new vaccines with greater efficacy against adult pulmonary tuberculosis may be needed to make substantial progress.[19]
Efficacy
A number of possible reasons for the variable efficacy of BCG in different countries have been proposed. None have been proven, some have been disproved, and none can explain the lack of efficacy in both low-tuberculosis burden countries (US) and high-tuberculosis burden countries (India). The reasons for variable efficacy have been discussed at length in a World Health Organization (WHO) document on BCG.[20]
- Genetic variation in BCG strains: Genetic variation in the BCG strains used may explain the variable efficacy reported in different trials.[21]
- Genetic variation in populations: Differences in genetic make-up of different populations may explain the difference in efficacy. The Birmingham BCG trial was published in 1988. The trial, based in Birmingham, United Kingdom, examined children born to families who originated from the Indian Subcontinent (where vaccine efficacy had previously been shown to be zero). The trial showed a 64% protective effect, which is very similar to the figure derived from other UK trials, thus arguing against the genetic variation hypothesis.[22]
- Interference by nontuberculous mycobacteria: Exposure to environmental mycobacteria (especially Mycobacterium avium, Mycobacterium marinum and Mycobacterium intracellulare) results in a nonspecific immune response against mycobacteria. Administering BCG to someone who already has a nonspecific immune response against mycobacteria does not augment the response already there. BCG will, therefore, appear not to be efficacious because that person already has a level of immunity and BCG is not adding to that immunity. This effect is called masking because the effect of BCG is masked by environmental mycobacteria. Clinical evidence for this effect was found in a series of studies performed in parallel in adolescent school children in the UK and Malawi.[23] In this study, the UK school children had a low baseline cellular immunity to mycobacteria which was increased by BCG; in contrast, the Malawi school children had a high baseline cellular immunity to mycobacteria and this was not significantly increased by BCG. Whether this natural immune response is protective is not known.[24] An alternative explanation is suggested by mouse studies; immunity against mycobacteria stops BCG from replicating and so stops it from producing an immune response. This is called the block hypothesis.[25]
- Interference by concurrent parasitic infection: In another hypothesis, simultaneous infection with parasites changes the immune response to BCG, making it less effective. As Th1 response is required for an effective immune response to tuberculous infection, concurrent infection with various parasites produces a simultaneous Th2 response, which blunts the effect of BCG.[26]
Mycobacteria
BCG has protective effects against some non-tuberculosis mycobacteria.
- Leprosy: BCG has a protective effect against leprosy in the range of 20 to 80%.[6]
- Buruli ulcer: BCG may protect against or delay the onset of Buruli ulcer.[6][27]
Cancer

BCG has been one of the most successful immunotherapies.[28] BCG vaccine has been the "standard of care for patients with bladder cancer (NMIBC)" since 1977.[28][29] By 2014 there were more than eight different considered biosimilar agents or strains used for the treatment of non–muscle-invasive bladder cancer (NMIBC).[28] [29]
- A number of cancer vaccines use BCG as an additive to provide an initial stimulation of the person's immune systems.
- BCG is used in the treatment of superficial forms of bladder cancer. Since the late 1970s, evidence has become available that instillation of BCG into the bladder is an effective form of immunotherapy in this disease.[30] While the mechanism is unclear, it appears a local immune reaction is mounted against the tumor. Immunotherapy with BCG prevents recurrence in up to 67% of cases of superficial bladder cancer.
- BCG has been evaluated in a number of studies as a therapy for colorectal cancer.[31] The US biotech company Vaccinogen is evaluating BCG as an adjuvant to autologous tumour cells used as a cancer vaccine in stage II colon cancer.
Method of administration
A tuberculin skin test is usually carried out before administering BCG. A reactive tuberculin skin test is a contraindication to BCG due to the risk of severe local inflammation and scarring; it does not indicate any immunity. BCG is also contraindicated in certain people who have IL-12 receptor pathway defects.
BCG is given as a single intradermal injection at the insertion of the deltoid. If BCG is accidentally given subcutaneously, then a local abscess may form (a "BCG-oma") that can sometimes ulcerate, and may require treatment with antibiotics immediately, otherwise without treatment it could spread the infection causing severe damage to vital organs. An abscess is not always associated with incorrect administration, and it is one of the more common complications that can occur with the vaccination. Numerous medical studies on treatment of these abscesses with antibiotics have been done with varying results, but the consensus is once pus is aspirated and analysed, provided no unusual bacilli are present, the abscess will generally heal on its own in a matter of weeks.[32]
The characteristic raised scar that BCG immunization leaves is often used as proof of prior immunization. This scar must be distinguished from that of smallpox vaccination, which it may resemble.
When given for bladder cancer, the vaccine is not injected through the skin, but is instilled into the bladder through the urethra using a soft catheter.[33]
Adverse effects
BCG immunization generally causes some pain and scarring at the site of injection. The main adverse effects are keloids—large, raised scars. The insertion to the deltoid muscle is most frequently used because the local complication rate is smallest when that site is used. Nonetheless, the buttock is an alternative site of administration because it provides better cosmetic outcomes.
BCG vaccine should be given intradermally. If given subcutaneously, it may induce local infection and spread to the regional lymph nodes, causing either suppurative (production of pus) and non-suppurative lymphadenitis. Conservative management is usually adequate for non-suppurative lymphadenitis. If suppuration occurs, it may need needle aspiration. For non-resolving suppuration, surgical excision may be required. Evidence for the treatment of these complications is scarce.[34]
Uncommonly, breast and gluteal abscesses can occur due to haematogenous (carried by the blood) and lymphangiomatous spread. Regional bone infection (BCG osteomyelitis or osteitis) and disseminated BCG infection are rare complications of BCG vaccination, but potentially life-threatening. Systemic anti-tuberculous therapy may be helpful in severe complications.[35]
When BCG is used for bladder cancer, approximately 2.9% of treated patients discontinue immunotherapy due to a genitourinary or systemic BCG-related infection,[36] however while symptomatic bladder BCG infection is frequent, the involvement of other organs is very uncommon.[37] When systemic involvement occurs, liver and lungs are the first organs to be affected (1 week [median] after the last BCG instillation).[38]
If BCG is accidentally given to an immuno-compromised patient (e.g., an infant with SCID), it can cause disseminated or life-threatening infection. The documented incidence of this happening is less than one per million immunizations given.[39] In 2007, The World Health Organization (WHO) stopped recommending BCG for infants with HIV, even if there is a high risk of exposure to tuberculosis,[40] because of the risk of disseminated BCG infection (which is approximately 400 per 100,000 in that higher risk context).[41][42]
Usage
The age of the person and the frequency with which BCG is given has always varied from country to country. The World Health Organization (WHO) currently recommends childhood BCG for all countries with a high incidence of tuberculosis and/or high leprosy burden.[6] This is a partial list of historic and current BCG practice around the globe. A complete atlas of past and present practice has been generated.[43]
Americas
- Brazil: Brazil introduced universal BCG immunization in 1967–1968, and the practice continues until now. According to Brazilian law, BCG is given again to professionals of the health sector and to people close to patients with tuberculosis or leprosy.
