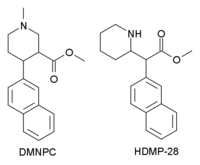
N,O-Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate
This is an old revision of this page, as edited by DePiep (talk | contribs) at 12:45, 28 June 2015 (Infobox drug: rm/replace deprecated params. Fix unk parameters (rm some Chembox-params) (via AWB script)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H21NO2 |
| Molar mass | 283.364 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| (verify) | |
N,O-Dimethyl-4β-(2-naphthyl)piperidine-3β-carboxylate (DMNPC) is a piperidine based stimulant drug which is synthesised from arecoline. It is similar to nocaine in chemical structure, and has two and a half times more activity than cocaine as a dopamine reuptake inhibitor. However it is also a potent serotonin reuptake inhibitor, with similar affinity to fluoxetine.[1] It is a structural isomer of another potent dopamine reuptake inhibitor, HDMP-28.
| Ki Affinity of Piperidine Based MAT Inhibitors | ||||||
| ∗∗ | X | N | 5HT | DA | NE | |
| SS | p-Vinyl | Me | 138 | 131 | 175 | |
| p-Ethyl | 255 | >1.7K | >1.1K | |||
| p-Allyl | 309 | 964 | >1K | |||
| p-Ethynyl | 175 | 187 | 364 | |||
| p-Phenyl | 62 | 173 | 203 | |||
| β-Naphthyl | 7.6 | 21 | 34 | |||
| 3R,4S | 42 | 947 | 241 | |||
| RR | 192 | 87 | 27 | |||
| 3S,4R | 12 | 271 | 38 | |||
| H2Cl | 3.5 | 90 | 30 | |||
| SS/RR | α-Naphthyl | Me | 101 | 304 | 281 | |
Clearly it is not just the SS enantiomer of the title compound that is an active MAT inhibitor.
Effect of N-demethylation
Interestingly, for the SR enantiomer, increased DAT affinity is seen upon demethylation.
This is the same choice of isomer used in the production of Paxil.
See also
References
This drug article relating to the nervous system is a stub. You can help Wikipedia by expanding it. |
- Chem-molar-mass both hardcoded and calculated
- Infobox-drug molecular-weight unexpected-character
- Infobox drug articles with non-default infobox title
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Wikipedia articles needing clarification from September 2024
- All stub articles