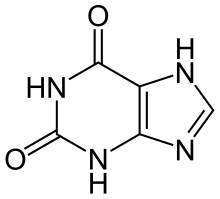
Xanthine
| |

| |
| Names | |
|---|---|
| IUPAC name
3,7-Dihydropurine-2,6-dione
| |
| Other names
1H-Purine-2,6-dione
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.653 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H4N4O2 | |
| Molar mass | 152.11 g/mol |
| Appearance | White solid |
| Melting point | decomposes |
| 1 g/ 14.5 L @ 16 °C 1 g/1.4 L @ 100 °C | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Xanthine (/ˈzænθiːn/ or /ˈzænθaɪn/; archaically xanthic acid) (3,7-dihydro-purine-2,6-dione), is a purine base found in most human body tissues and fluids and in other organisms. A number of stimulants are derived from xanthine, including caffeine and theobromine.[2]
Xanthine is a product on the pathway of purine degradation.
- It is created from guanine by guanine deaminase.
- It is created from hypoxanthine by xanthine oxidoreductase.
- It is also created from xanthosine by purine nucleoside phosphorylase (PNP).[3]
Xanthine is subsequently converted to uric acid by the action of the xanthine oxidase enzyme.
Studies reported in 2008, based on 12C/13C isotopic ratios of organic compounds found in the Murchison meteorite, suggested that xanthine and related chemicals, including the RNA component uracil, were formed extraterrestrially.[4][5] In August 2011, a report, based on NASA studies with meteorites found on Earth, was published suggesting xanthine and related organic molecules, including the DNA and RNA components adenine and guanine, were made in outer space.[6][7][8]
Pathology
People with the rare genetic disorder xanthinuria lack sufficient xanthine oxidase and cannot convert xanthine to uric acid.
Clinical significance of xanthine derivatives
Derivatives of xanthine (known collectively as xanthines) are a group of alkaloids commonly used for their effects as mild stimulants and as bronchodilators, notably in the treatment of asthma symptoms. In contrast to other, more potent stimulants like sympathomimetic amines, xanthines mainly act to oppose the actions of the sleepiness-inducing adenosine, and increase alertness in the central nervous system. They also stimulate the respiratory centre, and are used for treatment of infantile apnea. Due to widespread effects, the therapeutic range of xanthines is narrow, making them merely a second-line asthma treatment. The therapeutic level is 10-20 micrograms/mL blood; signs of toxicity include tremor, nausea, nervousness, and tachycardia/arrhythmia.
Methylated xanthines (methylxanthines), which include caffeine, aminophylline, IBMX, paraxanthine, pentoxifylline,[9] theobromine, and theophylline, affect not only the airways but stimulate heart rate, force of contraction, and cardiac arrhythmias at high concentrations. In high doses they can lead to convulsions that are resistant to anticonvulsants. Methylxanthines induce acid and pepsin secretions in the GI tract. Methylxanthines are metabolized by cytochrome P450 in the liver.
These drugs act as both:
- competitive nonselective phosphodiesterase inhibitors [10] which raise intracellular cAMP, activate PKA, inhibit TNF-α [9][11] and leukotriene [12] synthesis, and reduce inflammation and innate immunity [12] and
- nonselective adenosine receptor antagonists [13] which inhibit sleepiness-inducing adenosine.
But different analogues show varying potency at the numerous subtypes, and a wide range of synthetic xanthines (some nonmethylated) have been developed searching for compounds with greater selectivity for phosphodiesterase enzyme or adenosine receptor subtypes.[14][15][16][17][18][19][20][21][22][23][24][25][26] Xanthines are also found very rarely as constituents of nucleic acids.

Theobromine: R1 = H, R2 = R3 = CH3
Theophylline: R1 = R2 = CH3, R3 = H
| Name | R1 | R2 | R3 | R8 | IUPAC nomenclature | Found In |
|---|---|---|---|---|---|---|
| Xanthine | H | H | H | H | 3,7-dihydro-purine-2,6-dione | Plants, animals |
| Caffeine | CH3 | CH3 | CH3 | H | 1,3,7-trimethyl-1H-purine-2,6(3H,7H)-dione | Coffee, Guarana, Yerba mate, Tea, Kola, Guayusa, Holly |
| Theobromine | H | CH3 | CH3 | H | 3,7-dihydro-3,7-dimethyl-1H-purine-2,6-dione | Cacao (chocolate), Yerba mate, Kola, Guayusa, Holly |
| Theophylline | CH3 | CH3 | H | H | 1,3-dimethyl-7H-purine-2,6-dione | Tea, Cacao (chocolate), Yerba mate, Kola |
| Paraxanthine | CH3 | H | CH3 | H | 1,7-dimethyl-7H-purine-2,6-dione | Animals that have consumed caffeine |
| 8-Chlorotheophylline | CH3 | CH3 | H | Cl | Dimenhydrinate |
See also
References
- ^ Merck Index, 11th Edition, 9968.
- ^ Spiller, Gene A. (1998). Caffeine. Boca Raton: CRC Press. ISBN 0-8493-2647-8.
- ^ Voet, Donald; Voet, Judith; Pratt, Charlotte (2008). "The Major Pathways of Purine Catabolism in Animals", Fundamentals of Biochemistry: Life at the Molecular Level, p. 840.
- ^ Martins, Z.; Botta, O.; Fogel, M. L.; Sephton, M. A.; Glavin, D. P.; Watson, J. S.; Dworkin, J. P.; Schwartz, A. W.; Ehrenfreund, P. (2008). "Extraterrestrial nucleobases in the Murchison meteorite". Earth and Planetary Science Letters. 270: 130–136. arXiv:0806.2286. Bibcode:2008E&PSL.270..130M. doi:10.1016/j.epsl.2008.03.026.
