Wikipedia:Reference desk/Science: Difference between revisions
| Line 940: | Line 940: | ||
::::I graduated from high school in 2000 and last learned about HIV/AIDS in 1998. So, no, I don't remember everything they told us about AIDS back then, and even if they went into details such as these.--[[User:IndexOutOfBounds|IndexOutOfBounds]] ([[User talk:IndexOutOfBounds|talk]]) 15:15, 11 August 2009 (UTC) |
::::I graduated from high school in 2000 and last learned about HIV/AIDS in 1998. So, no, I don't remember everything they told us about AIDS back then, and even if they went into details such as these.--[[User:IndexOutOfBounds|IndexOutOfBounds]] ([[User talk:IndexOutOfBounds|talk]]) 15:15, 11 August 2009 (UTC) |
||
:::::It's kind of a big deal, you know, how to avoid getting various diseases. I'm just saying. And it's pretty easy to look up this information. You might as well be posting on here about whether the Earth is flat or not, and then complaining that you went to school a long time ago and can't be bothering to remember such a thing. To think that AIDs is "only spread by gay sex" is an extremely, extremely ignorant thing to believe still. If that's really what you were taught in 2000 (which is not what I was taught in the 1990s), then you were, as I said, seriously poorly served by whomever taught you about public health, STDs, etc. --[[Special:Contributions/98.217.14.211|98.217.14.211]] ([[User talk:98.217.14.211|talk]]) 16:13, 11 August 2009 (UTC) |
:::::It's kind of a big deal, you know, how to avoid getting various diseases. I'm just saying. And it's pretty easy to look up this information. You might as well be posting on here about whether the Earth is flat or not, and then complaining that you went to school a long time ago and can't be bothering to remember such a thing. To think that AIDs is "only spread by gay sex" is an extremely, extremely ignorant thing to believe still. If that's really what you were taught in 2000 (which is not what I was taught in the 1990s), then you were, as I said, seriously poorly served by whomever taught you about public health, STDs, etc. --[[Special:Contributions/98.217.14.211|98.217.14.211]] ([[User talk:98.217.14.211|talk]]) 16:13, 11 August 2009 (UTC) |
||
::::::Just so you know, this is a ''reference desk'' where you can ask questions. I'm not a doctor. I have few college degrees, but none of them are in biology or medicine. So, to call me ignorant is (ironically) ignorant.--[[User:IndexOutOfBounds|IndexOutOfBounds]] ([[User talk:IndexOutOfBounds|talk]]) 23:45, 11 August 2009 (UTC) |
|||
:::Are you currently in Africa? I thought we spent enough time and money in America and Europe to stamp out such ignorance. -- [[User:Kainaw|<font color='#ff0000'>k</font><font color='#cc0033'>a</font><font color='#990066'>i</font><font color='#660099'>n</font><font color='#3300cc'>a</font><font color='#0000ff'>w</font>]][[User talk:Kainaw|™]] 15:10, 11 August 2009 (UTC) |
:::Are you currently in Africa? I thought we spent enough time and money in America and Europe to stamp out such ignorance. -- [[User:Kainaw|<font color='#ff0000'>k</font><font color='#cc0033'>a</font><font color='#990066'>i</font><font color='#660099'>n</font><font color='#3300cc'>a</font><font color='#0000ff'>w</font>]][[User talk:Kainaw|™]] 15:10, 11 August 2009 (UTC) |
||
Revision as of 23:46, 11 August 2009
of the Wikipedia reference desk.
Main page: Help searching Wikipedia
How can I get my question answered?
- Select the section of the desk that best fits the general topic of your question (see the navigation column to the right).
- Post your question to only one section, providing a short header that gives the topic of your question.
- Type '~~~~' (that is, four tilde characters) at the end – this signs and dates your contribution so we know who wrote what and when.
- Don't post personal contact information – it will be removed. Any answers will be provided here.
- Please be as specific as possible, and include all relevant context – the usefulness of answers may depend on the context.
- Note:
- We don't answer (and may remove) questions that require medical diagnosis or legal advice.
- We don't answer requests for opinions, predictions or debate.
- We don't do your homework for you, though we'll help you past the stuck point.
- We don't conduct original research or provide a free source of ideas, but we'll help you find information you need.
How do I answer a question?
Main page: Wikipedia:Reference desk/Guidelines
- The best answers address the question directly, and back up facts with wikilinks and links to sources. Do not edit others' comments and do not give any medical or legal advice.
August 4
Bond Energy
Hello. Under which conditions (STP or SATP) is the bond energy of a single carbon-to-carbon bond 347 kJ mol-1? Thanks in advance. --Mayfare (talk) 00:54, 4 August 2009 (UTC)
- As it's chemistry/physics it will use the IUPAC conditions http://goldbook.iupac.org/goldbook/S06036.html
- If you want to KNOW FOR CERTAIN then you need to find and read the reference from which the data came.
- Also are STP and SATP actually different?83.100.250.79 (talk) 14:23, 4 August 2009 (UTC)
STP is 0°C and 101.325 kPa. SATP is 25°C and 100 kPa. --Mayfare (talk) 18:36, 4 August 2009 (UTC)
- Isn't it the other way round (1atm-101.325kPa) if A means 'atmospheric'.
- I'm not sure that the acronym STP has a single defined value - it may depend on whether you are a chemist, physicist or other type of scientist.83.100.250.79 (talk) 20:16, 4 August 2009 (UTC)
STP stands for Standard Temperature and Pressure. SATP stands for Standard Ambient Temperature and Pressure. Sorry for being unclear in the first place. --Mayfare (talk) 18:35, 5 August 2009 (UTC)
I read this article on standard conditions for temperature and pressure. I did not realize that I was using US standards. --Mayfare (talk) 17:50, 6 August 2009 (UTC)
Peter Pan Syndrome - refusing to heed written laws or just societal norms?
Reading the article in our link on the above, I was curious. When People refer to this, are they talking about people who refuse any type of restrictions, especially legal ones? Or, are they talking mostly cultural ones, not wanting to take the responsibilities that adults generally do.
What brought this up was a monarch - Ludwig II of Bavaria whom I had heard elsewhere might been like this (I hesitate to use 'suffered" since it's not an actual diagnosis), because of the fantasy world in which he often seemed to live. Although, I imagine just "living in a fantasy world" isn't the only criteria, it certainly seems to be part of it. Or, does the term generally refer to someone a little more dangerous or odious than just someone "living in a fantasy world"?209.244.30.221 (talk) 01:08, 4 August 2009 (UTC)
- In a social setting (i. e. not a medical one), I have only ever heard this term used to describe men who never seemed to be interested in taking on adult responsibility. (Please note that I am not suggesting that this only happens to males, but simply that "Peter Pan" is usually applied only to males.) I have never heard the use to mean anything illegal or odious, unless the idea of perpetual childhood is an odious one. Usually it is women making the complaint, and it is a complaint. // BL \\ (talk) 01:36, 4 August 2009 (UTC)
- As our article says - this is a "pop-psych" term - it's not a proper medical term with a hard definition. So what do people mean when they use it? Well, it's just some vague concept that an adult behaves like a kid. Different people are bound to use the word in different ways. SteveBaker (talk) 03:30, 4 August 2009 (UTC)
Old refrigerators making more ice
I have an old refrigerator and it makes more ice as when it was new. Now it needs to be defrosted at regular intervals. Why? Wouldn't it be much more logical if it made less ice as it gets older?--80.58.205.37 (talk) 11:01, 4 August 2009 (UTC)
- My guess is that it's because with age the seal becomes less effective, so more water vapour gets inside the fridge. AndrewWTaylor (talk) 11:05, 4 August 2009 (UTC)
- I agree, maybe the gasket in the door is old, so outside air leaks in and carries moisture with it. It could also be that different stuff give off different amonunts of moisture (if your fridge held two sixpacks of beer a few years ago and today it holds vegetables, home made baby food and leftovers you will probably see some difference in the amounts of ice). Also if you open the door more often you let more air in.Sjö (talk) 11:10, 4 August 2009 (UTC)
- Yep - your refrigerator is still getting cold enough to freeze the moisture out of the air. Usually, what happens is that the air inside the fridge is first cooled to the point where it can't hold so much water. That causes the water to condense onto the cold surfaces - just like the windows on your car fogging up when it's cold outside. The water droplets that end up on the freezer compartment then freeze into ice. However, if all was well, there would now be no more water in the air inside the fridge and no more ice would form. But if the door seal leaks (especially if it's towards the bottom of the door) then the cold/dry air (being denser) will slowly flow out of the fridge to be replaced by moist/warm air from outside. The warm air carries with it more moisture into the fridge and the cycle repeats, gradually building up the ice. Replacing the door seal should stop that and will save you money too. Failing that (as others have suggested) it would have to mean some change in life-style...perhaps different foods being stored, open containers of liquids - increased opening of the door - or perhaps a new family member who doesn't shut the fridge door firmly enough or soon enough. I suppose it's also possible that you have the thing dialled down to a colder setting than before, or that the thermostat has gone wonky...but that doesn't explain where the water to form the ice is coming from...so I'm still betting that the door seal is failing to keep the thing airtight. You should consider changing the seal though - it's a real waste of electricity. But at least you know that your old fridge is still capable of keeping things properly cool! SteveBaker (talk) 12:06, 4 August 2009 (UTC)
- To check the door seal, use a piece of ribbon. Close the door on it and see if it can be pulled out freely. Check several places around the door, including the hinged side. —Preceding unsigned comment added by 98.21.108.4 (talk) 13:48, 5 August 2009 (UTC)
- P.S. Instead of a ribbon, a narrow strip of paper would be better. This has the stiffness to allow it to be inserted easily in the sides of the door. —Preceding unsigned comment added by 98.21.106.176 (talk) 13:22, 6 August 2009 (UTC)
- No - you don't insert the paper into the closed door - you open the door, place the ribbon/paper against the seal, then close the door and see how hard it is to pull it back out again. If there is a significant gap in the seal, the ribbon/paper will come out easily. It's gonna be hard to find a problem that way though. I'd get one of those electronic temperature probes and just look for cold spots on the outside of the seal when the door has been closed for an hour or two. Personally - I wouldn't bother - if the problem is all that serious, it's going to be a defective seal for 100% sure...so just replace it already! SteveBaker (talk) 13:46, 6 August 2009 (UTC)
- P.S. Instead of a ribbon, a narrow strip of paper would be better. This has the stiffness to allow it to be inserted easily in the sides of the door. —Preceding unsigned comment added by 98.21.106.176 (talk) 13:22, 6 August 2009 (UTC)
does the acidity of aromatic rings, alkenes (or even alkanes) contribute to petroleum formation?
So, the pKa of an alkane is like 60, and that of alkene around 45, .... but that means a proton still comes off occasionally. That starts to make me think ... if I say stored a pure olefin (or maybe benzene) in a glass jar for 1000 years, would I eventually see some signs of chemical reactions (maybe on the ppm scale ... if someone was alive to run the jar through NMR later?). I'm thinking carbon-carbon bonds would be formed in this way ... well, let's say we had liquified 1-butene or something, ever so often, the boltzmann distribution apparently gives a lucky butene molecule enough energy to lose a proton ... a proton which then proceeds to readily protonate some other butene .... which makes it a cation, which then finds the anion, forming a C-C bond. (Well, it doesn't have to find the original molecule that lost the proton, the original anion would probably have pulled a proton off some other molecule later on, creating a new anion....)
Yeah, it probably would occur very very slowly ... but then it occurs to me, that would probably happen in an oil bed under high heat and pressure, given enough time (like 50 million years). I'm using a pure olefin as a purely hypothetical thought experiment of course (to allow ease of detection of new products), since I assume in peat or whatever you have a wide collection of organic olefins. Or is the breakage and reformation of C-C bonds (homolytically or hemolytically) under high heat and pressure the more predominant process? John Riemann Soong (talk) 11:42, 4 August 2009 (UTC)
- I don't know the answer, but Petroleum#Formation and Catagenesis (geology) say that C-C bonds are formed by living organisms, which die and become kerogen. Kerogen subsequently undergoes thermal decomposition to hydrocarbons.
- No mention of acid-base reactions. The pKa values of alkanes and alkenes are very approximate and in any case depend on the solvent they're in.
- The loss of a proton, or H radical could contribute to petroleum formation, as will all conceivable chemical processes including dehydration, other eliminations, pericyclic additions rearrangements and eliminations etc.83.100.250.79 (talk) 14:28, 4 August 2009 (UTC)
They could in principle, but do they in practice?
The answer may not be known.
Ben (talk) 14:34, 4 August 2009 (UTC)
By the way peat etc is predominately polysaccharides/cellulose (and lignin) and one of the major reactions to get hydrocarbon is loss of Oxygen (possible as water), additionally the carbon skeleton of 'peat' is not the same as oil/coal so C-C bond changes must be a major factor, along with C-H and C-OH bond changes. (It's heterolytic cleavage not hemolytic)83.100.250.79 (talk) 16:41, 4 August 2009 (UTC)

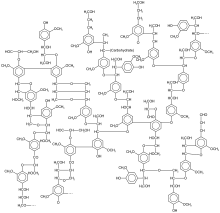
- Have a look at this lignin structure - and compare that with coal (a fossil fuel)..83.100.250.79 (talk) 16:44, 4 August 2009 (UTC)
Wow that lignin seems to have some ring strain ... I mean, I like how two substituents on a benzene ring can later "join up" later on.... and just how does it react that it fuses multiple aromatic rings together? That's amazing. John Riemann Soong (talk) 18:15, 4 August 2009 (UTC)
May I add that peat and lignite also form biologically, from plant matter? 98.234.126.251 (talk) 01:39, 5 August 2009 (UTC)
The contrast between the two structures is interesting... looking at the disulfide and nitrogen linkages in the coal structure, it's pretty clear that protein is a major precursor, while lignin is essentially purely carbohydrate, which is what you would expect from collulose-like matter. Hmm... I wonder how common these patterns are among the compounds. – ClockworkSoul 01:55, 5 August 2009 (UTC)
- ok Lignin is not pure carbohydrate at all (it's not a cellulose)- carbohydrate has not bezene rings/polyphenols (there are good structures of carbohydrates at cellulose)
- As for the N's and S's in coal - I would guess that they do derive from proteins - but possibly by decomposition (eg anaerobic bacteria action to give H2S, or NH3), though it could equally be by direct interaction of proteins and other plant matter. This article suggest that both methods may contribute [1] - (though don't take that as fact)
- There's a mention of the origins and types of sulphur in coal in this thesis http://witsetd.wits.ac.za:8080/dspace/bitstream/123456789/7060/4/EL%20Koper%20PhD%20(c)%202009%20-%2003%20Background%20and%20reviews.pdf (which I can't find a title for but seems to have been writen by a EL Koper) There probably are better descriptions on the web if you look, maybe in google books.83.100.250.79 (talk) 13:05, 5 August 2009 (UTC)
- I'm no sure the disulfide linkages in coal mean anything about it coming from "protein". Given the sort of chemical changes that occur between the living tissue and the fossil fuels, there really should not be any recognizable biomolecules there, and any "evidence" of such biomolecules should merely be the chemical equivalent of "convergent evolution". If there is sulfur and carbon present in the mix, then disulfide linkages are likely to form regardless of the source of the sulfur and carbon. I would say that it is impossible to consider that the disulfide linkages found in protein (via cystein) would remain unchanged through the harsh conditions that created coal from decayed biological material. --Jayron32 02:56, 6 August 2009 (UTC)
- I do see an amazing resemblance between chlorophyll and whatever residue was found (mentioned in the petroleum formation article). John Riemann Soong (talk) 03:21, 6 August 2009 (UTC)
"ok Lignin is not pure carbohydrate at all (it's not a cellulose)- carbohydrate has not bezene rings/polyphenols (there are good structures of carbohydrates at cellulose)" -- Benzene rings, polyphenols and other aromatic-type compounds in coal (lignite, whatever have you) form by thermal dehydration / dehydrogenation of carbohydrate units in lignin and cellulose (that's pretty obvious, when you think about it). FWiW 98.234.126.251 (talk) 06:02, 6 August 2009 (UTC)
- Lignin is a compound in wood - it's formed in the living tree, and already contains the phenols whilst it is still alive. (see that article)83.100.250.79 (talk) 14:20, 6 August 2009 (UTC)
- Yeah, I know that, what I'm saying is that cellulose is also subject to thermal dehydration/dehydrogenation to form polyphenolic and polyaromatic compounds. (In any case, wood contains more cellulose than lignin.) 98.234.126.251 (talk) 23:04, 6 August 2009 (UTC)
Interstellar travel
In the far future, when travel to distant stars is possible or even common, will it be easier/faster to travel along the comparatively crowded arms of the galaxy or along the emptier spaces in between?
