Osimertinib: Difference between revisions
+ |
→Medical application: per MEDMOS |
||
| Line 55: | Line 55: | ||
'''Osimertinib''' (previously known as '''mereletinib''' or '''AZD9291'''; trade name '''Tagrisso''')<ref>{{cite web|title=Osimertinib|url=http://adisinsight.springer.com/drugs/800037784|publisher=AdisInsight|accessdate=27 February 2017|language=en}}</ref><ref>{{cite journal|title=Proposed INN: List 113|journal=International Nonproprietary Names for Pharmaceutical Substances (INN)|date=2015|volume=29|issue=2|pages=285|url=http://www.who.int/medicines/publications/druginformation/INN_List113.pdf|accessdate=16 November 2015}}</ref> is a third-generation [[epidermal growth factor receptor]] [[tyrosine kinase inhibitor]] drug used to treat [[Non-small-cell lung carcinoma|non-small-cell lung carcinomas]] with a specific mutation.<ref>{{cite journal |authors=Ayeni D, Politi K, Goldberg SB |title=Emerging Agents and New Mutations in EGFR-Mutant Lung Cancer |journal=Clin. Cancer Res. |volume=21 |issue=17 |pages=3818–20 |year=2015 |pmid=26169963 |doi=10.1158/1078-0432.CCR-15-1211 |url=}}</ref><ref>{{cite journal |authors=Tan CS, Gilligan D, Pacey S |title=Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer |journal=Lancet Oncol. |volume=16 |issue=9 |pages=e447–59 |year=2015 |pmid=26370354 |doi=10.1016/S1470-2045(15)00246-6 |url=}}</ref> Developed by [[AstraZeneca]], the drug was fully approved as a cancer treatment in 2017 by both the [[Food and Drug Administration]] and the [[European Commission]]. |
'''Osimertinib''' (previously known as '''mereletinib''' or '''AZD9291'''; trade name '''Tagrisso''')<ref>{{cite web|title=Osimertinib|url=http://adisinsight.springer.com/drugs/800037784|publisher=AdisInsight|accessdate=27 February 2017|language=en}}</ref><ref>{{cite journal|title=Proposed INN: List 113|journal=International Nonproprietary Names for Pharmaceutical Substances (INN)|date=2015|volume=29|issue=2|pages=285|url=http://www.who.int/medicines/publications/druginformation/INN_List113.pdf|accessdate=16 November 2015}}</ref> is a third-generation [[epidermal growth factor receptor]] [[tyrosine kinase inhibitor]] drug used to treat [[Non-small-cell lung carcinoma|non-small-cell lung carcinomas]] with a specific mutation.<ref>{{cite journal |authors=Ayeni D, Politi K, Goldberg SB |title=Emerging Agents and New Mutations in EGFR-Mutant Lung Cancer |journal=Clin. Cancer Res. |volume=21 |issue=17 |pages=3818–20 |year=2015 |pmid=26169963 |doi=10.1158/1078-0432.CCR-15-1211 |url=}}</ref><ref>{{cite journal |authors=Tan CS, Gilligan D, Pacey S |title=Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer |journal=Lancet Oncol. |volume=16 |issue=9 |pages=e447–59 |year=2015 |pmid=26370354 |doi=10.1016/S1470-2045(15)00246-6 |url=}}</ref> Developed by [[AstraZeneca]], the drug was fully approved as a cancer treatment in 2017 by both the [[Food and Drug Administration]] and the [[European Commission]]. |
||
==Medical |
==Medical uses== |
||
Osimertinib is used to treat locally advanced or metastatic [[non-small-cell lung cancer]] (NSCLC), if the cancer cells are positive for the [[T790M]] mutation in the gene coding for [[epidermal growth factor receptor|EGFR]].<ref name =USlabel/><ref name=UKlabel>{{cite web|title=UK label|url=https://www.medicines.org.uk/emc/medicine/31496|publisher=UK Electronic Medicines Compendium|accessdate=27 February 2017|language=en|date=26 January 2017}}</ref> The T790M mutation may be [[De novo mutation|de novo]] or acquired following first-line treatment with other tyrosine kinase inhibitors (TKIs), such as [[gefitinib]] and [[afatinib]]. |
Osimertinib is used to treat locally advanced or metastatic [[non-small-cell lung cancer]] (NSCLC), if the cancer cells are positive for the [[T790M]] mutation in the gene coding for [[epidermal growth factor receptor|EGFR]].<ref name =USlabel/><ref name=UKlabel>{{cite web|title=UK label|url=https://www.medicines.org.uk/emc/medicine/31496|publisher=UK Electronic Medicines Compendium|accessdate=27 February 2017|language=en|date=26 January 2017}}</ref> The T790M mutation may be [[De novo mutation|de novo]] or acquired following first-line treatment with other tyrosine kinase inhibitors (TKIs), such as [[gefitinib]] and [[afatinib]]. |
||
Revision as of 16:26, 16 January 2018
 | |
| Clinical data | |
|---|---|
| Trade names | Tagrisso, Tagrix |
| Other names | AZD9291 |
| AHFS/Drugs.com | tagrisso |
| License data | |
| Routes of administration | Oral tablets |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Probably high[1] |
| Metabolism | Oxidation (CYP3A) |
| Elimination half-life | 48 hours |
| Excretion | Feces (68%), urine (14%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
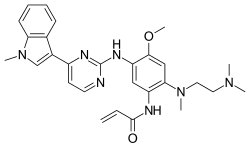
| Formula | C28H33N7O2 |
| Molar mass | 499.619 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Osimertinib (previously known as mereletinib or AZD9291; trade name Tagrisso)[2][3] is a third-generation epidermal growth factor receptor tyrosine kinase inhibitor drug used to treat non-small-cell lung carcinomas with a specific mutation.[4][5] Developed by AstraZeneca, the drug was fully approved as a cancer treatment in 2017 by both the Food and Drug Administration and the European Commission.