- Canada: Indigenous Canadian communities currently receive the BCG vaccine,[44] and in the province of Quebec the vaccine was offered to children until the mid-70s.[45]
- Most countries in Central and South America have universal BCG immunizations.[46]
- United States: The US has never used mass immunization of BCG due to the rarity of tuberculosis in the US, relying instead on the detection and treatment of latent tuberculosis.
Europe
| Country | Mandatory now | Mandatory in the past | Years vaccine was mandatory |
|---|---|---|---|
| Austria[43] | 1952–1990 | ||
| Belgium[43][47] | N/A | ||
| Bosnia and Herzegovina[43] | 1950–present | ||
| Bulgaria[43][48] | 1951–present | ||
| Croatia[43] | 1948–present | ||
| Czech Republic[43][49] | 1953–2010 | ||
| Denmark[43][50] | 1946–1986 | ||
| Estonia[43] | ?–present | ||
| Finland[43][51] | 1941–2006 | ||
| France[43][52][53][54] | 1950–2007 | ||
| Germany[43][55] | 1961–1998 (East Germany began 1951) | ||
| Greece[43][56][57] | ?–2016 | ||
| Hungary[43][58] | 1953–present | ||
| Ireland[59] | 1950s–2015 | ||
| Italy[43] | N/A | ||
| Latvia[43] | 1940s–present | ||
| Lithuania[43] | ?–present | ||
| Moldova[43] | ?–present | ||
| North Macedonia[43] | 1950–present | ||
| Norway[43][60] | 1947–1995, voluntary 1995-2009 | ||
| Poland[43] | 1955–present | ||
| Portugal[43][61] | ?–2016 | ||
| Romania[43][62] | 1928–present | ||
| Russia[43] | 1962–present | ||
| Serbia[43] | ?–present | ||
| Slovakia[43][6] | 1953–2012 | ||
| Slovenia[43] | 1947–2005 | ||
| Spain[43][63] | 1965–1981 | ||
| Sweden[43][64] | 1940–1975 | ||
| Switzerland[43] | 1960s–1987 | ||
| Turkey[43][65] | 1952–present | ||
| Ukraine[43][66] | ?–present | ||
| United Kingdom[43][67][68][69] | N/A |
Asia
- China: Introduced in 1930s. Increasingly widespread after 1949. Majority inoculated by 1979.[70]
- South Korea, Singapore, Taiwan and Malaysia. In these countries, BCG was given at birth and again at age 12. In Malaysia and Singapore from 2001, this policy was changed to once only at birth. South Korea stopped re-vaccination in 2008.
- Hong Kong: BCG is given to all newborns.[71]
- Japan: In Japan, BCG was introduced in 1951, given typically at age 6. From 2005 it is administered between five and eight months after birth, and no later than a child's first birthday. BCG was administered no later than the fourth birthday until 2005, and no later than six months from birth from 2005 to 2012; the schedule was changed in 2012 due to reports of osteitis side effects from vaccinations at 3–4 months. Some municipalities recommend an earlier immunization schedule.[72]
- Thailand: In Thailand, the BCG vaccine is given routinely at birth.[73]
- India and Pakistan: India and Pakistan introduced BCG mass immunization in 1948, the first countries outside Europe to do so.[74] In 2015, millions of infants were denied BCG vaccine in Pakistan for the first time due to shortage globally.[75]
- Mongolia: All newborns are vaccinated with BCG. Previously, the vaccine was also given at ages 8 and 15, although this is no longer common practice.[citation needed]
- Philippines: BCG vaccine started in the Philippines in 1979 with the Expanded Program on Immunization.
- Sri Lanka: In Sri Lanka, The National Policy of Sri Lanka is to give BCG vaccination to all newborn babies immediately after birth. BCG vaccination is carried out under the Expanded Programme of Immunisation (EPI).[76]
Middle East
- Israel: BCG was given to all newborns between 1955 and 1982.[77]
- Iran: Iran's vaccination policy implemented in 1984. Vaccination with the Bacillus Calmette–Guerin (BCG) is among the most important tuberculosis control strategies in Iran [2]. According to Iranian neonatal vaccination policy, BCG has been given as a single dose at children aged <6 years, shortly after birth or at first contact with the health services.[78][citation needed]
Africa
- South Africa: In South Africa, the BCG Vaccine is given routinely at birth, to all newborns, except those with clinically symptomatic AIDS. The vaccination site in the right shoulder.[79]
- Morocco: In Morocco, the BCG was introduced in 1949. The current policy is BCG Vaccination at birth, to all newborns[80]
South Pacific
- Australia: BCG vaccination was used between 1950s and mid 1980. BCG is not part of routine vaccination since mid 1980.[81]
- New Zealand: BCG Immunisation was first introduced for 13 yr olds in 1948. Vaccination was phased out 1963–1990.[43]
Manufacture
BCG is prepared from a strain of the attenuated (virulence-reduced) live bovine tuberculosis bacillus, Mycobacterium bovis, that has lost its ability to cause disease in humans. Because the living bacilli evolve to make the best use of available nutrients, they become less well-adapted to human blood and can no longer induce disease when introduced into a human host. Still, they are similar enough to their wild ancestors to provide some degree of immunity against human tuberculosis. The BCG vaccine can be anywhere from 0 to 80% effective in preventing tuberculosis for a duration of 15 years; however, its protective effect appears to vary according to geography and the lab in which the vaccine strain was grown.[16]
A number of different companies make BCG, sometimes using different genetic strains of the bacterium. This may result in different product characteristics. OncoTICE, used for bladder instillation for bladder cancer, was developed by Organon Laboratories (since acquired by Schering-Plough, and in turn acquired by Merck & Co.). A similar application is the product of Onko BCG[82] of the Polish company Biomed-Lublin, which owns the Brazilian substrain M. bovis BCG Moreau which is less reactogenic than vaccines including other BCG strains. Pacis BCG, made from the Montréal (Institut Armand-Frappier) strain,[83] was first marketed by Urocor in about 2002. Urocor was since acquired by Dianon Systems. Evans Vaccines (a subsidiary of PowderJect Pharmaceuticals). Statens Serum Institut in Denmark markets BCG vaccine prepared using Danish strain 1331.[84] Japan BCG Laboratory markets its vaccine, based on the Tokyo 172 substrain of Pasteur BCG, in 50 countries worldwide.