88.108.8.64 (talk) 13:15, 4 August 2009 (UTC)
- Maintaining a constant speed while travelling from one location to another requires no energy if no other forces act upon a body, so it should be equally easy for both (though travelleing intergalactically would take considerably longer). However, the common theme in sci-fi is that to travel faster than light you need to maintain something (a warp field, a subspace bubble, etc) which is a constant drain on energy. So going somewhere closer would be easier. I'm not sure exactly how crowded the arms of our galaxy are, but if I recall correctly, you could travel in a straight line to a nearby star without expecting to hit anything along the way.
- But all of that is kind of irrelevant: since we don't know how to travel faster than light, we can't say how difficult it is. Vimescarrot (talk) 13:22, 4 August 2009 (UTC)
- Assuming real-world (non warp magic) physics, which way you'd go depends greatly on the technology of your spaceship and its drive system. The advantage of the space between arms is that there's a bit less debris - once you're travelling at relativistic velocities then impacting even a modest sized particle can be damaging, forcing you to have quite a robustly constructed spaceship (this assuming that there really is less matter between the arms than in them). The advantage of being in the arm is that if you rely on there being free matter (e.g. if your ship is propelled by something like a Bussard ramjet) then you'll have more to chew on in the arms. But really we're so far from being able to to anything like this, that the engineering details are really anybody's guess (it'd be like arguing with Da Vinci about whether his hang-glider thing would be better than his helicopter thing; you don't know until you build one). -- Finlay McWalter • Talk 13:48, 4 August 2009 (UTC)
- How much variation is there in density between the arms and the 'gaps' (in the plane, at a given distance from the core)? I've gotten the impression that the bright 'arms' are merely where a pressure wave passed recently, creating young bright stars. —Tamfang (talk) 02:09, 11 August 2009 (UTC)
I was thinking more in terms of the effect of gravity, similar to how current space probes and such like are sent close to certain planets on their way somewhere else, helping them go faster. Though I suppose not crashing into things would also be useful. 88.108.8.64 (talk) 14:09, 4 August 2009 (UTC)
- The stars are probably too far apart for you to gravity assist in a useful fashion. A gravity assist will not get you faster then light, and if you have to travel 5 years to get to the star to do the gravity assist that will increase your speed by 10%, is that really worth it? Googlemeister (talk) 14:23, 4 August 2009 (UTC)
- In that case you might like to read gravity assist and Interplanetary Transport Network; but those only describe intra-solar system travel, and even then taking a decade to get anywhere. It'd take millennia to move between stars by this mechanism. -- Finlay McWalter • Talk 14:24, 4 August 2009 (UTC)
- I just want to point out, since it is assumed but not addressed directly in the comments above, that most people here have been answering in a way that assumes faster-than-light is probably the only way to do this efficiently. There are slower-than-light approaches but they require a ship that takes decades and decades if not centuries to get from point A to point B (which, even if it really is possible for humans to do that—which I'm not convinced—cannot certainly be a "common" activity). This is because the vastness of the universe is, well, VAST. The distances are HUGE. If we cannot travel faster-than-light, there is little likelihood of any kind of Star Trek future for us. Even traveling at the speed of light is pretty slow on intergalactic scales, compared to the span of human lives (or, worse, the span of human attentions). --98.217.14.211 (talk) 15:10, 4 August 2009 (UTC)
- Relativity can (mostly) fix the timespan issue for the passengers, though. If you posit a spaceship that can withstand debris at relativistic speeds (a Bussard ramjet above is a good starting point), then you don't need a multi-generational ship even if Earthbound observers note a multi-generational journey. This still prohibits a Star Trek-type future, but not a humans-in-space future. Poul Anderson's Starfarers is a good treatment of the subject. — Lomn 15:25, 4 August 2009 (UTC)
- It depends how far you are going to travel, obviously, and whether you want to have any kind of communication with Earth (which is basically prohibited in any useful way). Even with relativity, I find it unlikely that you could get many humans to sign up for 20 years on a ship. It's not Star Trek; you're going to read all the books you have pretty quickly, have all the conversations you can have pretty quickly, and the stars are going to get a bit dull. Even a five year trip would be quite disruptive in the course of one's life, if nothing was happening on it (it would be one thing if you were traveling around the world, a different port every night... but in space it's a lot more monotonous). (As you may be able to tell, I am quite pessimistic about space travel without the possibility of FTL. I tend to think that those who push most strongly for it are just being escapist.) --98.217.14.211 (talk) 18:00, 4 August 2009 (UTC)
- Relativity can (mostly) fix the timespan issue for the passengers, though. If you posit a spaceship that can withstand debris at relativistic speeds (a Bussard ramjet above is a good starting point), then you don't need a multi-generational ship even if Earthbound observers note a multi-generational journey. This still prohibits a Star Trek-type future, but not a humans-in-space future. Poul Anderson's Starfarers is a good treatment of the subject. — Lomn 15:25, 4 August 2009 (UTC)
So no chance then of any particular route across the galaxy being much better for long distance transit? I was hoping for some sort of trans-galactic path with lots of starships running back and forth through it.88.108.8.64 (talk) 17:16, 4 August 2009 (UTC)
- Not without faster than light technology. If you have that, well, that changes things, but since we don't know what that truly would look like (since there is not the slightest indication that it is possible), it's hard to say. --98.217.14.211 (talk) 18:00, 4 August 2009 (UTC)
- Certain routes may well turn out to be better than others, but I can't see lots of starships covering interstellar distances without faster-than-light travel. You don't start journeys that are going to last years very frequently. I would be surprised if craft passed other craft more than a handful of times during their journey. --Tango (talk) 20:57, 4 August 2009 (UTC)
- I've talked about this many times before - so I'll keep the explanation short. There IS a way...at least in theory. You have to transfer your brain into a computer...build a computer that can replicate in great detail the precise functioning of every neuron every chemical pathway. This concept doesn't violate any fundamental laws - and many people believe it will be possible in the not too distant future. You arrange that your entire psyche - everything that makes you be "you" is in the machine - and then you destroy your physical body. OK - now you can put your brain into a computer on board a very slow spaceship - and adjust the clock rate of the computer such that the computer program that is your brain runs very slowly (You could install Windows Vista, for example!)...you will be "thinking" very slowly. For you, inside the computer, time can now be speeded up and (to some degree) slowed down at will. So - off you go on your million year (thousand lightyear) trip - and it seems to you like it only took half an hour maybe...if you see something interesting along the way - you can temporarily speed up the clock on your computer...spend as long as you want observing whatever it is...and then slow the clock down again to 'fast forwards' over the boring parts. So long as we can make spacecraft that are reliable enough - speed is a relatively insignificant barrier for "humans" (gotta use the quotes to keep everyone happy!) to colonise the entire galaxy. When you get where you're going, you put your brain/computer into a realistic humaniod robot and you can carry on with life more or less as usual. Of course, for very long trips, you have the problem that the place you saw through the telescope (which is already a very out of date view) will have changed considerably by the time you get there. But it's not an impossible prospect. If the spacecraft you're using is unreliable - you can send out a spacecraft with an 'empty' computer. When it gets where it's going, it can send you a speed-of-light radio message to say it's OK - then you can send your brain software as a digital data stream back to the computer. In effect, you can travel at the speed of light...so long as there is a suitable computer at the other end. But for you, it would seem just like instantaneous teleportation. Your robotic self climbs into the booth - you dial up some far distant star that humanoid robots have already been to - you push the button and in literally zero time (for YOU), you can step out in an identical robotic body at the other end. Of course the actual elapsed time would be vast...but maybe you don't care about that. SteveBaker (talk) 22:26, 4 August 2009 (UTC)
- Rather than putting your consciousness in a computer, you could put your body in stasis for the duration of the journey. Quantumelfmage (talk) 21:15, 10 August 2009 (UTC)
- You don't really need any special computer technology to do this, as Lomn pointed out. Travelling in a ship at very near light speed, time goes by much slower for the passengers than for an observer on Earth. For example if you had a ship that could accelerate to 0.9999995c relative to its initial frame in a relatively short time period, you could travel 1000 light years from Earth and only experience 1 year passing. Of course if you made the return trip, everyone you knew on Earth would be long dead. Rckrone (talk) 00:45, 5 August 2009 (UTC)
- While there are no theoretical problems with near-light speed travel, there are several engineering problems that we are nowhere near solving (some means of propulsion and a way of surviving hitting interstellar dust particles at such high speeds being the main ones). Steve's idea may be more achievable. We have ever improving computer systems, it probably won't be long before they are powerful enough to simulate the human brain, we just need a better understanding of how the human brain works. Personally, I think we should just be content with travelling to nearby stars, where less exotic means of transport would suffice (travelling at 0.1c there are several stars within a lifetime's travel of us and at those speeds relativistic effects aren't too serious - you need to account for them in your calculations, but you don't need to worry too much). --Tango (talk) 01:07, 5 August 2009 (UTC)
- But, but...but...I want my instantaneous (to me) interstellar transporter! I want to teleport to some planet orbitting Proxima Centauri - spend a few days looking at the sights - then teleport back again. Sadly, I'll need 8 years off work in order to do it...but it would certainly be worth the trip! SteveBaker (talk) 01:20, 5 August 2009 (UTC)
- You're a computer graphics expert - make a virtual Proxima Centauri. What is the difference between a real you observing a virtual star and a virtual you observing a real star? They seem equally good to me, except the former is far easier and doesn't require 8 years off work. --Tango (talk) 02:02, 5 August 2009 (UTC)
- Do you take the red pill or the blue pill? SteveBaker (talk) 15:05, 5 August 2009 (UTC)
- You're a computer graphics expert - make a virtual Proxima Centauri. What is the difference between a real you observing a virtual star and a virtual you observing a real star? They seem equally good to me, except the former is far easier and doesn't require 8 years off work. --Tango (talk) 02:02, 5 August 2009 (UTC)
- But, but...but...I want my instantaneous (to me) interstellar transporter! I want to teleport to some planet orbitting Proxima Centauri - spend a few days looking at the sights - then teleport back again. Sadly, I'll need 8 years off work in order to do it...but it would certainly be worth the trip! SteveBaker (talk) 01:20, 5 August 2009 (UTC)
- While there are no theoretical problems with near-light speed travel, there are several engineering problems that we are nowhere near solving (some means of propulsion and a way of surviving hitting interstellar dust particles at such high speeds being the main ones). Steve's idea may be more achievable. We have ever improving computer systems, it probably won't be long before they are powerful enough to simulate the human brain, we just need a better understanding of how the human brain works. Personally, I think we should just be content with travelling to nearby stars, where less exotic means of transport would suffice (travelling at 0.1c there are several stars within a lifetime's travel of us and at those speeds relativistic effects aren't too serious - you need to account for them in your calculations, but you don't need to worry too much). --Tango (talk) 01:07, 5 August 2009 (UTC)
What's this about humans not wanting to sign up for 20 years on a ship? Guys, people in the medieval Age of Sail, or on the Silk Road had it far worse. Entire nomadic groups would take decades to move to one place to another. What's worse? Being imprisoned for 20 years on a galley. John Riemann Soong (talk) 07:55, 5 August 2009 (UTC)
- I don't think a nomadic group on the Silk Road is a very good comparison to interstellar travel. You could walk the entire silk road in about 1 year, and while it isn't a stroll through Paris, there is a whole lot more to see on the way then on a 20 year trip through space with nothing in it. Googlemeister (talk) 12:56, 5 August 2009 (UTC)
- Plus, with any kind of realistic technology, you can't even get out of the solar system in 20 years. People who signed up for 20 years on board ship would get shore leave every six months or so. The guys on the spaceship can't even look out of the window and see anything interesting. SteveBaker (talk) 15:05, 5 August 2009 (UTC)
- Plenty of realistic technologies can get out of the solar system in under 20 years. They don't exist yet, but they are perfectly realistic. Laser beam propulsion is probably the most likely to happen. I wouldn't be surprised if an unmanned flyby probe is sent to the Alpha Centauri system within my lifetime (it might not get there within my lifetime, though...). --Tango (talk) 20:45, 5 August 2009 (UTC)
- I sure wouldn't want to sign up on a laser-beam-propelled spaceship (or indeed on any spaceship without a self-contained propulsion system) -- there's always a serious risk that by the time you get to where you're going, the laser-beam apparatus would have been de-funded for financial / political reasons and you got no way to get back home again. 98.234.126.251 (talk) 02:24, 6 August 2009 (UTC)
- I think manned interstellar travel, at least at first, would be a one way trip anyway. While you can get to nearby stars in a lifetime at, say, 0.1c, you can't get back again (and if it is a generation ship you would probably be planning to establish a colony which could build its own beam emitter for any return journeys). More of an issue with beamed propulsion is deceleration at the other end. You can do that using a mirror to reflect the beam back at the front of your craft, but only if they turn the beam on at the right time. You could decelerate a little using a conventional solar sail (almost like parachuting into the new star system), but probably not from near-relativistic speeds. That's why the probe I think might be launched in my lifetime would be a flyby, it would be too difficult to slow it down so it could be captured. --Tango (talk) 02:51, 6 August 2009 (UTC)
- I sure wouldn't want to sign up on a laser-beam-propelled spaceship (or indeed on any spaceship without a self-contained propulsion system) -- there's always a serious risk that by the time you get to where you're going, the laser-beam apparatus would have been de-funded for financial / political reasons and you got no way to get back home again. 98.234.126.251 (talk) 02:24, 6 August 2009 (UTC)
- Plenty of realistic technologies can get out of the solar system in under 20 years. They don't exist yet, but they are perfectly realistic. Laser beam propulsion is probably the most likely to happen. I wouldn't be surprised if an unmanned flyby probe is sent to the Alpha Centauri system within my lifetime (it might not get there within my lifetime, though...). --Tango (talk) 20:45, 5 August 2009 (UTC)
- Plus, with any kind of realistic technology, you can't even get out of the solar system in 20 years. People who signed up for 20 years on board ship would get shore leave every six months or so. The guys on the spaceship can't even look out of the window and see anything interesting. SteveBaker (talk) 15:05, 5 August 2009 (UTC)
- The Migration Period lasted for centuries, and I don't mean to imply that permanent nomadism does not exist, but I doubt that anyone ever planned a definite route to take more than a few years. "We'll cross the Bering Strait, then our grandchildren will reach Vancouver Island, and their great-grandchildren will drink margaritas in Cancún" – nope. —Tamfang (talk) 02:09, 11 August 2009 (UTC)
This generation might not like to sign up .... but lifestyles can change, and culture can adapt. Would you really want to be a pioneer and own a log cabin out in the middle of nowhere, where you might not see town for another 30 years? Perhaps occasionally (as you're being flung forwards), you could get out of the ship and stretch (well, as long as you somehow maintained velocity with the ship). John Riemann Soong (talk) 02:30, 6 August 2009 (UTC)
- If I were planning a long interstellar voyage, especially if it were a generation ship, I would send about 3 ships at once. It would allow those on one ship to evacuate to the others in an emergency (perhaps not permanently, but for a couple of days while life support was repaired - what you do if it turns out to be irreparable, I'm not sure...). It would also help reduce the feeling of isolation since you wouldn't be alone in the interstellar void, you would be part of a formation and could travel between the ships. --Tango (talk) 02:51, 6 August 2009 (UTC)
- You mean, sort of like Columbus and his 3 caravels? 98.234.126.251 (talk) 03:18, 6 August 2009 (UTC)
- I've no idea. Not being American, I've never learnt much about Columbus. --Tango (talk) 04:00, 6 August 2009 (UTC)
- I'm curious -- can you perform gravity assists (not necessarily stars -- perhaps large Kupier Belt objects, etc.) for minor trajectory corrections for a fleet of ships of different masses while keeping them altogether? I know at a certain distance away from the object, their gravitational accelerations are all going to be the same, but their kinetic energies won't be. John Riemann Soong (talk) 05:21, 6 August 2009 (UTC)
- If their accelerations are the same, then they'll stay together. FWiW 98.234.126.251 (talk) 05:54, 6 August 2009 (UTC)
- You mean, sort of like Columbus and his 3 caravels? 98.234.126.251 (talk) 03:18, 6 August 2009 (UTC)