Medical uses
Osimertinib is used to treat locally advanced or metastatic non-small-cell lung cancer (NSCLC), if the cancer cells are positive for the T790M mutation in the gene coding for EGFR.[1][6] The T790M mutation may be de novo or acquired following first-line treatment with other tyrosine kinase inhibitors (TKIs), such as gefitinib and afatinib.
In the USA, T790M status of the patient prior to treatment with osimertinib must be detected by a federally approved companion diagnostic test. The Food and Drug Administration (FDA) has approved FoundationOne CDx for this purpose. In Europe and elsewhere, T790M mutations may be determined by a validated and suitably sensitive next-generation sequencing assay.[1][7]
The NSCLC in people receiving the drug has developed resistance mutations, namely C797S occurs in EGFR exon 20 or amplifications of the EGFR gene, even as T790M clones have been eliminated.[8]
It causes fetal harm, so should not be used in women who are pregnant, and women who take it should avoid becoming pregnant.[1][6]
Caution should be taken in people with a history of interstitial lung disease (ILD) were excluded from clinical trials, as the drug can cause severe ILD or pneumonia. Caution should also be taken in people with a predisposition to long QT syndrome as the drug can provoke this.[1]
Adverse effects
Very common (greater than 10% of clinical trial subjects) adverse effects include diarrhea, stomatitis, rashes, dry or itchy skin, infections where finger or toenails abut skin, low platelet counts, low leukocyte counts, and low neutrophil counts.[6]
Common (between 1% and 10% of clinical trial subjects) adverse effects include interstitial lung disease.[6]
Interactions
Osimertinib is metabolized by CYP3A4 and CYP3A5, so substances that strongly inhibit either enyzme, like macrolide antibiotics, antifungals, and antivirals may increase exposure to osimertinib, and substances like rifampicin that activate either enzyme will decrease the effectiveness of osimertinib.[1][6]
Pharmacology
Osimertinib binds irreversibly to epidermal growth factor receptor proteins expressed by EGFR with a T790M mutation;[6] it also binds irreversibly to EGFR with a L858R mutation and with an exon 19 deletion.[1]
It exhibits linear pharmacokinetics; the median time to Cmax is 6 hours (range 3–24 hours). The estimated mean half-life is 48 hours, and oral clearance (CL/F) is 14.2 (L/h). 68% of elimination is by feces and 14% by urine.[1]
Chemistry
Osimertinib is provided as the mesylate; the chemical formula is C28H33N7O2•CH4O3S, and the molecular weight is 596 g/mol. The chemical name is N-(2-{2-dimethylaminoethyl-methylamino}-4-methoxy-5-{[4-(1-methylindol-3-yl)pyrimidin-2-yl]amino}phenyl)prop-2-enamide mesylate salt.[1]
History
The drug discovery program that led to osimertinib started in 2009 and yielded the drug by 2012; the process was structure-driven and aimed to find a third generation EGFR inhibitor that would selectively target the T790M form of the EGFR receptor.[9]
Osimertinib was designated as a Breakthrough Therapy in April 2014 based on Phase I trial results,[9] and the drug was provisionally approved under the FDA accelerated approval program with a priority review voucher, in November 2015.[10]
In February 2016 the EMA provisionally approved osimertinib under an accelerated process—the first approval under the program.[9]
Society and culture
At launch, Astrazeneca priced the drug at $12,750 per month.[11]: 59
Research
As of December 2016 several clinical trials were ongoing.[8]
References
- ^ a b c d e f g h i "US Label" (PDF). FDA. November 2015. Index page for NDA 208065
- ^ "Osimertinib". AdisInsight. Retrieved 27 February 2017.
- ^ "Proposed INN: List 113" (PDF). International Nonproprietary Names for Pharmaceutical Substances (INN). 29 (2): 285. 2015. Retrieved 16 November 2015.
- ^ "Emerging Agents and New Mutations in EGFR-Mutant Lung Cancer". Clin. Cancer Res. 21 (17): 3818–20. 2015. doi:10.1158/1078-0432.CCR-15-1211. PMID 26169963.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ "Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer". Lancet Oncol. 16 (9): e447–59. 2015. doi:10.1016/S1470-2045(15)00246-6. PMID 26370354.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ a b c d e f "UK label". UK Electronic Medicines Compendium. 26 January 2017. Retrieved 27 February 2017.
- ^ "The latest therapeutic strategies after resistance to first generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in patients with non-small cell lung cancer (NSCLC)". Ann Transl Med. 3 (7): 96. 2015. doi:10.3978/j.issn.2305-5839.2015.03.60. PMC 4430733. PMID 26015938.
{{cite journal}}: Cite uses deprecated parameter|authors=(help) - ^ a b Minari, R; Bordi, P; Tiseo, M (December 2016). "Third-generation epidermal growth factor receptor-tyrosine kinase inhibitors in T790M-positive non-small cell lung cancer: review on emerged mechanisms of resistance". Translational lung cancer research. 5 (6): 695–708. PMC 5233880. PMID 28149764.
- ^ a b c Yver, A (June 2016). "Osimertinib (AZD9291)-a science-driven, collaborative approach to rapid drug design and development". Annals of Oncology. 27 (6): 1165–70. PMID 26961148.
- ^ "Approved Drugs - Osimertinib". FDA Center for Drug Evaluation and Research. November 13, 2015.
- ^ "AHRQ Healthcare Horizon Scanning System – Potential High-Impact Interventions Report Priority Area 02: Cancer" (PDF). AHRQ. December 2015.