According to a UNICEF report published in December 2015, on BCG vaccine supply security, global demand increased in 2015 from 123 to 152.2 million doses. To improve security and to [diversify] sources of affordable and flexible supply," UNICEF awarded seven new manufacturers contracts to produce BCG. Along with supply availability from existing manufacturers, and a "new WHO prequalified vaccine" the total supply will be "sufficient to meet both suppressed 2015 demand carried over to 2016, as well as total forecast demand through 2016–2018."[85]
Supply shortage
In 2011, the Sanofi Pasteur plant flooded, causing problems with mold.[86] The facility, located in Toronto, Ontario, Canada, produced BCG vaccine products made with substrain Connaught such as a tuberculosis vaccine and ImmuCYST, a BCG immunotherapeutic and bladder cancer drug.[87] By April 2012 the FDA had found dozens of documented problems with sterility at the plant including mold, nesting birds and rusted electrical conduits.[86] The resulting closure of the plant for over two years caused shortages of bladder cancer and tuberculosis vaccines.[88] On 29 October 2014 Health Canada gave the permission for Sanofi to resume production of BCG.[89] A 2018 analysis of the global supply concluded that the supplies are adequate to meet forecast BCG vaccine demand, but that risks of shortages remain, mainly due to dependence of 75 percent of WHO pre-qualified supply on just two suppliers.[90]
Preparation
A weakened strain of bovine tuberculosis bacillus, Mycobacterium bovis is specially subcultured in a culture medium, usually Middlebrook 7H9.[91]
Dried
Some BCG vaccines are freeze dried and become fine powder. Sometimes the powder is sealed with vacuum in a glass ampoule. Such a glass ampoule has to be opened slowly to prevent the airflow from blowing out the powder. Then the powder has to be diluted with saline water before injecting.[92]
History

The history of BCG is tied to that of smallpox. By 1865 Jean Antoine Villemin had demonstrated that rabbits could be infected with tuberculosis from humans;[93] by 1868 he had found that rabbits could be infected with tuberculosis from cows, and that rabbits could be infected with tuberculosis from other rabbits.[94] Thus, he concluded that tuberculosis was transmitted via some unidentified microorganism (or "virus", as he called it).[95][96] In 1882 Robert Koch regarded human and bovine tuberculosis as identical.[97] But in 1895, Theobald Smith presented differences between human and bovine tuberculosis, which he reported to Koch.[98][99] By 1901 Koch distinguished Mycobacterium bovis from Mycobacterium tuberculosis.[100] Following the success of vaccination in preventing smallpox, established during the 18th century, scientists thought to find a corollary in tuberculosis by drawing a parallel between bovine tuberculosis and cowpox: it was hypothesized that infection with bovine tuberculosis might protect against infection with human tuberculosis. In the late 19th century, clinical trials using M. bovis were conducted in Italy with disastrous results, because M. bovis was found to be just as virulent as M. tuberculosis.
Albert Calmette, a French physician and bacteriologist, and his assistant and later colleague, Camille Guérin, a veterinarian, were working at the Institut Pasteur de Lille (Lille, France) in 1908. Their work included subculturing virulent strains of the tuberculosis bacillus and testing different culture media. They noted a glycerin-bile-potato mixture grew bacilli that seemed less virulent, and changed the course of their research to see if repeated subculturing would produce a strain that was attenuated enough to be considered for use as a vaccine. The BCG strain was isolated after subculturing 239 times during 13 years from virulent strain on glycerine potato medium. The research continued throughout World War I until 1919, when the now avirulent bacilli were unable to cause tuberculosis disease in research animals. Calmette and Guerin transferred to the Paris Pasteur Institute in 1919. The BCG vaccine was first used in humans in 1921.[101]
Public acceptance was slow, and one disaster, in particular, did much to harm public acceptance of the vaccine. In the summer of 1930 in Lübeck, 240 infants were vaccinated in the first 10 days of life; almost all developed tuberculosis and 72 infants died. It was subsequently discovered that the BCG administered there had been contaminated with a virulent strain that was being stored in the same incubator, which led to legal action against the manufacturers of the vaccine.[102]
Dr. R. G. Ferguson, working at the Fort Qu'Appelle Sanatorium in Saskatchewan, was among the pioneers in developing the practice of vaccination against tuberculosis. In Canada, more than 600 children from residential schools were used as involuntary participants in BCG vaccine trials between 1933 and 1945.[103] In 1928, BCG was adopted by the Health Committee of the League of Nations (predecessor to the World Health Organization (WHO)). Because of opposition, however, it only became widely used after World War II. From 1945 to 1948, relief organizations (International Tuberculosis Campaign or Joint Enterprises) vaccinated over eight million babies in eastern Europe and prevented the predicted typical increase of tuberculosis after a major war.
BCG is very efficacious against tuberculous meningitis in the pediatric age group, but its efficacy against pulmonary tuberculosis appears to be variable. As of 2006, only a few countries do not use BCG for routine vaccination. Two countries that have never used it routinely are the United States and the Netherlands (in both countries, it is felt that having a reliable Mantoux test and therefore being able to accurately detect active disease is more beneficial to society than vaccinating against a condition that is now relatively rare there).[104][105]
Other names include "Vaccin Bilié de Calmette et Guérin vaccine" and "Bacille de Calmette et Guérin vaccine".