- "Would you really want to be a pioneer and own a log cabin out in the middle of nowhere, where you might not see town for another 30 years? Perhaps occasionally (as you're being flung forwards), you could get out of the ship and stretch (well, as long as you somehow maintained velocity with the ship)." -- That's not the issue that I was talking about. The problem specific to a laser-beam propelled ship is that it's dependent on an external laser installation inside the Solar System to propel it to its destination and back again (similar to, say, a cable car being dependent on a lineside winch to propel it forward). Now, it's a given that the journey will take many, many years, during which time the laser installation will have to be maintained by us Earthlings at considerable expense so it could generate the laser beam again to propel the ship back to the Solar System on its return journey. Well, during that time it's very likely that some cost-cutting politician will come along and say, "Why are we spending all that money maintaining that laser installation that ain't doing nothing? Let's cut it from the budget and save some cash" -- and if that happens, the astronauts on that spaceship will be stranded in some galaxy far far away with no way to get back home. As for "this generation might not like to sign up", etc., etc. -- well, for one thing I might sign up if they paid me enough for it, but ONLY IF THE SHIP GOT ITS OWN PROPULSION ENGINE ON BOARD!!! 98.234.126.251 (talk) 03:03, 6 August 2009 (UTC)
- I don't think John was replying to you. A reply is usually indented one tab more than what it is replying to, not indenting at all usually means it is a general reply to everything that has gone above or a completely new point. I've moved your reply to the bottom and undone the change you made to the indentation of my reply - people don't alter other people's comments and keep comments at the same level in chronological order. --Tango (talk) 04:00, 6 August 2009 (UTC)
- I thought he was. My mistake. (BTW, I added a quote from John's post that I was replying to, so's it's clear who's replying to whom.) 98.234.126.251 (talk) 04:31, 6 August 2009 (UTC)
- I don't think John was replying to you. A reply is usually indented one tab more than what it is replying to, not indenting at all usually means it is a general reply to everything that has gone above or a completely new point. I've moved your reply to the bottom and undone the change you made to the indentation of my reply - people don't alter other people's comments and keep comments at the same level in chronological order. --Tango (talk) 04:00, 6 August 2009 (UTC)
- For a ship going at a substantial fraction of 'c', the time dilation effects would mean that more time was passing on earth than on-board the ship. But even without that - over a period of decades, there are wars, natural disasters, all kinds of things that could stop that laser from working. But in any case, to be practical, the laser would have to be set up in it's own solar orbit with gigantic solar panels providing the power source for it. We're not talking about a small laser here. To transmit enough power to push a spacecraft along at a reasonable acceleration, we're talking about a laser that would look MUCH brighter than the sun at the distance of the earth's orbit with a beam diameter of a couple of kilometers to cover the large solar sail that the spacecraft would need in order to be able to keep it cool enough. Out in interstellar space, this thing is by far the brightest thing in the sky even when you're lightyears away from it. The laser would have to be pretty reliable because going out to fix it would be costly - and focussing it would require lenses out at the orbit of Mars or so because even lasers diverge by a tiny amount - and over lightyears, that dilutes it's power to a massive extent. Aiming the thing would be an interesting trick too...if the spacecraft ever somehow drifted out of the beam, it might have an impossible task finding it again and given the years it would take a radio message to return to earth to ask what happened to the laser...the mission would be in DEEP touble if that ever happened. SteveBaker (talk) 13:43, 6 August 2009 (UTC)
- Indeed, using a laser here to decelerate a craft to be captured by another star or to accelerate it back towards Earth would be very difficult. For a flyby it's relatively easy, though, there is no need to keep beaming when it is lightyears away. Just propelling it for the first couple of years would probably be enough. Some back of the edit form calculations: To accelerate a 1 tonne probe to 0.1c requires (ignoring relativity) 4.5x1017J. If we do that over 2 years that requires a power of 7GW. At Earth's distance from the Sun (I would put the laser at Sun-Earth L4 or L5) solar power generates 1.4kW/m2. Therefore we need solar panels 5 km2 in area. That's big, but it's not impossible. --Tango (talk) 22:29, 6 August 2009 (UTC)
- That seems to be assuming that the energy of an absorbed or reflected photon all turns into kinetic energy of the probe. I don't think you get within orders of magnitude of that. If you reflect the photon, most of its energy is carried away in the reflection; if you absorb it, it mostly just heats up the probe. Try doing it with momentum instead of energy. --Trovatore (talk) 22:40, 6 August 2009 (UTC)
- The scarce resource is energy. Momentum is just a technicality based on the details of the design, which I don't have. It is also a more difficult calculation - the momentum transfer is going to vary over time as the relative velocities change, with energy you can just compare the initial state and the final state. I was assuming 100% efficiency, which is obviously unrealistic, but I was hoping it would get the right order of magnitude. I'll go and look it up... --Tango (talk) 23:37, 6 August 2009 (UTC)
- You could use more of the energy if, say, you used it to power an ion drive, accelerating a propellant backwards at high velocity. The fraction of it you're going to get when you just bounce the photon backwards is tiny. 100% is not just unrealistic, it's wrong by orders of magnitude.
- You might get a bit more of the energy once the craft is already moving at relativistic speeds. Then the photon is redshifted when you reflect it. Maybe you get to keep some of the difference between the incoming and outgoing photon; not really sure about that. This is a billiard-ball physics question, but it has to be done relativistically, which obviously makes it a bit more difficult. --Trovatore (talk) 23:42, 6 August 2009 (UTC)
- Using the beam purely for power is an option, but it means you have to carry whatever you are going to use as exhaust. Even with the kind of exhaust velocities you get with an ion drive that's still a massive inefficiency on the scale we're talking about. If the photon being redshifted helped you could just start with a longer wavelength laser. I'm talking about speeds of around 0.1c, relativity isn't a big concern. If we only want orders of magnitude, Newtonian physics will suffice. --Tango (talk) 23:58, 6 August 2009 (UTC)
- I sense that you're still missing the point, which is a pretty simple point and you're going to groan once you see it. The energy analysis that you've done is completely bogus. When you have a mirror sitting in space, and a photon of energy E hits it, does the mirror's kinetic energy increase by anything remotely close to E. No. The reflected photon has energy almost exactly E, and that's where almost all the energy goes.
- Alternatively, you can absorb the photon, but in that case you get even less impulse, which should be obvious -- the photon's incoming momentum was E/c; if you reflect it, the impulse you get is the difference between that and the outgoing momentum −E/c, namely 2E/c. Whereas if you just eat the photon, the impulse you get is only E/c. So absorbing the photon is only half as good as reflecting it. --Trovatore (talk) 00:33, 7 August 2009 (UTC)
- Yeah, I got that point after your last message. I've been trying to come up with a loophole to get me out of it. It all comes down to wavelengths - the reflected photon will be slightly redshifted and that is where the energy comes from. What determines how much it gets redshifted by? If you could redshift it enough you could get a large proportion of the energy. As we said, it is collisions of billiard balls. If we assume perfect reflection with no absorption then it is a perfectly elastic collision. If I can remember my A-level mechanics lessons [snip]. Ok. I can't remember by A-level mechanics lessons! I've just spent ages on it and can't get a useful answer. It seems, though, that you get a greater proportion of the energy if you use low energy photons, but I don't think the limit is 100% efficiency and I can't work out what it actually is (it seems to be dependant on the mass of the spacecraft, although I'm not sure in what way). Could someone else have a go at the maths? Also, can someone explain how the hell I just got a First Class MMath degree when I apparently can't do maths? Thanks! --Tango (talk) 03:12, 7 August 2009 (UTC)
- Let's see. Using momentum instead of energy, 2E/c=mv, and plugging in the numbers gives 4.5*10^18 J: exactly 10 times the amount that Tango calculated. As for the redshift, a ship moving at 0.1c sees the laser photons coming in at approx. E(1-v/c); it reflects the photons, and Earth sees the reflected photons at E(1-v/c)^2. For v=0.1c, and considering the other uncertainties involved, we can simply forget about the redshifts and treat the photons as if they were reflecting off a stationary mirror. --Bowlhover (talk) 22:31, 7 August 2009 (UTC)
- No, we can't. You can't assume the thing you are propelling is stationary, that's never going to work! There must be a flaw in your calculation somewhere - since the craft starts off stationary that would mean it gets zero energy per photon, it would never go anywhere. --Tango (talk) 00:22, 8 August 2009 (UTC)
- The redshift can't be ignored for accurate computation, not even if you want a second significant figure, but for order-of-magnitude analysis (using momentum rather than energy) it can.
- It's true to first order that the kinetic energy is not increasing at the start of the trip. K.E. is (1/2)mv^2, so the first derivative is mv(dv/dt), that is mva, where a is the acceleration. The acceleration actually diminishes as the craft speeds up, because it's reflecting redshifted photons which don't carry as much momentum -- but the rate of increase of K.E. goes up. --Trovatore (talk) 00:30, 8 August 2009 (UTC)
- True, but I wasn't trying to determine the acceleration, I was trying to determine the efficiency. That is, of the energy that goes into sending the laser how much is used to accelerate the craft. That is all down to redshift. --Tango (talk) 17:24, 8 August 2009 (UTC)
- No, we can't. You can't assume the thing you are propelling is stationary, that's never going to work! There must be a flaw in your calculation somewhere - since the craft starts off stationary that would mean it gets zero energy per photon, it would never go anywhere. --Tango (talk) 00:22, 8 August 2009 (UTC)
- Let's see. Using momentum instead of energy, 2E/c=mv, and plugging in the numbers gives 4.5*10^18 J: exactly 10 times the amount that Tango calculated. As for the redshift, a ship moving at 0.1c sees the laser photons coming in at approx. E(1-v/c); it reflects the photons, and Earth sees the reflected photons at E(1-v/c)^2. For v=0.1c, and considering the other uncertainties involved, we can simply forget about the redshifts and treat the photons as if they were reflecting off a stationary mirror. --Bowlhover (talk) 22:31, 7 August 2009 (UTC)
- Yeah, I got that point after your last message. I've been trying to come up with a loophole to get me out of it. It all comes down to wavelengths - the reflected photon will be slightly redshifted and that is where the energy comes from. What determines how much it gets redshifted by? If you could redshift it enough you could get a large proportion of the energy. As we said, it is collisions of billiard balls. If we assume perfect reflection with no absorption then it is a perfectly elastic collision. If I can remember my A-level mechanics lessons [snip]. Ok. I can't remember by A-level mechanics lessons! I've just spent ages on it and can't get a useful answer. It seems, though, that you get a greater proportion of the energy if you use low energy photons, but I don't think the limit is 100% efficiency and I can't work out what it actually is (it seems to be dependant on the mass of the spacecraft, although I'm not sure in what way). Could someone else have a go at the maths? Also, can someone explain how the hell I just got a First Class MMath degree when I apparently can't do maths? Thanks! --Tango (talk) 03:12, 7 August 2009 (UTC)
- Using the beam purely for power is an option, but it means you have to carry whatever you are going to use as exhaust. Even with the kind of exhaust velocities you get with an ion drive that's still a massive inefficiency on the scale we're talking about. If the photon being redshifted helped you could just start with a longer wavelength laser. I'm talking about speeds of around 0.1c, relativity isn't a big concern. If we only want orders of magnitude, Newtonian physics will suffice. --Tango (talk) 23:58, 6 August 2009 (UTC)
- The scarce resource is energy. Momentum is just a technicality based on the details of the design, which I don't have. It is also a more difficult calculation - the momentum transfer is going to vary over time as the relative velocities change, with energy you can just compare the initial state and the final state. I was assuming 100% efficiency, which is obviously unrealistic, but I was hoping it would get the right order of magnitude. I'll go and look it up... --Tango (talk) 23:37, 6 August 2009 (UTC)
- That seems to be assuming that the energy of an absorbed or reflected photon all turns into kinetic energy of the probe. I don't think you get within orders of magnitude of that. If you reflect the photon, most of its energy is carried away in the reflection; if you absorb it, it mostly just heats up the probe. Try doing it with momentum instead of energy. --Trovatore (talk) 22:40, 6 August 2009 (UTC)
- Indeed, using a laser here to decelerate a craft to be captured by another star or to accelerate it back towards Earth would be very difficult. For a flyby it's relatively easy, though, there is no need to keep beaming when it is lightyears away. Just propelling it for the first couple of years would probably be enough. Some back of the edit form calculations: To accelerate a 1 tonne probe to 0.1c requires (ignoring relativity) 4.5x1017J. If we do that over 2 years that requires a power of 7GW. At Earth's distance from the Sun (I would put the laser at Sun-Earth L4 or L5) solar power generates 1.4kW/m2. Therefore we need solar panels 5 km2 in area. That's big, but it's not impossible. --Tango (talk) 22:29, 6 August 2009 (UTC)
- For a ship going at a substantial fraction of 'c', the time dilation effects would mean that more time was passing on earth than on-board the ship. But even without that - over a period of decades, there are wars, natural disasters, all kinds of things that could stop that laser from working. But in any case, to be practical, the laser would have to be set up in it's own solar orbit with gigantic solar panels providing the power source for it. We're not talking about a small laser here. To transmit enough power to push a spacecraft along at a reasonable acceleration, we're talking about a laser that would look MUCH brighter than the sun at the distance of the earth's orbit with a beam diameter of a couple of kilometers to cover the large solar sail that the spacecraft would need in order to be able to keep it cool enough. Out in interstellar space, this thing is by far the brightest thing in the sky even when you're lightyears away from it. The laser would have to be pretty reliable because going out to fix it would be costly - and focussing it would require lenses out at the orbit of Mars or so because even lasers diverge by a tiny amount - and over lightyears, that dilutes it's power to a massive extent. Aiming the thing would be an interesting trick too...if the spacecraft ever somehow drifted out of the beam, it might have an impossible task finding it again and given the years it would take a radio message to return to earth to ask what happened to the laser...the mission would be in DEEP touble if that ever happened. SteveBaker (talk) 13:43, 6 August 2009 (UTC)
- It's interesting that Tango comes up with the number 4.5x1017J...that's almost exactly the number some OP quoted in a thread below for the energy produced by a decent-sized nuclear weapon. A 100 Megaton nuke (appropriately applied) would theoretically provide 4.2x1017J - enough energy to put that 1 ton capsule into an 0.1 c trajectory. 50 Megaton hydrogen bombs are certainly buildable with 1960's technology...perhaps the Project Orion approach is the right one after all? If we're talking about "Brain in a computer" transportation systems - or robotic probes of a more conventional kind...maybe that's a better way. SteveBaker (talk) 01:29, 7 August 2009 (UTC)
- I heard they actually had a project for a nuclear-powered starship, called Project Daedalus. But the part that I just don't get is, how do they keep the nuclear explosions from melting the starship? 98.234.126.251 (talk) 02:18, 7 August 2009 (UTC)
- Nuclear power plants don't melt (unless something goes wrong), so I it can it done. --Tango (talk) 03:12, 7 August 2009 (UTC)
- It's a possible idea. You do, however, want to accelerate the ship without blowing it up. You would need lots of small nukes, not one big one. --Tango (talk) 03:12, 7 August 2009 (UTC)
- Wait - everyone is getting confused. Project Orion was the seemingly crazy idea to build a spacecraft with a gigantic, heavy "pusher plate" at the back, then a bunch of huge shock absorbers and springs - and the payload/crew-quarters at the front. The idea was to toss small nuclear bombs out of the back of the machine and to explode them against the pusher plate. Project Daedalus was to use a continuous controlled fusion reaction - akin to the ideas use in experimental fusion power generators. Daedalus is technologically exceedingly difficult - we know that controlling and confining fusion reactions is really difficult. Orion seems absolutely crazy...totally insane...but when you crunch the numbers, it's quite do-able, even with present day technology. Well...do-able, IF you had the launch facility to get all of the thousands of tons of material needed for the pusher plate and shock absorbers up into earth orbit - and the political will to violate every nuclear and orbital weapons treaty ever to be signed!! Realistically, you'd have to construct it from lunar materials out in orbit around the moon because that's the only reasonable way to get all of that tonnage of 'stuff' up there. Orion-style spacecraft are much beloved by science fiction writers simply because they just about the only hope of reaching a significant fraction of 'c' with believable technology. SteveBaker (talk) 18:31, 7 August 2009 (UTC)
- It might be better to build it out in the asteroid belt. Lots of resources out there and not even the Moon's gravity to contend with. Of course, a space elevator would make it all much easier. --Tango (talk) 19:27, 7 August 2009 (UTC)
- I heard they actually had a project for a nuclear-powered starship, called Project Daedalus. But the part that I just don't get is, how do they keep the nuclear explosions from melting the starship? 98.234.126.251 (talk) 02:18, 7 August 2009 (UTC)
I'll plug the book Interstellar Migration and the Human Experience, edited by Ben R. Finney & Eric M. Jones (U.Calif.Press 1985). —Tamfang (talk) 02:09, 11 August 2009 (UTC)
Birch tree with golden bark??