- ^ AFP Staff (13 June 2008). "We may all be space aliens: study". AFP. Retrieved 2011-08-14.
- ^ Callahan, M. P.; Smith, K. E.; Cleaves, H. J.; Ruzicka, J.; Stern, J. C.; Glavin, D. P.; House, C. H.; Dworkin, J. P. (2011). "Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases". Proceedings of the National Academy of Sciences. 108 (34): 13995–8. doi:10.1073/pnas.1106493108. PMC 3161613. PMID 21836052.
- ^ Steigerwald, John (8 August 2011). "NASA Researchers: DNA Building Blocks Can Be Made in Space". NASA. Retrieved 2011-08-10.
- ^ ScienceDaily Staff (9 August 2011). "DNA Building Blocks Can Be Made in Space, NASA Evidence Suggests". ScienceDaily. Retrieved 2011-08-09.
- ^ a b Deree J, Martins JO, Melbostad H, Loomis WH, Coimbra R. (2008). "Insights into the regulation of TNF-alpha production in human mononuclear cells: the effects of non-specific phosphodiesterase inhibition". Clinics (São Paulo). 63 (3): 321–8. doi:10.1590/S1807-59322008000300006. PMC 2664230. PMID 18568240.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Essayan DM. (2001). "Cyclic nucleotide phosphodiesterases". J Allergy Clin Immunol. 108 (5): 671–80. doi:10.1067/mai.2001.119555. PMID 11692087.
- ^ Marques LJ, Zheng L, Poulakis N, Guzman J, Costabel U (February 1999). "Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages". Am. J. Respir. Crit. Care Med. 159 (2): 508–11. doi:10.1164/ajrccm.159.2.9804085. PMID 9927365.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b Peters-Golden M, Canetti C, Mancuso P, Coffey MJ. (2005). "Leukotrienes: underappreciated mediators of innate immune responses". J Immunol. 174 (2): 589–94. doi:10.4049/jimmunol.174.2.589. PMID 15634873.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Daly JW, Jacobson KA, Ukena D. (1987). "Adenosine receptors: development of selective agonists and antagonists". Prog Clin Biol Res. 230 (1): 41–63. PMID 3588607.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ MacCorquodale DW. THE SYNTHESIS OF SOME ALKYLXANTHINES. Journal of the American Chemical Society. 1929 July;51(7):2245–2251. doi:10.1021/ja01382a042
- ^ WO patent 1985002540, Sunshine A, Laska EM, Siegel CE, "ANALGESIC AND ANTI-INFLAMMATORY COMPOSITIONS COMPRISING XANTHINES AND METHODS OF USING SAME", granted 1989-03-22, assigned to RICHARDSON-VICKS, INC.
- ^ Constantin Koulbanis, Claude Bouillon, Patrick Darmenton,"Cosmetic compositions having a slimming action", US patent 4288433, granted 1981-09-04 , assigned to L'Oréal
- ^ Daly JW, Padgett WL, Shamim MT (July 1986). "Analogues of caffeine and theophylline: effect of structural alterations on affinity at adenosine receptors". Journal of Medicinal Chemistry. 29 (7): 1305–8. doi:10.1021/jm00157a035. PMID 3806581.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Daly JW, Jacobson KA, Ukena D (1987). "Adenosine receptors: development of selective agonists and antagonists". Progress in Clinical and Biological Research. 230: 41–63. PMID 3588607.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Choi OH, Shamim MT, Padgett WL, Daly JW (1988). "Caffeine and theophylline analogues: correlation of behavioral effects with activity as adenosine receptor antagonists and as phosphodiesterase inhibitors". Life Sciences. 43 (5): 387–98. doi:10.1016/0024-3205(88)90517-6. PMID 2456442.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Shamim MT, Ukena D, Padgett WL, Daly JW (June 1989). "Effects of 8-phenyl and 8-cycloalkyl substituents on the activity of mono-, di-, and trisubstituted alkylxanthines with substitution at the 1-, 3-, and 7-positions". Journal of Medicinal Chemistry. 32 (6): 1231–7. doi:10.1021/jm00126a014. PMID 2724296.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Daly JW, Hide I, Müller CE, Shamim M (1991). "Caffeine analogs: structure-activity relationships at adenosine receptors". Pharmacology. 42 (6): 309–21. doi:10.1159/000138813. PMID 1658821.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Ukena D, Schudt C, Sybrecht GW (February 1993). "Adenosine receptor-blocking xanthines as inhibitors of phosphodiesterase isozymes". Biochemical Pharmacology. 45 (4): 847–51. doi:10.1016/0006-2952(93)90168-V. PMID 7680859.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Daly JW (July 2000). "Alkylxanthines as research tools". Journal of the Autonomic Nervous System. 81 (1–3): 44–52. doi:10.1016/S0165-1838(00)00110-7. PMID 10869699.
- ^ Daly JW (August 2007). "Caffeine analogs: biomedical impact". Cellular and Molecular Life Sciences : CMLS. 64 (16): 2153–69. doi:10.1007/s00018-007-7051-9. PMID 17514358.
- ^ González MP, Terán C, Teijeira M (May 2008). "Search for new antagonist ligands for adenosine receptors from QSAR point of view. How close are we?". Medicinal Research Reviews. 28 (3): 329–71. doi:10.1002/med.20108. PMID 17668454.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Baraldi PG, Tabrizi MA, Gessi S, Borea PA (January 2008). "Adenosine receptor antagonists: translating medicinal chemistry and pharmacology into clinical utility". Chemical Reviews. 108 (1): 238–63. doi:10.1021/cr0682195. PMID 18181659.
{{cite journal}}: CS1 maint: multiple names: authors list (link)