Research
Tentative evidence exists for a beneficial non-specific effect of BCG vaccination on overall mortality in low income countries, or for its reducing other health problems including sepsis and respiratory infections when given early,[106] with greater benefit the earlier it is used.[107]
In rhesus macaques, BCG shows improved rates of protection when given intravenously.[108][109] Some risks must be evaluated before it can be translated to human.[110]
Type 1 diabetes
As of 2017[update], BCG vaccine is in the early stages of being studied in type 1 diabetes.[111][112]
COVID-19
Use of the BCG vaccine may provide protection against COVID‑19.[113][114][115] However, epidemiologic observations in this respect are ambiguous.[116] The WHO does not recommend its use for prevention as of 12 January 2021[update].[117]
As of January 2021[update], twenty BCG trials are in various clinical stages.[118]
References
- ^ "Summary for ARTG Entry:53569 BCG VACCINE Mycobacterium bovis (Mycobacterium bovis (Bacillus Calmette and Guerin (BCG) strain) (BCG) strain) 1.5mg powder for injection multidose vial with diluent vial". Therapeutic Goods Administration (TGA).
- ^ "BCG Vaccine AJV - Summary of Product Characteristics (SmPC)". (emc). Retrieved 21 September 2020.
- ^ "BCG Vaccine- bacillus Calmette–Guerin substrain TICE live antigen injection, powder, lyophilized, for solution". DailyMed. 3 September 2020. Retrieved 21 September 2020.
- ^ a b "FREEZE - DRIED GLUTAMATE BCG VACCINE (JAPAN) FOR INTRADERMAL USE" (PDF). World Health Organization. Retrieved 18 November 2021.
- ^ Yamamoto S, Yamamoto T (November 2007). "Historical review of BCG vaccine in Japan". Jpn J Infect Dis. 60 (6): 331–6. PMID 18032829.
- ^ a b c d e f g h i j k l m n o p q r s "BCG vaccines: WHO position paper – February 2018". Weekly Epidemiological Record. 93 (8): 73–96. February 2018. hdl:10665/260307. PMID 29474026.
{{cite journal}}: Unknown parameter|lay-url=ignored (help) - ^ Hawgood, Barbara J. (August 2007). "Albert Calmette (1863-1933) and Camille Guérin (1872-1961): the C and G of BCG vaccine". Journal of Medical Biography. 15 (3): 139–146. doi:10.1258/j.jmb.2007.06-15. ISSN 0967-7720. PMID 17641786. S2CID 41880560.
- ^ LUCA, Simona; MIHAESCU, Traian (March 2013). "History of BCG Vaccine". Mædica. 8 (1): 53–58. ISSN 1841-9038. PMC 3749764. PMID 24023600.
- ^ Fuge O, Vasdev N, Allchorne P, Green JS (May 2015). "Immunotherapy for bladder cancer". Research and Reports in Urology. 7: 65–79. doi:10.2147/RRU.S63447. PMC 4427258. PMID 26000263.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Houghton BB, Chalasani V, Hayne D, Grimison P, Brown CS, Patel MI, et al. (May 2013). "Intravesical chemotherapy plus bacille Calmette–Guérin in non-muscle invasive bladder cancer: a systematic review with meta-analysis". BJU International. 111 (6): 977–83. doi:10.1111/j.1464-410X.2012.11390.x. PMID 23253618. S2CID 24961108.
- ^ a b Roy A, Eisenhut M, Harris RJ, Rodrigues LC, Sridhar S, Habermann S, et al. (August 2014). "Effect of BCG vaccination against Mycobacterium tuberculosis infection in children: systematic review and meta-analysis". BMJ. 349: g4643. doi:10.1136/bmj.g4643. PMC 4122754. PMID 25097193.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ "BCG Vaccine: WHO position paper" (PDF). Weekly Epidemiological Record. 4 (79): 25–40. 23 January 2004. Archived (PDF) from the original on 21 September 2015.
- ^ a b Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F (March 1994). "Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature". JAMA. 271 (9): 698–702. doi:10.1001/jama.1994.03510330076038. PMID 8309034.
- ^ a b Fine PE (November 1995). "Variation in protection by BCG: implications of and for heterologous immunity". Lancet. 346 (8986): 1339–45. doi:10.1016/S0140-6736(95)92348-9. PMID 7475776. S2CID 44737409.
- ^ a b Venkataswamy MM, Goldberg MF, Baena A, Chan J, Jacobs WR, Porcelli SA (February 2012). "In vitro culture medium influences the vaccine efficacy of Mycobacterium bovis BCG". Vaccine. 30 (6): 1038–49. doi:10.1016/j.vaccine.2011.12.044. PMC 3269512. PMID 22189700.
- ^ Aronson NE, Santosham M, Comstock GW, Howard RS, Moulton LH, Rhoades ER, Harrison LH (May 2004). "Long-term efficacy of BCG vaccine in American Indians and Alaska Natives: A 60-year follow-up study". JAMA. 291 (17): 2086–91. doi:10.1001/jama.291.17.2086. PMID 15126436.
- ^ Rodrigues LC, Diwan VK, Wheeler JG (December 1993). "Protective effect of BCG against tuberculous meningitis and miliary tuberculosis: a meta-analysis". International Journal of Epidemiology. 22 (6): 1154–8. doi:10.1093/ije/22.6.1154. PMID 8144299.
- ^ Kupz, Andreas. "Tuberculosis kills as many people each year as COVID-19. It's time we found a better vaccine". The Conversation.
- ^ Fine PE, Carneiro IA, Milstein JB, Clements CJ (1999). "Chapter 8: Reasons for variable efficacy" (PDF). Issues relating to the use of BCG in immunization programmes: a discussion document (Report). Geneva, Switzerland: World Health Organization. hdl:10665/66120. WHO/V&B/99.23.