There is a tree groing at my workplace that I cannot identify for the life of me. It is definitvely not native to where I live and is intriguing to say the least. It cunningly resembles a birch tree and has a similar peeling type of bark. However, the bark isn't with or gray, it's golden-brown. The leafs also do not seem to be that of a birch. Anyone know what it could be? I'm going to try to attach photos later on too. It has been bugging me all summer long!! Veronika Stolbikova (talk) 15:53, 4 August 2009 (UTC)
- Some eucalyptus trees shed bark, might be one of those. Is the tree indoors, or outside, and where on earth is it if it is outside? Googlemeister (talk) 16:03, 4 August 2009 (UTC)
- The bark of different birch trees can vary in color. The Yellow Birch has yellow-bronze bark. The bark of the Alaska Birch ranges in color "from pure white to red, yellowish, pinkish, or gray". -- 128.104.112.100 (talk) 16:52, 4 August 2009 (UTC)
- Pacific Madrone is what comes to mind for me. --jpgordon::==( o ) 23:33, 4 August 2009 (UTC)
why do some nuts sqeak as you eat them?
why do some nuts sqeak as you eat them? thanks. —Preceding unsigned comment added by 82.234.207.120 (talk) 16:05, 4 August 2009 (UTC)
- I haven't actually noticed this myself. I imagine it is just parts of the nuts rubbing against each other, or your teeth, and the "squeak" has just something to do with the surface of the nut. --98.217.14.211 (talk) 23:12, 4 August 2009 (UTC)
- It's horrible. SGGH ping! 11:56, 6 August 2009 (UTC)
In space
I see often in science fiction shows that people are exposed to space for maybe 5 seconds or so without spacesuits. In reality, how long would a person stay alive when exposed to space, and what would the effects be of a 5 second exposure (assuming they're "beamed up" to their spaceship almost immediately after exposure)? —Preceding unsigned comment added by 82.43.91.27 (talk) 16:05, 4 August 2009 (UTC)
- See Human adaptation to space. DMacks (talk) 16:13, 4 August 2009 (UTC)
- Also Space exposure. Googlemeister (talk) 16:14, 4 August 2009 (UTC)
- Survival might be possible. Damage to ears or lungs is possible. Unconsciousness would occur after a few seconds. Edison (talk) 03:01, 5 August 2009 (UTC)
- Not to mention decompression sickness... 98.234.126.251 (talk) 03:37, 5 August 2009 (UTC)
- Survival might be possible. Damage to ears or lungs is possible. Unconsciousness would occur after a few seconds. Edison (talk) 03:01, 5 August 2009 (UTC)
- Also Space exposure. Googlemeister (talk) 16:14, 4 August 2009 (UTC)
independent variable in x axis
hi friends,
in my basics i've learned that independent variable is marked in x axis and the dependent variable in y axis.in refrigeration i learned about T-s and P-H plots where entropy(S) and enthalpy(H) are in x axis.kindly explain me how it is made. SCI-hunter (talk) —Preceding undated comment added 16:32, 4 August 2009 (UTC).
- I’m not sure I understand what the confusion is. The convention when plotting a function is to show the dependant variable vertically, and the independent variable horizontally. A function y=f(x), where y is a function of x, is plotted with y (the dependant variable) vertically, and x (the independent variable) horizontally. In a T-s plot, T is a function of s, so T (the dependant variable) is vertical, and s (the independent variable) is horizontal. In a p-h plot, p is a function of h, so p (the dependant variable) is vertical, and h (the independent variable) is horizontal. Does that help at all? Red Act (talk) 18:05, 4 August 2009 (UTC)
- The above explanation is not exactly correct. Even in the simplest thermodynamic applications there will be functions of more than one variable. For instance, the ideal gas law PV=nRT can be used to obtain a relationship bewtween pressure, temperature and density for some gas, therefore one of them can be taken as a function of the other two. That means that there isn't a unique relationship between temperature and entropy that could be used to plot the temperature as a function of the entropy. More information needs to be specifyed. You could, for instance, specify what kind of thermodynamic process is being used. It could be isothermic, isobaric, isocoric, adiabatic, some other kind, or a combination of these. A carnot cycle, for instance, is given as a sequence of four different thermodynamic processes (Isothermic expansion, Adiabatic expansion, Isotermic contraction, Adiabatic contraction) which show up on a T-s plot as a rectangle, not as a function. Dauto (talk) 19:44, 4 August 2009 (UTC)
- Ah. I had looked at Fig. 25.10 here, and the T-s plot and p-h plot look very much like the plots of two functions. However, reading further, I see that those two plots are actually phase diagrams, which are a different beast. The 2D phase diagrams section of that article might be a helpful read. Red Act (talk) 20:06, 4 August 2009 (UTC)
thanks a lot for the discussions on above topic.let me clear my doubt is that how could entropy become the independent variable(in case of T-S plot).we never change entropy for a system.what we usually do is to vary the temperature of a system and that causes a change in entropy of the system. SCI-hunter (talk) —Preceding undated comment added 22:34, 4 August 2009 (UTC).
- No, what you do is transfer/take heat or compress/expand the system and, that way, change both the entropy and the temperature. The distinction between dependent and independent variables is often quite arbitrary since many of the functions that show up in practice can be inverted. In the T-s diagram, though, that doesn't really matter since you are not actually plotting a function. As I said, what you are doing is plotting the path taken by the system through the T-s space as some kind of thermodynamic process is being performed. Dauto (talk) 23:13, 4 August 2009 (UTC)
- Keep in mind that x-independent/y-dependent convention is only that - a convention. There's no reason to have it that way, except that that's what everyone always does, and anyone looking at your graph will initially expect that's the way it's set up. As mentioned, there isn't a clear dependent/independent distinction in thermodynamics, so the first researchers set up the graphs the way that they thought looked best or made the most sense in context. (For example, the plots could have been made by a theoretician who thinks of the entropy as the core concept, and the measured temperature is simply a derived result of the intrinsic entropy of the system.) Others repeated that same sort of display in other contexts, and that then became the convention used for those graphs. Now everyone sets up T-S and P-H plots that way because that's how researchers expect the plots to be set up. By doing so their not necessarily claiming that one variable is dependent and one is independent, they're just showing the relation between the two in the conventional fashion. -- 128.104.112.100 (talk) 17:37, 5 August 2009 (UTC)
Moldy Bones
What would be the best way to wash this green, smelly stuff that I think might be a kind of mold off of some (real, animal) bones (which I think are for a comparitive collection)? I tried scrubbing with a wet toothbrush (per boss' suggestion), but that didn't really work. 138.192.58.227 (talk) 17:04, 4 August 2009 (UTC)
- Like all good bosses would say "try scrubbing harder"
- Alternatively why not search for "bone cleaning" - I think you need to get all the organic matter out of the bone to stop it going green/smelling.83.100.250.79 (talk) 18:13, 4 August 2009 (UTC)
- You could try bleaching it with hydrogen peroxide.CalamusFortis 18:32, 4 August 2009 (UTC)
- It still needs cleaning (degreasing) first. 83.100.250.79 (talk) 19:33, 4 August 2009 (UTC)
- What about boiling the bones in water? That usually removes almost everything from them. // BL \\ (talk) 22:17, 4 August 2009 (UTC)
- Somewhere I read about preserving bone for bone handled knives. You have to remove all the marrow. Prolonged soaking in ammonia solution can remove the fat and protein. Caustic soda in water could do this dissolving too but is more hazardous and needs more cleanup, it will leave the mineral component behind. Graeme Bartlett (talk) 22:48, 4 August 2009 (UTC)
- On prolonged boiling I would imagine caustic soda to attack the bone (it would), though it is good at defattting. Has anyone tried using washing powder - maybe a biological washing powder?83.100.250.79 (talk) 22:57, 4 August 2009 (UTC)
- Sodium carbonate (washing soda) would prob'ly work pretty good. FWiW 98.234.126.251 (talk) 01:45, 5 August 2009 (UTC)
- Per National Public Radio (U.S), museums use maggots to clean bones of small animals. Books on 19th century medicine say that a doctor might take human bones and put them in a wire mesh cage submerges in a pond for a year, so the small fish etc would clean them. Edison (talk) 03:00, 5 August 2009 (UTC)
- A classic way to get clean bones is to use ants. At least one friend of mine has, on several occasions, taken a small dead animal and buried it in an ant hill for a couple of weeks or so. They pick the skeleton clean. This time-lapse video of ants eating a dead gecko demonstrates this ability fairly well. -- Captain Disdain (talk) 10:16, 5 August 2009 (UTC)
- Most natural history museums use dermestid beetles to clean bone samples. They do an extremely thorough job, but controlling the process can be tricky simply because of how thorough they are. You likely wouldn't want them chewing your carpet away, for example. I don't know how readily ants or maggots would eat mold; I don't know if the dermestids even do that, but that's where I'd put my money. Matt Deres (talk) 16:22, 5 August 2009 (UTC)
- The ants in the video not only clean the bones, but the disarticulate the skeleton and carry away some of the bones. The gecko is left in a Napoleon condition. ("bone-apart") Edison (talk) 17:18, 5 August 2009 (UTC)
- Per National Public Radio (U.S), museums use maggots to clean bones of small animals. Books on 19th century medicine say that a doctor might take human bones and put them in a wire mesh cage submerges in a pond for a year, so the small fish etc would clean them. Edison (talk) 03:00, 5 August 2009 (UTC)
- Sodium carbonate (washing soda) would prob'ly work pretty good. FWiW 98.234.126.251 (talk) 01:45, 5 August 2009 (UTC)
- On prolonged boiling I would imagine caustic soda to attack the bone (it would), though it is good at defattting. Has anyone tried using washing powder - maybe a biological washing powder?83.100.250.79 (talk) 22:57, 4 August 2009 (UTC)
- Somewhere I read about preserving bone for bone handled knives. You have to remove all the marrow. Prolonged soaking in ammonia solution can remove the fat and protein. Caustic soda in water could do this dissolving too but is more hazardous and needs more cleanup, it will leave the mineral component behind. Graeme Bartlett (talk) 22:48, 4 August 2009 (UTC)
- What about boiling the bones in water? That usually removes almost everything from them. // BL \\ (talk) 22:17, 4 August 2009 (UTC)
- It still needs cleaning (degreasing) first. 83.100.250.79 (talk) 19:33, 4 August 2009 (UTC)
- You could try bleaching it with hydrogen peroxide.CalamusFortis 18:32, 4 August 2009 (UTC)
electrical grounding
My son has just asked a good question (at least I thought so, with my limited scientific knowledge). I explained to him why birds don't get electrocuted while standing on an electrical wire - because the bird is not grounded and does not complete an electrical circuit. So he asked - what happens if we touch a live (exposed) wire inside an aeroplane - are we 'grounded' (earthed) or not - will we get electrocuted? Hmm! Sandman30s (talk) 19:28, 4 August 2009 (UTC)
- It's all about forming a circuit, rather than grounding per se - if a (very) big bird managed to put one foot on one overhead wire, and another foot on another wire then it would get zzzapped.
- The same applies in a plane or anywhere - if you make a circuit you get 'it'.
- However if you touch a live wire which has the 'ground' connection also connected to the ground (ie the soil) the you can form the electrical circuit without touching two wires - the circuit being through the wire, through you, and the through the earth (soil) to the grounded connection.83.100.250.79 (talk) 19:37, 4 August 2009 (UTC)
- I think the key concept here is that electrical grounding isn't "are you touching the ground?" but "are you touching something at a different voltage?" (still an approximation). Birds are OK not because they're sitting up high but because they don't provide an interesting electrical path. Even assuming an uninsulated power line, electricity will continue to flow through a short highly-conductive wire than through a long poorly-conducting bird. Since the endpoints (and end voltages) are the same, there's no reason for electricity to flow through the bird. On an airplane, though, there's a complete electrical circuit. The power supplies in the airplane are at 28 volts or 400 volts or whatever they happen to be, relative to 0 volts as defined by the plane itself. If you're touching the plane (and you are, you're in a seat or in the aisle or what have you), you're grounded, and subject to shock. You may also be interested in a past discussion of birds here or at the Straight Dope. — Lomn 19:42, 4 August 2009 (UTC)
- Note also that the usual procedure for jump starting an automobile involves connecting the positive terminals of the batteries and then connecting the negative terminal of the charged battery to some metallic portion of the vehicle with the dead battery. This is called "connecting to ground" even though an automobile is actually insulated from the earth by its rubber tires. Deor (talk) 20:36, 4 August 2009 (UTC)
- Your explanation of why the bird doesn't get zapped isn't correct anyway - the correct answer is actually that the bird DOES get zapped - but not by enough to bother it. The electricity has two paths it can take - the short path down an inch or so of copper wire - or the longer path: up one leg of the bird, past it's "naughty bits" and back down the other leg. The amount of current that flows down each path is inversely proportional to the resistance. Since nice thick copper wire has very little resistance - and birds have (relatively) high resistance, a huge amount of current flows down the wire - and very little of it flows through the bird. BUT that amount isn't zero! The bird is indeed being very slightly electrocuted...but the current flow, even with a soaking wet bird on a very high voltage wire is so slight that it doesn't seem to harm them. SteveBaker (talk) 22:07, 4 August 2009 (UTC)
- Here's a question for you SteveBaker (without going to any reference materials!): If the line were suddenly cut so that one foot of the bird was on one side, the other foot on the other side, would the bird get electrocuted? 82.234.207.120 (talk) 23:49, 4 August 2009 (UTC)
- If cutting the wire caused the two sides of the copper wire to reach different voltages, then the bird would be zapped. The crucial bit is that voltage loss along a conducting wire is "negligibly small" - so when the bird stands with two feet only inches apart on the wire, there's a small voltage drop across the bird. As Steve and others mentioned, the bird has a high resistance, so the small voltage yields an even smaller current (V = IR, or Ohm's Law). Cutting the wire could cause the two parts of the wire to reach different voltages - one half is still attached to the power source, while the other is loaded to ground (via the electric delivery network - but it might take a few seconds to decay that energy out through the grid - it's hard to say in the purely hypothetical case). But if the bird continued to stand on the two wire parts, its feet are now at very different voltages - and it will get zapped. Nimur (talk) 00:32, 5 August 2009 (UTC)
- Yep. If the wire was cleanly cut - then the electricity still has two paths to choose from...through the bird - or through an inch of air. Air is a pretty good insulator - and birds are mostly salty water - which conducts electricity reasonably well (but not as well as copper wire) so the bird represents a lower resistance path than the air - so the current goes that way and the bird is undoubtedly zapped. If the process of cutting the wire allowed a spark to be produced between the ends of the wire, the air would be ionized - ionized air has a much lower resistance than regular air...so perhaps the the bird stands a better chance in that case...but I'm pretty sure the heat from the spark would still fry the poor thing. SteveBaker (talk) 01:09, 5 August 2009 (UTC)
- If cutting the wire caused the two sides of the copper wire to reach different voltages, then the bird would be zapped. The crucial bit is that voltage loss along a conducting wire is "negligibly small" - so when the bird stands with two feet only inches apart on the wire, there's a small voltage drop across the bird. As Steve and others mentioned, the bird has a high resistance, so the small voltage yields an even smaller current (V = IR, or Ohm's Law). Cutting the wire could cause the two parts of the wire to reach different voltages - one half is still attached to the power source, while the other is loaded to ground (via the electric delivery network - but it might take a few seconds to decay that energy out through the grid - it's hard to say in the purely hypothetical case). But if the bird continued to stand on the two wire parts, its feet are now at very different voltages - and it will get zapped. Nimur (talk) 00:32, 5 August 2009 (UTC)
- Here's a question for you SteveBaker (without going to any reference materials!): If the line were suddenly cut so that one foot of the bird was on one side, the other foot on the other side, would the bird get electrocuted? 82.234.207.120 (talk) 23:49, 4 August 2009 (UTC)
- Correct. Birds do get electrocuted by creating a complete circuit. Usually it is by touching one wing to a power source and another to ground. When I lived in the Mojave, the power poles were being redesigned because the birds there were getting zapped far too often. -- kainaw™ 00:14, 5 August 2009 (UTC)
- On power poles here, the lines are far enough apart so that birds' wings do not touch two wires. But occasionally a big bat will make that mistake, and be electocuted. Because their feet are designed to lock them upside down automatically when they are asleep, you can sometimes see electrocuted ones hanging on a wire, dead as a doornail. Bolshy birds like Currawongs will amuse themselves all day attacking this 'interloper' who should have been at home during the day. Myles325a (talk) 01:25, 5 August 2009 (UTC)
- If a power line is at a sufficiently high voltage, a bird touching it even with a single foot would get a painful and perhaps dangerous jolt, even without being grounded or without touching a wire at a different voltage. The bird acts as a capacitor, and alternating current can flow through a capacitor. Higher voltage means more current. Birds will sit on power lines of 4 thousand or maybe 12 thousand volts, but I have not seen one sitting on a 69 thousand or 138 thousand volt conductor. Edison (talk) 02:57, 5 August 2009 (UTC)
Thanks for all the answers. Steve, when I google 'bird on electric wire grounding', there are lots of answers that agree with my explanation about the bird needing to be grounded, although there are just as many that talk about the potential difference/voltage between its legs. Some argue that the grounding explanation is more correct. Surely there is some compromise between these different opinions? Sandman30s (talk) 07:59, 5 August 2009 (UTC)
- The potential difference between two spots an inch apart on a power line will be negligible. Take the full rated current of the conductor, multiply it by the resistance of one inch, and you have the voltage. A 4kv conductor might be bare #1/0 copper, with .105 ohms per thousand feet. One inch would be resistance of .00000875 ohm. At 300 amperes of load current, the voltage would be .0026 volts. At 1000 amps, which would overload the circuit, the voltage would still only be .009 volt across the birds feet 1 inch apart. The conductor would literally be too hot to stand on, and on its way to burning down, before it electrocuted the bird just due to the potential difference between two spots 1 inch apart. Edison (talk) 14:28, 5 August 2009 (UTC)
- The "grounding" explanation is an over-simplification - and that vague and fuzzy thinking explains why you were confused about touching wires inside airplanes. Suppose there were two power lines strung parallel to each other. One is at 10,000 volts and the other one isn't quite adjusted correctly so it's at 10,100 volts. If the bird puts one foot on each wire, it'll get a 100 volt jolt and die without ever being within 50 feet of "ground". It's a matter of "potential difference" and relative resistance. If the difference between the voltages at the bird's left and right foot are sufficiently different, it dies - if not, it doesn't - irrespective of this fuzzy concept of "ground". The potential difference between 10,000 volts and "ground" is 10,000 volts - so of course the bird gets zapped if it has one foot on the ground and the other on the wire. But if you had your electricity transmission lines made out of (say) plastic instead of copper - then the bird would get zapped just standing on the plastic wire because the resistance through its body and the resistance through the plastic would be almost the same - roughly half the current would travel through the bird and it would die.
- The concept of "ground" is mostly a notational matter for electrical engineers. When you talk about "grounding" a wire in a car for example - you're attaching it to the bodywork - which is typically attached to the negative side of the battery. But on some older british cars (like my '63 Mini), they opted for "Positive ground" - so the positive side of the battery is connected to the body and all of the electrical systems run on -12 volts supplied from the '-' terminal of the battery.
- However, when you step out of either kind of car so that your feet are "grounded" by touching the ground - you can still get a brief but painful 'zap' (a 'static shock') from touching the door handles because the "ground" of the car isn't the same voltage as the "ground" of the planet earth. There is really no such thing as an absolute zero of voltage to use as a reference. In a sense, the bird on the 10,000 volt wire isn't getting zapped because as far as it is concerned, "ground" is the wire it's standing on.
- In telecommunications and some computer applications, you have to talk about "signal ground" and "frame ground" and treat them separately because again, it's just a notational convenience. Check out (for example) RS232#Pinouts - where you'll see a "Common ground" and a "Protective ground". Note the comment there: "Use of a common ground is one weakness of RS-232: if the two devices are far enough apart or on separate power systems, the ground will degrade between them and communications will fail, which is a difficult condition to trace.". SteveBaker (talk) 14:55, 5 August 2009 (UTC)
- Further... If you've worked with high-end audio equipment, you know that ground is not ground. If you assume ground is ground and connect all your equipment to the closest ground, you will get a hum on your speakers. That is because ground in one area is not necessarily the same potential as ground in another area. You need to ensure that all your equipment is using the same ground to remove difference in potential. -- kainaw™ 15:12, 5 August 2009 (UTC)
- Grounding of communication lines or control wires is indeed a confusing subject. Sometimes the ground braids are only connected to earth ground at one end of the line, to avoid ground current travelling over the braid and having a "ground loop." Edison (talk) 17:14, 5 August 2009 (UTC)
- And in some power-transmission systems, the earth serves as the conductor that closes the circuit, see Single-wire earth return. For a real-world situation similar to the "one foot on each side of a cut wire"-situation mentioned above, see the end of the section Tram#Electric (trolley cars). --NorwegianBlue talk 18:23, 5 August 2009 (UTC)
- Speaking of trams... I thought that the engineer could lower the pantograph from the cab without having to jump out of the tram. Is that not so? 98.234.126.251 (talk) 04:56, 6 August 2009 (UTC)
- And in some power-transmission systems, the earth serves as the conductor that closes the circuit, see Single-wire earth return. For a real-world situation similar to the "one foot on each side of a cut wire"-situation mentioned above, see the end of the section Tram#Electric (trolley cars). --NorwegianBlue talk 18:23, 5 August 2009 (UTC)
COCONUT OIL
DOES IT CONTAIN OMEGA 3 OR 6 ? —Preceding unsigned comment added by 209.252.144.42 (talk) 21:55, 4 August 2009 (UTC)
- According to the analysis at Oleic acid it contains Omega-6 Linoleic acid and Omega-9 Oleic acid. 83.100.250.79 (talk) 22:07, 4 August 2009 (UTC)
- You can look it up in the USDA's nutrient database - there it says that it contains 5.8% monounsaturated fatty acids and all of this is 18:1 fatty acids (most likely all of that is oleic acid); and 1.8% polyunsaturated fatty acids and all of this is 18:2 (most likely linoleic acid) - so it seems likely that there is 1.8% omega-6 fatty acids and 0.0% omega-3 fatty acids. Icek (talk) 17:11, 9 August 2009 (UTC)
- I think it's an unhealthy kind of oil. Imagine Reason (talk) 21:41, 11 August 2009 (UTC)
August 5
Knowing Allsorts
I've just been watching the film 'Knowing'. Is it possible that a solar flare would burn up the earth to such an extent as detailed in the film. When I say possible, I am referring to the likelihood of it occuring, rather that the damage caused by such a large-scale event. --russ (talk) —Preceding undated comment added 00:18, 5 August 2009 (UTC).
- "Solar flare" is a pop-science term for a coronal mass ejection (it can also mean the less-severe, closed-loop solar prominence and some related phenomena). Gross quantities of solar ejecta are unlikely to ever reach Earth's orbital radius in any significant way. (Read: no giant flameballs will reach us). However, the density and flux of charged particles will increase; and an electromagnetic effect is common; these effects can significantly harm objects in space near Earth. It is very important to understand that solar wind is a charged plasma - as such, it is "deflected" (rather, trapped in cyclotron resonance) by Earth's magnetic field - and the result is the Van Allen belts. During periods of high solar activity, these regions increase in size, energy, and particle content. Also, it's worth noting that 2007-2009 has been "the quietest Solar Minimum ever"[2] - so if there were going to be a catastrophic solar flare (or even any medium-large ones), it would be really unlikely timing. Nimur (talk) 00:36, 5 August 2009 (UTC)
- I haven't seen that movie but there is a story called Inconstant Moon on a similar theme. 70.90.174.101 (talk) 07:32, 5 August 2009 (UTC)
- I also have not seen the movie, but the book Death from the Skies has a chapter on the actual threat posed by CMEs. I don't have it in front of me, but here's what I recall: the most likely result of a severe CME directed at Earth is rendering satellites inoperative. More severe CMEs could potentially cause widespread damage to the power grid. A particularly severe CME in 2003 was the most violent flare recorded in modern times: it is described here, but there's a good chance you had no idea that anything happened. CMEs are common, and obviously we get smacked by them from time to time. No CME will incinerate the world as in Inconstant Moon; that more properly describes the sun going nova (story claims notwithstanding). We have no reason to expect the sun to go nova. In about one billion years, the sun's energy output will have risen enough (10%) to render Earth uninhabitable; in about five billion years, it will become a red giant. See our article on the sun for details. — Lomn 13:13, 5 August 2009 (UTC)
- I've read that Earth will have frozen over in 100 million years due to the cooling of the radioactive core. Imagine Reason (talk) 20:47, 8 August 2009 (UTC)
- If earth will survive over white dwarf we don't know for sure. This all depends on how big sun will be. The latter model shows earth might be destroy by tidal force. Original models shows earth could escape to a highier orbit by sun's loss mass and gravity. A good way to state it is earth will be swallow up by sun about a 55% chance. Doesn't matter. Earth will end up been lifeless.--69.229.108.245 (talk) 23:31, 12 August 2009 (UTC)
Steel yourself for this question
When I was a youngster, my family watched too much TV, as evidenced by the fact that we even watched crap shows like That's Incredible! and Real People. Anyway, it was on one of those shows (or similar) that I saw a segment about this dude that could (briefly) touch molten steel with his bare hand. I believe he worked in a steel mill or something of the sort. Anyway, they showed him on camera, quickly flicking his fingers across the liquid metal, flinging globs of steel. I don't think it was a video trick (though I was very young at the time). Does anyone remember the name of that guy or how he did what he did? Firewalking works because ash is actually a poor conductor of heat, but the same is definitely not true of iron/steel! Matt Deres (talk) 00:24, 5 August 2009 (UTC)
- I've never seen it done with steel - but I've seen people stick their hands up to the wrists in molten lead - which is probably just as bad. The trick with molten lead is to have your hand be wet - as the water flashes to steam, it insulates your hand from the heat of the metal. It's still insanely hot - and you can't hold your hand there for more than a second or so...but if you know what you're doing it's possible. However, iron melts at 1,370 degC and lead at only 320 degC - that's a very different matter! So I'm frankly a little skeptical about the steel thing - but perhaps if your memory is imperfect, it could have been some lower melting point metal than steel. I certainly don't recommend experimenting! This is an incredibly dangerous thing to try. SteveBaker (talk) 01:01, 5 August 2009 (UTC)
- Is there a physical chemist in the room? Does liquid metal have the same thermal conductivity properties as solid metal? If you don't have the same delocalised electrons as you do in a metallic lattice (I'm not sure if you do or not) then the conductivity would be much lower and it would be far easier to touch molten metal than you would expect based on solid metal. --Tango (talk) 01:28, 5 August 2009 (UTC)
- 76.21.37.87 here (with a new IP address); while I'm a petroleum chemist, not a physical chemist, I'm pretty sure that molten metal has the same delocalized electrons as solid metal, so there's no reason for thermal conductivity to be much lower -- if anything, it'll prob'ly be higher. Besides, with molten metal you also got convection adding to the heat transfer, so you got a lot more of the heat going from the metal to your hand! My advice is, DON'T TRY THIS AT HOME!!! 98.234.126.251 (talk) 01:59, 5 August 2009 (UTC)
- I read a book by a 19th century magician, perhaps Robert Houdin, who asserted that molten metal would roll of the hands as described here. At the time I suspected it was a ploy by him to get his rivals to burn their hands to smoking cinders so he would get all the bookings for magic shows. DO NOT ATTEMPT ANYTHING REMOTELY LIKE WHAT IS DESCRIBED. Edison (talk) 02:52, 5 August 2009 (UTC)
- As everyone else already says, don't try it. But I've heard the Leidenfrost effect as an explanation of what you're describing. 70.90.174.101 (talk) 07:36, 5 August 2009 (UTC)
- It was actually demonstrated on Time Warp (TV series) very recently (I think the episode title is "Hot Stuff and Cold Steel") - the guy gets his hand soaking wet - then calmly dunks it up to the wrist in molten lead - and pulls it back out again within about a second. I've heard of this being done by many people in many situations - it certainly works (although it's obviously dangerous). This is a very different thing than (say) a blob of molten metal landing on you (where the speed of it's arrival could cause it to penetrate the layer of water - or which might allow the steam to escape around the sides. The demonstration is very specific and precise details matter. The guy who did it said that it does hurt quite a bit - and he has what looks like a severe sun-tan on his skin afterwards. SteveBaker (talk) 14:17, 5 August 2009 (UTC)
I think it's a little funny that everyone feels so important to add "don't try this at home!!!" As though, you know, we all have molten iron (at 1300 degrees C) around the house :) 82.234.207.120 (talk) 09:04, 5 August 2009 (UTC)
- I guess what I'm getting at is that anyone who has molten steel around would already know :) —Preceding unsigned comment added by 82.234.207.120 (talk) 09:06, 5 August 2009 (UTC)
- Of course don't try it. But molten metal touches skin often by accident. (i) When soldering connections in electronics, excess solder may fall on the hand i.e. tin/lead at about 200 °C. (ii) When welding, molten steel may fall on an unprotected part of the body i.e. steel at 1400 °C or more. I can report that (i) hurts and leaves a burn. (ii) hasn't happened to me yet. Cuddlyable3 (talk) 09:26, 5 August 2009 (UTC)
- To this day I do not understand how it happened, but once when I was around 15 I was near my uncle while he was welding a steel fence post together. I was supposed to paint over the welds after they cooled. However, after the third one a bit of molten steel flew nearly 15 feet and hit me on the arm. I felt like I had been shot (and yes, I know what that feels like as well, but thats a different story). Considering the molten steel flew a good distance, thus cooling quite a bit, I would say that unless the guys nerves where dead he probably wouldn't be able to touch the molten steel without at least showing some sign of pain.Drew Smith What I've done 10:16, 5 August 2009 (UTC)
- We can speculate on how it happened: molten metal dripped from the weld point onto a surface which was either wet, or painted. The heat of the metal vaporized the water or paint, which provided sufficient impetus to launch it in your direction. We'll not, owever interested, speculate on the shooting ;) --Tagishsimon (talk) 13:03, 5 August 2009 (UTC)
- There is no question that in general, molten metal will burn your skin. The particular trick that's being described here requires an extremely special set of circumstances for it to work. SteveBaker (talk) 14:17, 5 August 2009 (UTC)
- A less special set of circumstances would be for the molten metal in question to be mercury. ;) --Tango (talk) 04:04, 6 August 2009 (UTC)
- Of course, sticking your hands in mercury is not a great idea either. --Trovatore (talk) 04:18, 6 August 2009 (UTC)
- A less special set of circumstances would be for the molten metal in question to be mercury. ;) --Tango (talk) 04:04, 6 August 2009 (UTC)
- There is no question that in general, molten metal will burn your skin. The particular trick that's being described here requires an extremely special set of circumstances for it to work. SteveBaker (talk) 14:17, 5 August 2009 (UTC)
- Question: was colour was this molten steel? red orange?83.100.250.79 (talk) 16:09, 5 August 2009 (UTC)
- As Steve noted, it's been a long time since I saw the piece (~quarter century). Any details I might recall are obviously susceptible to imagination, etc. My recollection is that the metal was lighter than that, more yellow-ish than orange. Steve also mentioned getting the hands wet before performing the stunt. Upon seeing that comment, I do recall the guy dipping his hands in a barrel of water. I had assumed or conflated the idea that he was dipping them in water afterwards, but it very well could have been beforehand; my memory is just not that clear on it. Is the TV show scene ringing any bells for anyone? If I could determine the guy's name, it might help me research it. And don't worry folks, I've taken both AAA batteries out of my Acme U-Smelt-It just to prevent curiosity from getting the better of me. Sheesh! :-P Matt Deres (talk) 16:38, 5 August 2009 (UTC)
- It's not just the heat- but the density of the molten steel - assuming he could flick it without being burnt, it would (I imagine) be like flicking a concrete bollard - sorry for my unbelieviness.