- ^ Brosch R, Gordon SV, Garnier T, Eiglmeier K, Frigui W, Valenti P, et al. (March 2007). "Genome plasticity of BCG and impact on vaccine efficacy". Proceedings of the National Academy of Sciences of the United States of America. 104 (13): 5596–601. Bibcode:2007PNAS..104.5596B. doi:10.1073/pnas.0700869104. PMC 1838518. PMID 17372194.
- ^ Packe GE, Innes JA (March 1988). "Protective effect of BCG vaccination in infant Asians: a case-control study". Archives of Disease in Childhood. 63 (3): 277–81. doi:10.1136/adc.63.3.277. PMC 1778792. PMID 3258499.
- ^ Black GF, Weir RE, Floyd S, Bliss L, Warndorff DK, Crampin AC, et al. (April 2002). "BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies". Lancet. 359 (9315): 1393–401. doi:10.1016/S0140-6736(02)08353-8. PMID 11978337. S2CID 24334622.
- ^ Palmer CE, Long MW (October 1966). "Effects of infection with atypical mycobacteria on BCG vaccination and tuberculosis". The American Review of Respiratory Disease. 94 (4): 553–68. doi:10.1164/arrd.1966.94.4.553 (inactive 31 October 2021). PMID 5924215.
{{cite journal}}: CS1 maint: DOI inactive as of October 2021 (link) - ^ Brandt L, Feino Cunha J, Weinreich Olsen A, Chilima B, Hirsch P, Appelberg R, Andersen P (February 2002). "Failure of the Mycobacterium bovis BCG vaccine: some species of environmental mycobacteria block multiplication of BCG and induction of protective immunity to tuberculosis". Infection and Immunity. 70 (2): 672–8. doi:10.1128/IAI.70.2.672-678.2002. PMC 127715. PMID 11796598.
- ^ Rook GA, Dheda K, Zumla A (March 2005). "Do successful tuberculosis vaccines need to be immunoregulatory rather than merely Th1-boosting?" (PDF). Vaccine. 23 (17–18): 2115–20. doi:10.1016/j.vaccine.2005.01.069. PMID 15755581. Archived (PDF) from the original on 22 September 2017.
- ^ Tanghe A, Content J, Van Vooren JP, Portaels F, Huygen K (September 2001). "Protective efficacy of a DNA vaccine encoding antigen 85A from Mycobacterium bovis BCG against Buruli ulcer". Infection and Immunity. 69 (9): 5403–11. doi:10.1128/IAI.69.9.5403-5411.2001. PMC 98650. PMID 11500410.
- ^ a b c Rentsch CA, Birkhäuser FD, Biot C, Gsponer JR, Bisiaux A, Wetterauer C, et al. (October 2014). "Bacillus Calmette–Guérin strain differences have an impact on clinical outcome in bladder cancer immunotherapy". European Urology. 66 (4): 677–88. doi:10.1016/j.eururo.2014.02.061. PMID 24674149.
- ^ a b Brandau S, Suttmann H (July 2007). "Thirty years of BCG immunotherapy for non-muscle invasive bladder cancer: a success story with room for improvement". Biomedicine & Pharmacotherapy. 61 (6): 299–305. doi:10.1016/j.biopha.2007.05.004. PMID 17604943.
- ^ Lamm DL, Blumenstein BA, Crawford ED, Montie JE, Scardino P, Grossman HB, et al. (October 1991). "A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette–Guérin for transitional-cell carcinoma of the bladder". The New England Journal of Medicine. 325 (17): 1205–9. doi:10.1056/NEJM199110243251703. PMID 1922207.
- ^ Mosolits S, Nilsson B, Mellstedt H (June 2005). "Towards therapeutic vaccines for colorectal carcinoma: a review of clinical trials". Expert Review of Vaccines. 4 (3): 329–50. doi:10.1586/14760584.4.3.329. PMID 16026248. S2CID 35749038.
- ^ Nick Makwana and Andrew Riordan (2004), "Is medical therapy effective in the treatment of BCG abscesses?", Birmingham Heartlands Hospital "BestBets: Is medical therapy effective in the treatment of BCG abscesses?". Archived from the original on 16 February 2007. Retrieved 1 April 2007.
- ^ American Cancer Society. Intravesical Therapy for Bladder Cancer.
- ^ Cuello-García CA, Pérez-Gaxiola G, Jiménez Gutiérrez C (January 2013). "Treating BCG-induced disease in children". The Cochrane Database of Systematic Reviews. 1 (1): CD008300. doi:10.1002/14651858.CD008300.pub2. PMC 6532703. PMID 23440826.
- ^ Govindarajan KK, Chai FY (April 2011). "BCG Adenitis-Need for Increased Awareness". The Malaysian Journal of Medical Sciences. 18 (2): 66–9. PMC 3216207. PMID 22135589. Malaysian Journal of Medical Sciences Archived 26 March 2012 at the Wayback Machine
- ^ Nummi, Antti; Järvinen, Riikka; Sairanen, Jukka; Huotari, Kaisa (4 May 2019). "A retrospective study on tolerability and complications of bacillus Calmette–Guérin (BCG) instillations for non-muscle-invasive bladder cancer". Scandinavian Journal of Urology. 53 (2–3): 116–122. doi:10.1080/21681805.2019.1609080. ISSN 2168-1805. PMID 31074322. S2CID 149444603.
- ^ Liu, Yuqing; Lu, Jian; Huang, Yi; Ma, Lulin (10 March 2019). "Clinical Spectrum of Complications Induced by Intravesical Immunotherapy of Bacillus Calmette–Guérin for Bladder Cancer". Journal of Oncology. 2019: 1–11. doi:10.1155/2019/6230409. ISSN 1687-8450. PMC 6431507. PMID 30984262.
- ^ Cabas, Paolo; Rizzo, Michele; Giuffrè, Mauro; Antonello, Roberta Maria; Trombetta, Carlo; Luzzati, Roberto; Liguori, Giovanni; Di Bella, Stefano (February 2021). "BCG infection (BCGitis) following intravesical instillation for bladder cancer and time interval between treatment and presentation: A systematic review". Urologic Oncology: Seminars and Original Investigations. 39 (2): 85–92. doi:10.1016/j.urolonc.2020.11.037. PMID 33308969. S2CID 229179250.