- Unless of course it was this guy [3]83.100.250.79 (talk) 17:36, 5 August 2009 (UTC)
- As Steve noted, it's been a long time since I saw the piece (~quarter century). Any details I might recall are obviously susceptible to imagination, etc. My recollection is that the metal was lighter than that, more yellow-ish than orange. Steve also mentioned getting the hands wet before performing the stunt. Upon seeing that comment, I do recall the guy dipping his hands in a barrel of water. I had assumed or conflated the idea that he was dipping them in water afterwards, but it very well could have been beforehand; my memory is just not that clear on it. Is the TV show scene ringing any bells for anyone? If I could determine the guy's name, it might help me research it. And don't worry folks, I've taken both AAA batteries out of my Acme U-Smelt-It just to prevent curiosity from getting the better of me. Sheesh! :-P Matt Deres (talk) 16:38, 5 August 2009 (UTC)
I'm sure I heard somewhere that a guy rolled molten lead around in his mouth. Is this possible? Vimescarrot (talk) 18:28, 5 August 2009 (UTC)
- There are a handful of YouTube videos of people doing this with lead...a bit more tentatively than the guy on Time Warp...[4]. SteveBaker (talk) 20:42, 5 August 2009 (UTC)
- Hmm. On the one hand, it seems that the stunt is much more likely to be done with lead than with iron or steel (assuming it wasn't a literal "trick"). On the other hand, I have a very low resolution video in my memory banks of a guy playing with yellowish orange molten metal, flicking small globs of it with the tips of his fingers. The more I consider it, the less likely that seems. Unfortunately that makes me all the more curious about the original stunt. Did I assume it was steel because it was shiny and metallic and then over the years alter my memory to make it appear as I now know molten steel to be like? Or was it someone actually playing with molten steel? Or was it an actual trick in the normal sense? Matt Deres (talk) 23:41, 5 August 2009 (UTC)
- "My recollection is that the metal was lighter than that, more yellow-ish than orange." -- In that case, it could not have been molten steel: at the temperatures required to melt iron, the metal is hot enough to glow a brilliant white that hurts your eyes just to look at it. It could've been copper, or maybe manganese, but not iron. 98.234.126.251 (talk) 02:41, 6 August 2009 (UTC)
- As someone said earlier (though its somehow been ignored) this is probably through the Leidenfrost effect. If you pour a drop of water on a really hot stove the bottom of the water drop will boil immediately and you will have some water vapor between the drop of water and the stove, insulating the water drop. If you put water on your hand and dip it in the metal it will insulate your hand temporarily. It's easy to demonstrate. Take a normal hot plate (the one's at my school worked fine and they're old) and heat it up for a while. Then pour a drop of water on it. If it's hot enough the water will skittle across the plate, like it's bouncing. It's really cool you should try it. 66.133.202.209 (talk) 04:01, 6 August 2009 (UTC)
- ...But would the steam provide enough insulation to keep out the heat of molten copper? 98.234.126.251 (talk) 04:52, 6 August 2009 (UTC)
- Yes, obviously...but the question is: for how long? Evidently (from the dunking your hand into molten lead demonstrations) - it provides enough insulation to allow you to withstand 400 degC temperatures for a second or two with only slight damage (the guy said it felt like sunburn). The effect isn't going to last much longer than a second or two anyway because there isn't a continually replenished supply of water - and in any case, the steam itself is hot enough to do you some pretty severe damage. It's not impossible that this trick would work with steel or copper...but we only have actual evidence of it being done at much lower temperatures using lead. It might be (for example) that because molten steel isn't a dense as molten lead, that the steam layer would be at lower pressure in the case of steel - that would provide a larger volume of protection than in the case of lead - and that might be enough to allow you to survive the much higher temperatures. It's a tough thing to guesstimate - so I wouldn't completely rule out the possibility of doing the trick with molten steel - but my gut feel is that it wouldn't work. However, gut feels are not science. SteveBaker (talk) 13:24, 6 August 2009 (UTC)
- ...But would the steam provide enough insulation to keep out the heat of molten copper? 98.234.126.251 (talk) 04:52, 6 August 2009 (UTC)
- As someone said earlier (though its somehow been ignored) this is probably through the Leidenfrost effect. If you pour a drop of water on a really hot stove the bottom of the water drop will boil immediately and you will have some water vapor between the drop of water and the stove, insulating the water drop. If you put water on your hand and dip it in the metal it will insulate your hand temporarily. It's easy to demonstrate. Take a normal hot plate (the one's at my school worked fine and they're old) and heat it up for a while. Then pour a drop of water on it. If it's hot enough the water will skittle across the plate, like it's bouncing. It's really cool you should try it. 66.133.202.209 (talk) 04:01, 6 August 2009 (UTC)
Catching flu twice
Is it possible that someone suffering from the current swine flu pandemic strain could fall ill twice? Should one fall ill once, would the immunity gained from combatting it be sufficient to prevent them from falling ill a second time? --russ (talk) —Preceding undated comment added 00:25, 5 August 2009 (UTC).
- It might be possible...or at least it might seem to be so. The problem is that identifying the flu strain is pretty tough - the symptoms of H1N1 are pretty similar to 'normal' flu strains - it's possible that the first dose someone got wasn't swine flu at all and they'd just been misdiagnosed - it's also possible that the virus is mutating and mixing with other flu strains - and that too could result in a "new" Swine flu which even people who recovered from the original strain might not have immunity to. So it would be risky to assume that you had immunity. On the other hand, it's believed that people over the age of 52 may have some degree of immunity left over from the 1958 Asian flu - so it's certainly possible that someone who had it once before is now immune. SteveBaker (talk) 00:53, 5 August 2009 (UTC)
- There are lots of different strains of 'flu and catching one often gives partial immunity to others. There are strains of H1N1 that are endemic in human populations and have been since long before the recent "swine flu" came on the scene. If at some point in your life you have caught one of those strains (which isn't unlikely, especially for someone in their 50s or older than has had plenty of time to be exposed to various strains of flu), that could well mean you won't get as seriously ill if you now catch swine flu. You shouldn't be able to catch exactly the same strain twice and if you catch a slightly mutated strain you'll still have partial immunity. If you do get flu twice in quick succession, chances are it was a completely different strain. Now, all that aside, there is one important thing to remember - it's just the flu, the cure is bedrest, simple as that (unless you are in a vulnerable group). If in doubt, ask a doctor (or a call centre worker who did a 2 hour course on how to read a script and is now qualified to dispense prescription drugs to people that don't need them). --Tango (talk) 01:19, 5 August 2009 (UTC)
Help identify this aquatic creature

We received this inquiry via e-mail. The image was donated to us, but part of the stipulation was that we help identify what it is. So please, go at it! It looks like a green sand dollar, only it has long legs (maybe like a sea star), photo was taken at Bali, at Nusa Dua beach. Thanks. Please send me a note on my talk page if you think you know the answer. -Andrew c [talk] 04:46, 5 August 2009 (UTC)
- Here is what looks like a picture of it. Bus stop (talk) 04:56, 5 August 2009 (UTC)
- This too looks like it. Bus stop (talk) 05:03, 5 August 2009 (UTC)
- It's called a brittle star, and is in the class Ophiuroidea. —Preceding unsigned comment added by CalamusFortis (talk • contribs) 05:17, 5 August 2009 (UTC)
- Hey, thanks everyone!-Andrew c [talk] 13:49, 6 August 2009 (UTC)
- It's called a brittle star, and is in the class Ophiuroidea. —Preceding unsigned comment added by CalamusFortis (talk • contribs) 05:17, 5 August 2009 (UTC)
- This too looks like it. Bus stop (talk) 05:03, 5 August 2009 (UTC)
Forces
Why do forces obey the superposition principal? —Preceding unsigned comment added by 76.69.240.190 (talk) 05:06, 5 August 2009 (UTC)
- We observe that this is empirically the case, and set up a mathematical framework based on that assumption. To date, everything which we define as a force tends to obey this mathematical rule, so there's no reason to assume it's invalid. On the deep subatomic scales, more precise definitions of force are necessary (usually a more complex set of physics, like Hamiltonian mechanics is used - where forces are defined as a gradient of a potential field. In the case of certain nuclear interactions, a potential field cannot be defined, so the simple linearly adding forces are probably not applicable to the deep sub-nuclear scale, where really strange quantum physics applies. Nimur (talk) 05:21, 5 August 2009 (UTC)
- Also, gravity, which is generally considered to be one of the four basic kinds of force, doesn’t really obey the superposition principle except in the Newtonian approximation. Red Act (talk) 07:21, 5 August 2009 (UTC)
- This is not true when one approaches light speed. Imagine Reason (talk) 20:49, 8 August 2009 (UTC)
Thermal Conductivity
According to Thermal Conductivity, the thickness of a material has an influence on thermal conductivity; k. I modelled the equation in Excel using the formula:
k = Q/t * 1/A * x/T as stated in Thermal Conductivity. However, when everything else is constant, increasing the thickness (x) causes an increase in the thermal conductivity and a decrease in thermal resistivity (the reciprocal of k). This seems counter intuitive - I would have thought increasing the thickness of a particular material would cause a decrease in the thermal conductivity as the distance between the source of heat and the colder area would be greater.
Is this an error in my maths or is there something else I am missing? —Preceding unsigned comment added by 157.203.42.175 (talk) 12:29, 5 August 2009 (UTC)
- You need (heat flow) Q/t = kA (T2-T1) / x
- Increasing the thickness decreases the heat flow all other things being equal.
- Q/t (the heat flow) differs depending on the thickness. So it's not independent of x
- Thermal conductivity is constant for a given material - the heat flow differs.
- You're mixing up thermal conductivity k and heat flow Q/t
- 83.100.250.79 (talk) 12:40, 5 August 2009 (UTC)
- And, of course, whether the thermal resistance (or conductance) of the material increases or decreases depends whether the direction of heat flow is alomg the direction of increased thickness or at right angles to it. Dbfirs 01:47, 7 August 2009 (UTC)
Transverse Processes
Hi everyone I am a little confused about where transverse processes are located on a human vertebrae. The article on transverse processes states: (Hope its ok for me to copy and paste this) 'The transverse or costal processes of a vertebra, two in number, project one at either side from the point where the lamina joins the pedicle, between the superior and inferior articular processes.' Take a look at Grays picture of a cervical vertebrae in the article. I don't know if its just me but I think the Grays picture and the description don't match! The transverse processes don't project at either side from the point where the lamina joins the pedicle. The point where the lamina joins the pedicle is where the articular processes are. I would say that the transverse processes are posterior to the pedicle. This is just a subjective speculation. Can someone else offer an input? Thanks in advance to anyone who helps RichYPE (talk) 12:54, 5 August 2009 (UTC)
- I agree that the description does not easily match a cervical vertebra but a cervical vertebra is significantly different from a thoracic vertebra where the description does seem to tally quite well. I guess it is not easy to describe transverse processes on vertebrae without qualifying which type of vertebra you are describing. 86.4.181.14 (talk) 13:34, 5 August 2009 (UTC)
- Last's anatomy shows the cervical vertebrae with the "true" transverse processes projecting forwards (anteriorly) from the posterior end of the pedicle. The "anterior" part of the transverse process is described as the anterior/costal bar. The transverse processes of the cervical vertebrae are, if anything, anterior to the pedicles. However you shouldn't be too worked up about this. It's only important if you're going to become a spinal surgeon. Axl ¤ [Talk] 17:55, 5 August 2009 (UTC)
- I agree that the description does not easily match a cervical vertebra but a cervical vertebra is significantly different from a thoracic vertebra where the description does seem to tally quite well. I guess it is not easy to describe transverse processes on vertebrae without qualifying which type of vertebra you are describing. 86.4.181.14 (talk) 13:34, 5 August 2009 (UTC)
PiCCO cardiac monitoring
I am looking for information for an essay regarding PiCCO cardiac monitoring.
I am also looking for the following information for the same essay:
(i) What does 'stroke volume variation" tell us? Especially a value of 15% , and a value of 9%?
(ii) normal valyues for extra vascular lung water. I have fgound articles about this but no 'normal' reference figures, or information about what a value of 7ml/kg or 5ml/kg would indicate.....
(iii) Intrathoracic blood volume (ITBV). Again, I have found articles about this but no 'normal' reference figures, or information about what a value of 820ml/m2 or900ml/m2 would indicate.....
I would be very grateful for any information that may help me. I would love to be able to complate this essay this week if at all possible.....
Many many thanks
- I didn't find a good quality free online journal publication. However this web page has a helpful guide and includes the answers to your questions. Axl ¤ [Talk] 18:12, 5 August 2009 (UTC)
sunspot cycle
The sun seems to have an 11 year sunspot cycle. While we probably have not been able to directly observe sunspots on other stars it seems unlikely that our star would be unique to have sunspots. Would other stars also have an 11 year cycle? Would a red giant have sunspots? Googlemeister (talk) 14:16, 5 August 2009 (UTC)
- It is in fact possible to observe starspots on other stars. Other stars have cycles, but not necessarily with the same period as our sun. anonymous6494 14:34, 5 August 2009 (UTC)
- Astronomers may have detected a brightness change in a star, but I doubt they have actually "observed" a sunspot by creating an image of a star showing a spot on the disc of the star, like we have long been able to make images of sunspots. "Detect" or "measure the effect of" might be more accurate than "observe." Edison (talk) 17:08, 5 August 2009 (UTC)
- The above statement that starspots have been observed is idiomatic in observational astronomy—see the lede of that article for the general sense of what is considered "observation". If they meant to say that they directly formed a picture of the star with spots on it, like we do for the sun, they would have said that they "imaged" or "directly imaged" the starspots. See, for example, this press release concerning the first direct imaging of extrasolar planets. -- Coneslayer (talk) 17:21, 5 August 2009 (UTC)
- BTW the sunspot cycle is really 22 years, not 11. It's not really significant but that's what it is. 66.133.202.209 (talk) 04:03, 6 August 2009 (UTC)
- The above statement that starspots have been observed is idiomatic in observational astronomy—see the lede of that article for the general sense of what is considered "observation". If they meant to say that they directly formed a picture of the star with spots on it, like we do for the sun, they would have said that they "imaged" or "directly imaged" the starspots. See, for example, this press release concerning the first direct imaging of extrasolar planets. -- Coneslayer (talk) 17:21, 5 August 2009 (UTC)
- Astronomers may have detected a brightness change in a star, but I doubt they have actually "observed" a sunspot by creating an image of a star showing a spot on the disc of the star, like we have long been able to make images of sunspots. "Detect" or "measure the effect of" might be more accurate than "observe." Edison (talk) 17:08, 5 August 2009 (UTC)
Knives
what kind of metal does a knife have to be in order for it to be so sharp, that a gently falling piece paper with easily cut in two in the air? Or, does this metal even exist? --Reticuli88 (talk) 19:26, 5 August 2009 (UTC)
- I would think that a lot of different metals could be sharpened to the point where they would do that, but I guess what you're asking is for one that would hold that edge for a useful period of time. For that I think you'd need as hard a steel as possible, which means a high carbon content and perhaps some more exotic things like nickel or chromium thrown in, especially if you don't want it to rust (see stainless steel). TastyCakes (talk) 19:33, 5 August 2009 (UTC)
- Surgical scalpels are usually made of obsidian as it is sharper then one made of steel. It would not corrode. That is not metal though. Googlemeister (talk) 19:36, 5 August 2009 (UTC)
- It doesn't exist - paper is quite strong for its weight.83.100.250.79 (talk) 19:40, 5 August 2009 (UTC)
Scalpels are usually made of steel. Obsidian scalpels are uncommon. Some surgeons consider obsidian scalpels superior to steel. However the evidence is questionable. Axl ¤ [Talk] 19:51, 5 August 2009 (UTC)
- I'm not sure it has too much to do with the sharpness of the knife anyway. When the paper hits the blade, there are perhaps three possibilities:
- The paper is cut.
- The paper stops moving and rests on the knife.
- The paper spins off to one side and continues to fall.