- ^ Centers for Disease Control and Prevention (April 1996). "The role of BCG vaccine in the prevention and control of tuberculosis in the United States. A joint statement by the Advisory Council for the Elimination of Tuberculosis and the Advisory Committee on Immunization Practices" (PDF). MMWR. Recommendations and Reports. 45 (RR-4): 1–18. PMID 8602127.
- ^ "Revised BCG vaccination guidelines for infants at risk for HIV infection". Weekly Epidemiological Record. 82 (21): 193–6. May 2007. hdl:10665/240940. PMID 17526121.
- ^ Trunz BB, Fine P, Dye C (April 2006). "Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness". Lancet. 367 (9517): 1173–80. doi:10.1016/S0140-6736(06)68507-3. PMID 16616560. S2CID 40371125.
- ^ Mak TK, Hesseling AC, Hussey GD, Cotton MF (September 2008). "Making BCG vaccination programmes safer in the HIV era". Lancet. 372 (9641): 786–7. doi:10.1016/S0140-6736(08)61318-5. PMID 18774406. S2CID 6702107.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah "Database of Global BCG Vaccination Policies and Practices". The BCG World Atlas. Retrieved 21 October 2020.
- ^ "Bacille Calmette–Guerin (BCG) Information for Health Professionals". toronto.ca. January 2020. Retrieved 8 April 2020.
- ^ Rousseau MC, Conus F, Kâ K, El-Zein M, Parent MÉ, Menzies D (August 2017). "Bacillus Calmette–Guérin (BCG) vaccination patterns in the province of Québec, Canada, 1956-1974". Vaccine. 35 (36): 4777–4784. doi:10.1016/j.vaccine.2017.06.064. PMID 28705514.
- ^ "Ficha metodológica". Archived from the original on 2 April 2015. Retrieved 12 March 2015.
- ^ "Prevention". sciensano.be.
- ^ "Задължителни и препоръчителни имунизации" [Mandatory and recommended immunizations]. mh.government.bg.
- ^ "Kopie – cem05_14.p65" (PDF). Retrieved 11 April 2020.
- ^ Rieckmann A, Villumsen M, Sørup S, Haugaard LK, Ravn H, Roth A, et al. (April 2017). "Vaccinations against smallpox and tuberculosis are associated with better long-term survival: a Danish case-cohort study 1971-2010". International Journal of Epidemiology. 46 (2): 695–705. doi:10.1093/ije/dyw120. PMC 5837789. PMID 27380797. S2CID 3792173.
- ^ "THL". BCG- eli tuberkuloosirokote. THL. Retrieved 16 April 2020.
- ^ Loi n° 50-7 du 5 janvier 1950
- ^ décret n° 2007-1111 du 17 juillet 2007
- ^ "relatif à l'obligation de vaccination par le BCG des professionnels listés aux articles L" (PDF). Archived (PDF) from the original on 30 July 2012. Retrieved 2 February 2014.
- ^ BCG World Atlas. "A Database of Global BCG Vaccination Policies and Practices". Archived from the original on 12 June 2009. Retrieved 4 April 2020.
- ^ Gabriele F, Katragkou A, Roilides E (October 2014). "BCG vaccination policy in Greece: time for another review?". The International Journal of Tuberculosis and Lung Disease. 18 (10): 1258. doi:10.5588/ijtld.14.0282. PMID 25216844.
- ^ "Διακοπή της εφαρμογής καθολικού αντιφυματικού εμβολιασμού (εμβόλιο BCG) στα παιδιά της Α΄ Δημοτικού 2016".
- ^ "A tuberkulózis és a tuberkulózis elleni védőoltás (BCG)" [Tuberculosis and tuberculosis vaccination]. ÁNTSZ. 17 July 2015.
- ^ O'Sullivan K. "Coronavirus: More 'striking' evidence BCG vaccine might protect against Covid-19". The Irish Times. Retrieved 6 April 2020.
- ^ "Tuberkulosevaksinasjon – veileder for helsepersonell" [Tuberculosis vaccination - guide for healthcare professionals]. FHI.no (in Norwegian). Archived from the original on 7 March 2016.
- ^ "Portugal data" (PDF). venice.cineca.org. Archived from the original (PDF) on 7 April 2020. Retrieved 11 April 2020.
- ^ "Vaccinări cu obligativitate generală" [Vaccinations with general obligation]. Archived from the original on 19 August 2013. Retrieved 14 April 2020.
- ^ "Vacunas disponibles | Vacunas / Asociación Española de Vacunología" [Vaccines available; Vaccines / Spanish Association of Vaccination].
- ^ "Tuberkulos (TB) – om vaccination – Folkhälsomyndigheten" [Tuberculosis (TB) - on vaccination - Public Health Agency]. folkhalsomyndigheten.se.
- ^ Zwerling, A.; Behr, M. A.; Verma, A.; Brewer, T. F.; Menzies, D.; Pai, M. (2011). "The BCG World Atlas: A Database of Global BCG Vaccination Policies and Practices". PLOS Medicine. 8 (3): e1001012. doi:10.1371/journal.pmed.1001012. PMC 3062527. PMID 21445325.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Ukraine search.ligazakon.ua
- ^ Styblo K, Meijer J (March 1976). "Impact of BCG vaccination programmes in children and young adults on the tuberculosis problem". Tubercle. 57 (1): 17–43. doi:10.1016/0041-3879(76)90015-5. PMID 1085050.
- ^ "School 'TB jabs' to be scrapped". BBC News Online. 6 July 2005. Archived from the original on 6 March 2012. Retrieved 24 September 2014.
- ^ "BCG tuberculosis (TB) vaccine overview". NHS.uk. Archived from the original on 1 April 2016. Retrieved 27 March 2016.
- ^ Chen ZR, Wei XH, Zhu ZY (June 1982). "BCG in China". Chinese Medical Journal. 95 (6): 437–42. PMID 6813052.
- ^ "Child health – Immunisation". Retrieved 6 January 2020.
- ^ "結核とBCGワクチンに関するQ&A|厚生労働省" [Q & A about tuberculosis and BCG vaccine, Ministry of Health, Labour and Welfare]. mhlw.go.jp (in Japanese). Archived from the original on 16 April 2017. Retrieved 10 July 2017.
- ^ "Thai Pediatrics". Thai Pediatrics. Archived from the original on 19 November 2015.
- ^ Mahler HT, Mohamed Ali P (1955). "Review of mass B.C.G. project in India". Ind J Tuberculosis. 2 (3): 108–16. Archived from the original on 13 February 2007.
- ^ Chaudhry A (24 February 2015). "Millions of infants denied anti-TB vaccination". Dawn. Pakistan. Retrieved 8 April 2020.
- ^ "Role of BC Vaccination". The National Programme for tuberculosis Control & Chest Diseases. Archived from the original on 6 June 2013.
- ^ SARS-CoV-2 Rates in BCG-Vaccinated and Unvaccinated Young Adults 13 May 2020 jamanetwork.com, accessed 21 October 2020
- ^ Sadeghi-Shanbestari, Mahnaz; Ansarin, Khalil; Maljaei, Seyed Hudieh; Rafeey, Mandana; Pezeshki, Zakaria; kousha, Ahmmad; Baradaran, Reza; Casanova, Jean; Feinberg, Jacqueline; de Villartay, Jean (2009). "Immunologic aspects of patients with disseminated bacille Calmette–Guerin disease in north-west of Iran". Italian Journal of Pediatrics. 35 (1): 42. doi:10.1186/1824-7288-35-42. PMC 2806263. PMID 20030825.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ "BCG" (PDF). South African National Department of Health. 2006. Archived from the original (PDF) on 11 May 2013.
- ^ "Évolution du calendrier vaccinal au Maroc". 29 May 2006.
- ^ "BCG vaccine for TB". Royal Children's Hospital Melbourne. Retrieved 31 March 2020.
- ^ "Onko BCG 100 Biomed Lublin". biomedlublin.com. Retrieved 3 March 2021.
- ^ "Pharmaceutical Information – PACIS". RxMed. Archived from the original on 22 February 2014. Retrieved 2 February 2014.
- ^ "BCG Vaccine Danish Strain 1331 – Statens Serum Institut". Ssi.dk. 19 September 2013. Archived from the original on 18 February 2014. Retrieved 2 February 2014.
- ^ "Bacillus Calmette–Guérin Vaccine Supply & Demand Outlook" (PDF), UNICEF Supply Division, p. 5, December 2015, archived (PDF) from the original on 5 February 2016, retrieved 29 January 2016
- ^ a b "April 2012 Inspectional Observations (form 483)", U.S. Food and Drug Administration, Vaccines, Blood & Biologics, 12 April 2012, archived from the original on 6 February 2016, retrieved 29 January 2016
- ^ "Sanofi Pasteur Product Monograph – Immucyst" (PDF). Sanofi Pasteur Canada. Retrieved 11 February 2016.
- ^ Palmer E (10 September 2014). "UPDATED: Merck again shipping BCG cancer treatment but Sanofi still is not". FiercePharma.
- ^ Palmer E (31 March 2015), "Sanofi Canada vax plant again producing ImmuCyst bladder cancer drug", FiercePharma, archived from the original on 5 February 2016, retrieved 29 January 2016
- ^ Cernuschi T, Malvolti S, Nickels E, Friede M (January 2018). "Bacillus Calmette–Guérin (BCG) vaccine: A global assessment of demand and supply balance". Vaccine. 36 (4): 498–506. doi:10.1016/j.vaccine.2017.12.010. PMC 5777639. PMID 29254839.
- ^ Atlas, Ronald M.; James W. Snyder (2006). Handbook of media for clinical microbiology. CRC Press. ISBN 978-0-8493-3795-6.
- ^ Ungar J, Muggleton PW, Dudley JA, Griffiths MI (October 1962). "Preparation and properties of a freeze-dried B.C.G. vaccine of increased stability". Br Med J. 2 (5312): 1086–9. doi:10.1136/bmj.2.5312.1086. PMC 1926490. PMID 13995378.
- ^ Villemin JA (1865). "Cause et nature de la tuberculose" [Cause and nature of tuberculosis]. Bulletin de l'Académie Impériale de Médecine (in French). 31: 211–216.
- ^ Villemin JA (1868). Études sur la Tuberculose [Studies of Tuberculosis] (in French). Paris, France: J.-B. Baillière et fils. pp. 528–597. (§ "Seizième Étude: La tuberculose est inoculable" (Sixteenth study: Tuberculosis can be transmitted by inoculation))
- ^ (Villemin, 1868), pp. 598–631. From p. 598: "La tuberculose est inoculable, voilà maintenant un fait incontestable. Désormais cette affection devra se placer parmi les maladies virulentes, … " (Tuberculosis [can be transmitted by] inoculation; that's now an incontestable fact. Henceforth this malady should be placed among the virulent maladies [i.e., those diseases that are transmitted via microorganisms], … ) From p. 602: "Les virus, comme les parasites, se multiplient eux-même, nous ne leur fournissons que les moyens de vivre et de se reproduire, jamais nous les créons." (Viruses, like parasites, multiply themselves; we merely furnish them with the means of living and reproducing; we never create them.)
- ^ Villemin, J.-A. (1868a). De la virulence et de la spécificité de la tuberculose [On the virulence [i.e., infectious nature] and specificity of tuberculosis] (in French). Paris, France: Victor Masson et fils.
- ^ Koch, Robert (10 April 1882). "Die Aetologie der Tuberculose" [The etiology of tuberculosis]. Berliner Klinische Wochenschrift (in German). 19 (15): 221–230. From p. 230: "Die Perlsucht ist identisch mit der Tuberculose des Menschen und also eine auf diesen übertragbare Krankheit." (Pearl disease [i.e., bovine tuberculosis] is identical with the tuberculosis of humans and thus [is] a disease that can be transmitted to them.)
- ^ Smith, Theobald (1895). "Investigations of diseases of domesticated animals". Annual Report of the Bureau of Animal Industry (U.S. Department of Agriculture). 12/13: 119–185. See § "Two varieties of the tubercle bacillus from mammals." pp. 149-161.
- Smith, Theobald (1896). "Two varieties of the tubercle bacillus from mammals". Transactions of the Association of American Physicians. 11: 75–95.