- Which of these happens depends on the forces involved. In the case (1), it takes a certain amount of energy for the bonds holding the paper together to be broken. If that energy is greater than the kinetic energy of the falling paper - then the paper will simply stop moving (2) or spin off to the side (3). I don't think it matters (beyond a certain point) how sharp the knife is. Here is a thought experiment for you. A GIGANTIC piece of paper that's 100 feet wide by 100 feet tall comes hurtling out of the sky, landing edge-on onto a 6" wide concrete beam. Obviously - the weight of the paper is huge - and it'll tear in half as it falls past our super-amazingly-blunt concrete "knife". On the other hand, if you dropped a sheet of copier paper onto a 6" concrete beam - there is no way it'll tear. So there is a relationship between the kinetic energy of the falling paper and the sharpness of the knife that is not easy to explain.
- Now imagine a large blob of jello falling onto a knife blade - you can imagine it hitting the blade and the blade penetrating a couple of inches - then stopping with the jello balanced on the blade with a couple of inches deep cut on the underside. What happens in that thought-experiment is that the Jello is initially moving quite fast - it has lots of kinetic energy - and that's enough for the knife to start breaking the bonds and cutting into it. However, the act of doing that cutting absorbs some of the kinetic energy - so the Jello slows down a bit. Now it has insufficient kinetic energy for the knife to cut any deeper and everything stops.
- With the paper, we could imagine a small cut occurring just at the edge of the paper - then it rolling to one side and falling off...that's when there is just enough kinetic energy to get the cut started - but not enough to go all the way through the sheet.
- If you did the experiment in a vacuum - the paper could fall much faster - and I'd expect it to have enough kinetic energy to let the knife cut all the way through it. But the terminal velocity of a sheet of paper is just pathetically low - so there is no way for it to fall fast enough to impart enough energy to make a decent cut.
- SteveBaker (talk) 20:49, 5 August 2009 (UTC)
- Well, if the knife is sharp enough, you only ever need to break one bond at a time - this effect is why a knife works better at cutting than a brick. --Stephan Schulz (talk) 21:06, 5 August 2009 (UTC)
- That's the key thing. The energy involved is the same regardless of the sharpness of the blade, the difference is whether that energy goes into breaking bonds or stopping/deflecting the paper. A standard way to test the sharpness of a blade is to try and cut paper with it. A sharp blade should be able to cut paper by just sliding the blade over the edge of the paper with minimal weight behind it. If you do that with a blunt knife the paper just bends. It has everything to do with the sharpness of the blade and very little to do with energy (there is a minimum amount of energy required to cut the paper, but that is very small indeed). --Tango (talk) 03:10, 6 August 2009 (UTC)
- Well, if the knife is sharp enough, you only ever need to break one bond at a time - this effect is why a knife works better at cutting than a brick. --Stephan Schulz (talk) 21:06, 5 August 2009 (UTC)
- But that's the problem. That "minimal amount of energy" is still larger than a normal-sized sheet of paper falling at it's terminal velocity. Hence, you can't drop a sheet of paper onto a knife and have it be cut in two because there isn't enough kinetic energy present to break enough bonds to make a noticable depth of cut. But no matter how sharp the knife - the energy required to break bonds is the same - and each bond that's broken consumes more of that kinetic energy. It slows the paper down to the point where no more bonds can be broken - even with a one-atom-thick blade. For a large, fast-moving sheet of paper to move past a brick (to pick a canonically blunt object) - only TWO bonds at a time have to be broken - one on either side of the brick...and even those won't have to be broken simultaneously. I really don't think it much matters about the knife. As for your "knife sharpness test" - it's completely bogus. I can poke an unsharpened pencil through a sheet of paper - and using enough force, make a jagged tear through it. It's simply a matter of the amount of force and energy in the system. I don't think a sheet of 8"x11" paper, falling at it's terminal velocity has anything like enough energy to break the bonds of more than maybe a quarter inch length of cut. That being the case, it doesn't matter a damn how sharp the knife is. On the other hand - if the paper has enough kinetic energy (eg if you dropped it from enough height in a vacuum) - then a brick could cut it in half almost as easily as a super-sharp knife. SteveBaker (talk) 13:16, 6 August 2009 (UTC)
- This youtube video and this website make me think that Tango's knife sharpness test isn't bogus, although I'm not sure it's exactly what he was describing. TastyCakes (talk) 14:11, 6 August 2009 (UTC)
- Those are complicated devices for testing it. My test just requires a piece of paper. --Tango (talk) 22:39, 6 August 2009 (UTC)
- The test is not bogus, it's a standard test that I have used plenty of times. As I said, you apply minimal force, you just stroke the blade along the edge of the paper. I think we've interpreting the OP's question differently, though - I'm imagining the blade moving and slicing the paper as it falls like the proverbial samurai slicing a handkerchief in an intimidating manner. --Tango (talk) 22:39, 6 August 2009 (UTC)
- This youtube video and this website make me think that Tango's knife sharpness test isn't bogus, although I'm not sure it's exactly what he was describing. TastyCakes (talk) 14:11, 6 August 2009 (UTC)
- But that's the problem. That "minimal amount of energy" is still larger than a normal-sized sheet of paper falling at it's terminal velocity. Hence, you can't drop a sheet of paper onto a knife and have it be cut in two because there isn't enough kinetic energy present to break enough bonds to make a noticable depth of cut. But no matter how sharp the knife - the energy required to break bonds is the same - and each bond that's broken consumes more of that kinetic energy. It slows the paper down to the point where no more bonds can be broken - even with a one-atom-thick blade. For a large, fast-moving sheet of paper to move past a brick (to pick a canonically blunt object) - only TWO bonds at a time have to be broken - one on either side of the brick...and even those won't have to be broken simultaneously. I really don't think it much matters about the knife. As for your "knife sharpness test" - it's completely bogus. I can poke an unsharpened pencil through a sheet of paper - and using enough force, make a jagged tear through it. It's simply a matter of the amount of force and energy in the system. I don't think a sheet of 8"x11" paper, falling at it's terminal velocity has anything like enough energy to break the bonds of more than maybe a quarter inch length of cut. That being the case, it doesn't matter a damn how sharp the knife is. On the other hand - if the paper has enough kinetic energy (eg if you dropped it from enough height in a vacuum) - then a brick could cut it in half almost as easily as a super-sharp knife. SteveBaker (talk) 13:16, 6 August 2009 (UTC)
You are right, Tango --Reticuli88 (talk) 14:43, 7 August 2009 (UTC)
- There is no answer to this question, without knowing particular details. How large is the paper? How stiff is the paper? What is its shear strength? Is there an atmosphere through which it falls (as pointed out by SteveBaker)? At what angle does it encounter the knife? Is it's weight equally distributed on either side of the blade? And only finally, how sharp is the knife? Even after knowing all these details it may take empirical tests, because the calculations are probably beyond those applicable to already known experiences. It is possible to stack the odds in favor of achieving a nice sliced in half rectangle of paper. That I think may be possible. Strength of materials seems to be an article that may have applicability here. Bus stop (talk) 15:04, 7 August 2009 (UTC)
- I'm getting a bit sick of these kind of answers, Bus stop. Every question on this desk could have more information added to it, it's not really helpful to list a random list of things you'd like to know, and then even if you are given all this information, you still can't answer it (as you point out). So ask for the details? And most of the details you could reasonably well guess: How big? I'd assume ~A4. How stiff? Assume 80gsm standard printer paper. How stiff? As stiff as paper, ffs! And finally, how sharp? THAT'S pretty much the question! I'm sorry if I'm offending you, but really when you have nothing useful to contribute, you don't need to post. Aaadddaaammm (talk) 13:18, 12 August 2009 (UTC)
Gravity and Mass
I understand that objects of differing mass will accelerate toward the ground at the same velocity, i.e. a ping pong ball and a metal ball bearing (excluding aerodynamics) This can be shown in NASA's vimit comet, when the aeroplane will drop at the same rate as its passengers giving the illusion of zero gravity. What I can't understand is why planets of different mass have different gravitational pulls. I.e. Jupiter would pull us toward it with much greater force than Earth due to it's greater mass! Does this mean that there is a correlation between mass and gravity? Is it simply the case that the difference between the ping pong ball and the heavier metal ball bearing is insignificant in comparison to the mass of the planet earth. Does the origin of the gravitational force come from within the particles or the forces that hold them together? Can it be associated with the possitive and negative charge? From Stuart McPhee86.174.230.38 (talk) 22:41, 5 August 2009 (UTC)
- This is an easy mistake to make. The thing to get straight is this: a ping pong ball heads towards earth at an acceleration of roughly 9.8 meters per second per second. This is not relative (at least in Newtonian mechanics); it is indisputable fact that it is the ping pong ball that is accelerating towards the earth, not the earth towards the ping pong ball. The earth is also accelerating towards the ping pong ball, but it is doing so by a much tinier amount, so we only perceive the acceleration of the ping pong ball.
- However, suppose we increase the mass of the ping pong ball. Let's replace it with a small planet (but we still want to keep it at the same distance from the center of the earth). The new planet's acceleration towards the earth is still about 9.8 m/s2, but the earth's acceleration towards the new planet has increased significantly, to the point that we can actually perceive and measure it.
- The earth's acceleration towards an object based on that object's gravity is directly proportional to its mass. For example, if the new "planet" has a mass equal to one tenth of that of earth, then earth will accelerate towards the new planet at a rate of .98 m/s2 (one tenth of the new planet's acceleration towards earth).
- Similarly, if earth's mass were increased, then the acceleration at which objects fall towards the earth would also increase proportionately. However, the earth's still imperceptible accelerations toward falling objects would remain unchanged (assuming the same distances from the centers of mass are unchanged), since its accelerations toward ping pong balls depends only on the mass of the ping pong ball and the distance between their centers of mass.
- You probably don't have the background to understand the equations involved, but here they are for reference. That is, those are the equations Newton formulated over 300 years ago; they have been replaced nowadays by general relativity, which is significantly more complicated. --COVIZAPIBETEFOKY (talk) 23:14, 5 August 2009 (UTC)
- To bring that back to your example about Jupiter, the thing to realize is this: Jupiter's acceleration towards earth is exactly the same as that of a hammer if we replace Jupiter with that hammer. It is earth's acceleration towards Jupiter or the hammer that changes in these two scenarios. --COVIZAPIBETEFOKY (talk) 23:28, 5 August 2009 (UTC)
the above answer is pure semantics. The simple answer is: everything attracts everything. (The exception is things that have no mass). And you guess the key already; you say: "Does this mean that there is a correlation between mass and gravity?" YES. Gravity is proportional to mass and inversely proportional to distance. Just remember: everything attracts everything (but the farther away from each other the things, the weaker the attraction). Then you'll be all set. 82.234.207.120 (talk) 01:26, 6 August 2009 (UTC)
- I'm not sure how you came to the conclusion that my answer was "pure semantics", but maybe I should clarify the key point in my answer, since I did kind of ramble a bit. Your answer doesn't really seem to address the main question being asked, which was: why should Jupiter have a stronger pull on the earth than earth has on everything else? The error in this 86.174.230.38's train of thought comes from thinking about the acceleration between two bodies, rather than the acceleration of one body due to another. The former is a sum of two instances of the latter (ie. the ping pong ball accelerating due to the earth's gravitational pull and the earth accelerating due to the ping pong ball's gravitational pull), and it is important to distinguish between those two separate accelerations.
- Failure to make that distinction can lead to the conclusion that the earth-ping pong ball system should behave identically to the earth-Jupiter system: just increase the mass of the ping pong ball to that of Jupter. The acceleration "between the two bodies" (remember, this is the wrong way to think about it) remains unchanged. Clearly, the earth-ping pong ball system should behave identically to the earth-Jupiter system, at least as far as gravity is concerned. The problem here is that we didn't make the important distinction between the acceleration of one body and that of the other.
- Hopefully that's a bit clearer. --COVIZAPIBETEFOKY (talk) 02:10, 6 August 2009 (UTC)
- The key detail is that mass appears in two places in the relevant calculations. It appears in the formula for gravitational force, and it appears in F=ma. The reasons hammers and feathers fall at the same rate is because that mass cancels out. However, only the mass of the object we are measuring the acceleration of cancels out, the mass of the other object doesn't cancel out (since it doesn't appear in F=ma, since that is only concerned with the object in question and the force, not the source of that force) so it still has an effect. (That gravitational mass and inertial mass are equal is far from trivial and has been the subject of much discussion.) --Tango (talk) 02:57, 6 August 2009 (UTC)
August 6
can ethers act like alcohols in acid-catalyzed addition?
My lecturer never really seemed to cover this (or I must have forgotten) and a quick google search isn't helping. Basically I'm wondering since ethers also have lone pairs to donate, whether they can interact with carbocations ...
So let's say we have an (R2)+ carbocation after the addition of H+ to a C=C alkene bond, and a R1-O-R1 ether, and say R1 was a shorter alkyl chain than R2. Shouldn't a R2-(O+)-(R1)_2 intermediate develop, with the resulting ejection of one of the alkyl groups? I'm basically wondering if you can exchange alkyl groups in an ether this way. Shouldn't the lone pairs on an ether oxygen be even more reactive than an HOH or ROH, since the ether oxygen will have more electron density. I know alkyl groups aren't that labile, so would that just make it kinetically unfavourable to form the ether cation?
I assume one of the difficulties is that acids don't like to dissolve into ethers -- and since the ether doesn't have an H group, it can't "return" the H+, so H+ is getting consumed....and I suppose nature would prefer to have H+ ions than R+ ions? But to address the first problem, we could choose a mixture of methylene chloride and ether to try to solvate the acid, and choose a strong acid whose conjugate base makes a poor nucleophile... (magic acid?). John Riemann Soong (talk) 02:15, 6 August 2009 (UTC)
- Alkyl groups are not great leaving groups in these sorts of reactions. And the lone pairs are LESS reactive on the ether, because the long alkyl chain acts as a "soft" electron sink, basically giving the electrons a place to disipate to, making them less concentrated on the oxygen. This is in contrast to the "hard" hydrogen, which cannot absorb extra electron density. These two effects (poor leaving group + less electron density around oxygen) probably make the reaction unlikely to happen to a great extent. --Jayron32 02:30, 6 August 2009 (UTC)
- That's not quite right - the lone pairs on ethers are less reactive maybe due to steric effect - but they have a greater ability to stabilise a cation compare to water when R=alkyl,benzyl, ethenyl etc..83.100.250.79 (talk) 13:04, 6 August 2009 (UTC)
- In other words: definitely not (at least under anything like "normal" conditions). 98.234.126.251 (talk) 02:46, 6 August 2009 (UTC)
- Well yes, but carbocations aren't too great either -- would that in fact prevent the H+ from attacking the alkene? (As in, the intermediate will convert back to the alkene + H+ before an ether would attach?)