- ^ Palmer, Mitchell V.; Waters, W. Ray (May 2011). "Bovine tuberculosis and the establishment of an eradication program in the United States: Role of veterinarians". Veterinary Medicine International. 2011 (1): 816345. doi:10.4061/2011/816345. PMC 3103864. PMID 21647341. S2CID 18020962.
{{cite journal}}: CS1 maint: unflagged free DOI (link) From p. 2: "In 1895, Smith visited Koch in Europe and described his findings." - ^ Koch, Robert (27 July 1901). "An address on the combatting of tuberculosis in the light of experience that has been gained in the successful combatting of other infectious diseases". The Lancet. 158 (4065): 187–191. doi:10.1016/S0140-6736(01)85122-9. From p. 189: "Considering all these facts, I feel justified in maintaining that human tuberculosis differs from bovine and cannot be transmitted to cattle."
- ^ Fine PE, Carneiro IA, Milstein JB, Clements CJ (1999). Issues relating to the use of BCG in immunization programs. Geneva: World Health Organization (WHO).
- ^ Rosenthal SR. (1957). BCG vaccination against tuberculosis. Boston: Little, Brown & Co.
- ^ Blackburn, Mark (24 July 2013). "First Nation infants subject to "human experimental work" for TB vaccine in 1930s-40s". APTN News. Retrieved 31 March 2021.
- ^ "Fact Sheets: BCG Vaccine". Centers for Disease Control and Prevention. 28 October 2011. Archived from the original on 20 July 2013. Retrieved 18 July 2013.
- ^ Vaccination of young children against tuberculosis (PDF). The Hague:Health Council of the Netherlands. 2011. ISBN 978-90-5549-844-4. Archived (PDF) from the original on 19 February 2014. Retrieved 12 July 2013.
- ^ Aaby P, Roth A, Ravn H, Napirna BM, Rodrigues A, Lisse IM, et al. (July 2011). "Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period?". The Journal of Infectious Diseases. 204 (2): 245–52. doi:10.1093/infdis/jir240. PMID 21673035.
- ^ Biering-Sørensen S, Aaby P, Napirna BM, Roth A, Ravn H, Rodrigues A, et al. (March 2012). "Small randomized trial among low-birth-weight children receiving bacillus Calmette–Guérin vaccination at first health center contact". The Pediatric Infectious Disease Journal. 31 (3): 306–8. doi:10.1097/inf.0b013e3182458289. PMID 22189537. S2CID 1240058.
- ^ Darrah PA, Zeppa JJ, Maiello P, Hackney JA, Wadsworth MH, Hughes TK, et al. (January 2020). "Prevention of tuberculosis in macaques after intravenous BCG immunization". Nature. 577 (7788): 95–102. Bibcode:2020Natur.577...95D. doi:10.1038/s41586-019-1817-8. PMC 7015856. PMID 31894150.
{{cite journal}}: Unknown parameter|lay-url=ignored (help) - ^ Behar SM, Sassetti C (January 2020). "Tuberculosis vaccine finds an improved route". Nature. 577 (7788): 31–32. Bibcode:2020Natur.577...31B. doi:10.1038/d41586-019-03597-y. PMID 31894152. S2CID 209528484.
- ^ "The trick that could inject new life into an old tuberculosis vaccine". Nature. 577 (7789): 145. January 2020. Bibcode:2020Natur.577..145.. doi:10.1038/d41586-020-00003-w. PMID 31911698. S2CID 210044794.
- ^ Kühtreiber WM, Tran L, Kim T, Dybala M, Nguyen B, Plager S, et al. (2018). "Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations". NPJ Vaccines. 3: 23. doi:10.1038/s41541-018-0062-8. PMC 6013479. PMID 29951281.
- ^ Kowalewicz-Kulbat M, Locht C (July 2017). "BCG and protection against inflammatory and auto-immune diseases". Expert Review of Vaccines. 16 (7): 699–708. doi:10.1080/14760584.2017.1333906. PMID 28532186. S2CID 4723444.
- ^ Weng CH, Saal A, Butt WW, Bica N, Fisher JQ, Tao J, Chan PA (July 2020). "Bacillus Calmette–Guérin vaccination and clinical characteristics and outcomes of COVID-19 in Rhode Island, United States: a cohort study". Epidemiology and Infection. 148: e140. doi:10.1017/S0950268820001569. PMC 7360951. PMID 32641191.
- ^ Escobar LE, Molina-Cruz A, Barillas-Mury C (July 2020). "BCG vaccine protection from severe coronavirus disease 2019 (COVID-19)". Proceedings of the National Academy of Sciences of the United States of America. 117 (30): 17720–17726. doi:10.1073/pnas.2008410117. PMC 7395502. PMID 32647056. S2CID 220469598.
- ^ Klinger, Danielle; Blass, Ido; Rappoport, Nadav; Linial, Michal (11 July 2020). "Significantly Improved COVID-19 Outcomes in Countries with Higher BCG Vaccination Coverage: A Multivariable Analysis". Vaccines. 8 (3): 378. doi:10.3390/vaccines8030378. PMC 7563451. PMID 32664505. S2CID 220528079.
- ^ Szigeti, Reka; Kellermayer, Domos; Trakimas, Giedrius; Kellermayer, Richard (7 October 2020). "BCG epidemiology supports its protection against COVID-19? A word of caution". PLOS ONE. 15 (10): e0240203. Bibcode:2020PLoSO..1540203S. doi:10.1371/journal.pone.0240203. PMC 7540851. PMID 33027297.
- ^ "Situation Report 13 April 2020 – COVID-19" (PDF). World Health Organisation. 13 April 2020. Retrieved 14 April 2020.
- ^ "Studies found for BCG Recruiting, Active, not recruiting Studies Covid19". Clinical Trials.gov.
{{cite web}}: CS1 maint: url-status (link)
External links
- "BCG Vaccine". Drug Information Portal. U.S. National Library of Medicine.
- "BCG Vaccine". U.S. Food and Drug Administration (FDA). 24 April 2019.
- Frequently Asked Questions about BCG Professor P D O Davies, Tuberculosis Research Unit, Cardiothoracic Centre, Liverpool, UK.
- BCG Vaccine at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