- Why is that ether oxygen lone pairs are less reactive? Because alcohol oxygens are still reactive (the reaction proceeds merrily), and I note the pKa of an alcohol is higher than that of water ... and the pKa of a tert alcohol tends to be highest. My interpretation of this is that the electron-donating effects of the alkyl chain were greater than any electron-withdrawing effects -- that is, the alkyl chain destablised the lone pair conjugate base. John Riemann Soong (talk) 03:16, 6 August 2009 (UTC)
- Carbocation formation is different cause there ain't nothing being actually ejected from the molecule in this case (i.e. no C-C or C-O bonds being broken, as they would be in your scenario). It's the strength of the bonds to be broken that accounts for the reaction not happening. FWiW 98.234.126.251 (talk) 03:25, 6 August 2009 (UTC)
- So what would happen after the acid addition step? Would we have a "stabilised" carbocation solvated in solution? (Assuming the acid's conjugate base is a very poor nucleophile, e.g. SbF6-.) After we evaporate the ether and the methylene chloride off, would I get a strange crystalline salt of alkyl carbocations and SbF6- anions? (Granted, it would probably be hygroscopic and quite reactive to water, but it would be interesting....) John Riemann Soong (talk) 05:03, 6 August 2009 (UTC)
- I think what will really happen is the anions would add to the carbocations forming a neutral covalent compound. Or if not, then the carbocations will instantly react with any moisture available (even the moisture in the air, no matter how slight) and form a sec / tert alcohol and regenerate the acid (and prob'ly give off a whole bunch of noxious vapors and maybe splatter the reaction mixture all over the place cause this final step is very exothermic). FWiW 98.234.126.251 (talk) 05:50, 6 August 2009 (UTC)
- So what would happen after the acid addition step? Would we have a "stabilised" carbocation solvated in solution? (Assuming the acid's conjugate base is a very poor nucleophile, e.g. SbF6-.) After we evaporate the ether and the methylene chloride off, would I get a strange crystalline salt of alkyl carbocations and SbF6- anions? (Granted, it would probably be hygroscopic and quite reactive to water, but it would be interesting....) John Riemann Soong (talk) 05:03, 6 August 2009 (UTC)
- Carbocation formation is different cause there ain't nothing being actually ejected from the molecule in this case (i.e. no C-C or C-O bonds being broken, as they would be in your scenario). It's the strength of the bonds to be broken that accounts for the reaction not happening. FWiW 98.234.126.251 (talk) 03:25, 6 August 2009 (UTC)
- Mmmm, but diborane is extremely reactive with water as well, and yet we can still observe it meaningfully without too much hassle. And one could just view it in dehumidified air... John Riemann Soong (talk) 06:27, 6 August 2009 (UTC)
- You said you formed the carbocation by protonation of an alkene. That's essentially an equilibrium process--loss of H+ adjacent to a carbocation forms an alkene as the standard E1 reaction mechanism. On the other hand, reaction of carbocation(-like species) and ethers is known, and leads to alkylation of the ether as you suggest (an O+ compound). These compounds can be isolated in some cases, for example, triethyloxonium tetrafluoroborate. DMacks (talk) 05:58, 6 August 2009 (UTC)
- So where exactly would the carbocation add to the ether? And would the alkylation product be stable? 98.234.126.251 (talk) 06:12, 6 August 2009 (UTC)
- It attatchs at the ether oxygen, and the resultant compound may or may not be stable, depending on what exactly it is, and the temperature, and other factors.83.100.250.79 (talk) 13:45, 6 August 2009 (UTC)
- So where exactly would the carbocation add to the ether? And would the alkylation product be stable? 98.234.126.251 (talk) 06:12, 6 August 2009 (UTC)
- Well, I see several scenarios: the ether is alkylated and doesn't eject any of the alkyl groups, and forms a stable cation, assuming again that the conjugate base of the acid used is a poor nucleophile; an alkyl group is eventually ejected (would the ether oxygen prefer to retain the longer-chain, and if so, could we exchange alkyl groups on an ether this way?); though much less labile than H+, the alkyl groups act somewhat like H+, being constantly exchanged; the ether doesn't react, leaving just the carbocation (the strange salt described above); the ether doesn't react and the carbocation isn't stabilised enough to keep the H+; so the equilibrium favours the reactants and most of the alkene is left unreacted. John Riemann Soong (talk) 06:27, 6 August 2009 (UTC)
- You said you formed the carbocation by protonation of an alkene. That's essentially an equilibrium process--loss of H+ adjacent to a carbocation forms an alkene as the standard E1 reaction mechanism. On the other hand, reaction of carbocation(-like species) and ethers is known, and leads to alkylation of the ether as you suggest (an O+ compound). These compounds can be isolated in some cases, for example, triethyloxonium tetrafluoroborate. DMacks (talk) 05:58, 6 August 2009 (UTC)
- Comment In the above discussion - only DMacks (and to an extent the orignal questioner) above is really correct in what they say.83.100.250.79 (talk) 13:11, 6 August 2009 (UTC)
- Assuming you have produced a carbocation, it will and can react with an ether - but you need to consider steric effects, which will tend to hinder their production in general.
- Compounds R1R2R3O+ are called oxonium ions. eg the triethyl oxonium ion mentioned above, which can actually be bought as the tetrafluoroborate in solution (needs to be kept cold)
- Yes you can/could exchange alkyl groups this way. As you expect the reaction will tend to favour the most stable carbocation being ejected (all other things being equal)
- see Triethyloxonium tetrafluoroborate 83.100.250.79 (talk) 12:57, 6 August 2009 (UTC)
- hovever bear in mind that if you try to protonate an alkene in ether solution with an acid, you'll end up protonating the ether instead83.100.250.79 (talk) 13:09, 6 August 2009 (UTC)
- Wouldn't the protonated ether then protonate the alkene? Or would the H+ prefer to stick with the oxygen? I mean H3O+ protonates alkenes in the classic acid addition of water setup... John Riemann Soong (talk) 13:17, 6 August 2009 (UTC)
- Yes, I was assuming you wanted pure solutions of carbocation - the reaction will still work.
- By the way did you know that one of the common reactions of ethers is acid catalysed decomposition - eg
- Wouldn't the protonated ether then protonate the alkene? Or would the H+ prefer to stick with the oxygen? I mean H3O+ protonates alkenes in the classic acid addition of water setup... John Riemann Soong (talk) 13:17, 6 August 2009 (UTC)
Et2O + H+ >>>EtOH + ethene + H+
- This can be a problem if the reaction is warm83.100.250.79 (talk) 13:49, 6 August 2009 (UTC)
How important is the consumption of H+? I guess it's not acid-catalysed addition anymore, is it, since H+ is being consumed? What determines whether the oxonium ion is stable or not? And even if alkyl exchange occurs, we'll still get an interesting crystalline species once we evaporate off the solvents... John Riemann Soong (talk) 13:15, 6 August 2009 (UTC)
- The same things that stabilise carbocations, stabilise oxonium ions.
- The concentration of H+ (from which the concentration of C+ derives via H+ addition to alkenes) will affect the rate of reaction. But it's not catalytic if oxonium cations are the product - since H+ is used up.
- However if alkyl exchange is the only reaction and the products are different ethers and alkenes - then H+ is catalytic83.100.250.79 (talk) 13:42, 6 August 2009 (UTC)
- The oxonium cation will be the product if the ether oxygen is the strongest base in the system - most oxonium cations will be stable at low enough temperatures - at high enough temperatures - decomposition occurs.
- It's probably worth noting that ethers can interact (form bonds) with a wide variety of lewis acids such as BF3to form Diethyl ether boron trifluoride adduct (aka Boron trifluoride etherate), and in general can act as ligands (as lone pair donors) or lewis bases with a wide variety of compounds (not just carbocations, and H+) 83.100.250.79 (talk) 14:53, 6 August 2009 (UTC)
- Interesting -- normally I assume high temperature favours carbocation formation .... but I guess this principle applies only as long as we want the carbocation to be an intermediate? I assume this is the same for oxonium too. Re: oxonium stabilisation: I suppose we can't do anything about trying out allylic-ish oxonium cations, since the pi bonds are going to be attacked. And if oxonium stability is analogous to carbocation stability, I guess actually alkylated ethers are more stable than H3O+?
- This makes me very interested now -- well, if tri-alkyl oxonium cations are more stable than say, a protonated alcohol or a protonated ether, why don't most textbooks mention ethers as something you could play around with during acid addition? I assume the ether would be a very good competitor for the cation compared to other nucleophiles. Is the fact that H+ might not be returned, potentially making a reaction scheme infeasible?
- Also, so many different possibilities -- I'm trying to work them out. I guess a useful reaction might be mixing a straight-chain alkene, an ether with tertiary alkyl groups and the strong acid with a poor nucleophile conjugate base; the ether solvent binds to the carbocation and expels one tertiary alkyl group. So if we run the reaction at high enough temperatures, this favours both the decomposition of oxonium and the alkyl group into a new ether and a new alkene...but then it occurs that some of the ether molecules might react twice with the protonated alkene -- I guess it depends on just how much alkene is there compared to ether, isn't it? (And what would be a general principle of separating two different ethers? I assume that if tert groups are being ejected in favour of primary groups, the boiling point of our products will be higher and we can evaporate off the original solvent -- but that's one case.)
- To me, I'm seeing this as a game of which type of cation is more stable -- protonated ether, tri-alkyl oxonium, or the alkyl carbocation. What if we had straight chain alkenes and ethers? I assume in general the longer-chain primary carbocation would be more stable? And now I realise that tertiary carbocations are more likely to be ejected, but primary carbocations are more likely to convert back into alkenes. Aaaahh, so many different considerations! John Riemann Soong (talk) 11:43, 7 August 2009 (UTC)
Why is the Mandelbrot Set so complicated?
Infinitely complicated in a way that is awesome, distressing, beautiful, fearful, samey, completely obsessed and obsessive, suggestive of an infinitely talented but hermetically sealed divine lunatic of a geometer. Pray answer me in such a way as this. Suppose that I had asked: “Master, why is this river so quiet and this other river so wild and noisy.” And the Master replied: “Grasshopper, the first river has little water and flows over land only slightly tilted so it runs slowly and quietly, but this other river runs over steep ground with many rocks and crevasses, and there is much water so it is churned up as it runs, and thus the noise and the spectacle.” You see, Grasshopper can understand that. And I am such a one as that Grasshopper. I am Termite. And I would appreciate you answering me in just such a way as the Master answered Grasshopper. Why is the Mandelbrot Set so complex, and why is a triangle so simple? Myles325a (talk) 05:50, 6 August 2009 (UTC)
- I think you should try the Mathematics desk, they may be of more help to you. John Riemann Soong (talk) 06:29, 6 August 2009 (UTC)
- I don't think the mathematicians are going to help you. You may get an answer there - but I'd bet good money that you'd be unable to understand it. This is a case of observer bias. The fact is that if the Mandelbrot set wasn't all of the things you describe, nobody would have given it a second glance. There are an infinite number of mathematical objects - and only the interesting ones are ever investigated or written about. The Mandelbrot set (and it's close relatives) is well known BECAUSE it's complicated. The triangle is known because it's useful. Anything significantly simpler than the Mandelbrot set is simply too boring to have made it into your stream of thought. There are many other things that are as complex as the Mandelbrot set (a Julia set, a Sierpinski sponge, or a Koch snowflake for example) - but somehow we don't see them as beautiful - so most people never hear about them. Check out List of fractals by Hausdorff dimension for lots more examples. SteveBaker (talk) 13:04, 6 August 2009 (UTC)
- Triangles are one of the abstractions of Euclidean geometry that Buckminster Fuller pointed out cannot exist[5]. The Mandelbrot set reveals the form in 2-D of an iterative oscillation that actually exists, in the same way that prime numbers actually exist. Triangles are useful for designing things and bracing them, while the other two are more intriguing than useful. No one promised that reality would not be complicated. Cuddlyable3 (talk) 20:56, 6 August 2009 (UTC)
- Huh? Triangles and prime numbers are both ideal objects; I don't see any basis for saying one really exists and not the other. Of course triangles are completed infinite totalities, and it's possible that finite ideal objects exist but infinite ones don't. But empirically this doesn't seem to be a very productive assumption to make. --Trovatore (talk) 21:01, 6 August 2009 (UTC)
- None of the Euclidean primitives point/line/plane can exist. Try to make one of them if you can. Cuddlyable3 (talk) 21:18, 7 August 2009 (UTC)
- As soon as you make a prime number. --Trovatore (talk) 08:01, 8 August 2009 (UTC)
- None of the Euclidean primitives point/line/plane can exist. Try to make one of them if you can. Cuddlyable3 (talk) 21:18, 7 August 2009 (UTC)
- Huh? Triangles and prime numbers are both ideal objects; I don't see any basis for saying one really exists and not the other. Of course triangles are completed infinite totalities, and it's possible that finite ideal objects exist but infinite ones don't. But empirically this doesn't seem to be a very productive assumption to make. --Trovatore (talk) 21:01, 6 August 2009 (UTC)
- Triangles are one of the abstractions of Euclidean geometry that Buckminster Fuller pointed out cannot exist[5]. The Mandelbrot set reveals the form in 2-D of an iterative oscillation that actually exists, in the same way that prime numbers actually exist. Triangles are useful for designing things and bracing them, while the other two are more intriguing than useful. No one promised that reality would not be complicated. Cuddlyable3 (talk) 20:56, 6 August 2009 (UTC)
- I consider the Julia set to be more beautiful than the Mandelbrot set. I have some home-generated prints produced on a BBC Archimedes computer many years ago. Most of the set is not particularly inspiring, but I spent many hours looking for the beautiful bits. I think the same is true to some extent of Mandelbrot: we are shown just the attractive bits. Dbfirs 01:59, 7 August 2009 (UTC)
- Which Julia set? The M-set is a sort of catalog of an uncountable family of J-sets. —Tamfang (talk) 03:21, 11 August 2009 (UTC)
- I consider the Julia set to be more beautiful than the Mandelbrot set. I have some home-generated prints produced on a BBC Archimedes computer many years ago. Most of the set is not particularly inspiring, but I spent many hours looking for the beautiful bits. I think the same is true to some extent of Mandelbrot: we are shown just the attractive bits. Dbfirs 01:59, 7 August 2009 (UTC)
- Yes, do ask at the mathematics desk. But the Mandelbrot set is complicated because of the phenomenon of sensitive dependence on initial conditions (SDIC). If you're really a grasshopper, then among your insect relations you have probably encountered a few butterflies, so you can understand the butterfly effect, another example of SDIC. A triangle is simple because moving from one point to another in one operation doesn't have SDIC. If you start from a slightly different place, you end at a slightly different place, and there you are. The SDIC in the Mandelbrot set comes from the repeated iteration of the squaring function, like in the butterfly effect it comes from evolving the chaotic system for a long period of time. Points in the Mandelbrot set can behave much differently from very nearby points under the iteration, because of the SDIC. The exact pattern of differences is the complexity that you see. 70.90.174.101 (talk) 07:09, 7 August 2009 (UTC)
Biology has its own version of such sets, in even more abstract form. Deuterostome gestation arguably follows a fractal pattern (radial cleavage) -- one cell's signals ends up determining the positions of other new cells, but the conditions for such patterns must all be stored within a single zygote cell. Mysterious, because there are no such things as "arm genes" or "elephant trunk genes" -- just a whole lot of signalling genes cooperating together to encourage division in the right direction and arrest division in the wrong direction (over and over). What's especially fascinating is how did such a gestation pattern evolve? Some dynamic patterns you might be interested in might include Conway's Game of Life. John Riemann Soong (talk) 08:35, 7 August 2009 (UTC)
amper-current and relativity
hello, this is a passage from article and i can't understand it,the context is that scientists didn't want to deal with theories that they couldn't measure.
"One can see an analogous sort of indeterminateness at the basis of A. Einstein's (1952, p. 37) complaint against pre-relativistic mechanics and electrodynamics. His criticism was that they lead "to asymmetries which do not appear to be inherent in the phenomena." Whether one assigns an absolute velocity of zero to a conductor and a non-zero velocity to a magnet, or vice versa, the measurable result (current) is the same. Hence, the absolute velocity is indeterminate: "The phenomena . . . possess no properties corresponding to the idea of absolute rest," as Einstein put it. Interpreted a la Glymour, the point is not the naive one that absolute velocity is not measurable-that would be alright-but that it cannot even be computed from measured quantities via any seriously proposed (let alone well-tested) hypotheses.
why does Einstein says there is no symmetry? why it is not the same to rotate object around another which stand still and then swap the rolls? why can't we measure current according to the text? if so, what is ammeter??
thank you —Preceding unsigned comment added by מני111 (talk • contribs) 07:10, 6 August 2009 (UTC)
"why does Einstein says there is no symmetry?" He means that Maxwell's equations are not invariant under the Galilean transformation. In simple terms : In Einsteins time, people thought they could transform from a "stationary" frame of reference to a moving frame of reference simply by adding velocities. The equations describing electromagnetism change when you do this. In reality, the observed phenomena stay the same for both observers. It is this observation that eventually led to the discovery of the Lorentz transformation, which does have the required symmetry : the Maxwell equations are invariant under the Lorentz transformation. http://en.wikipedia.org/wiki/Maxwell%27s_equations
http://en.wikipedia.org/wiki/Galilean_transformation
http://en.wikipedia.org/wiki/Lorentz_transformation —Preceding unsigned comment added by 81.11.170.162 (talk) 10:48, 6 August 2009 (UTC)
- I'm not really too sure what you're asking, but to give you I think a suitable analogy, take the Earth-Sun system. If they were stationary relative to one-another, the Earth would be sucked into the Sun. And there is a suitable rotating reference frame in which they are stationary relative to one another, but even in this frame the Earth is not sucked into the Sun. This is an asymmetry; the laws of physics do depend on your frame of reference. Incidentally, even in Special Relativity, acceleration is still absolute, it's only in General relativity that there are no inertial frames. I'm not too sure if I've addressed your clearly, so please post again if I haven't.--Leon (talk) 10:51, 6 August 2009 (UTC)
- There was an asymmetry in the physical theories of the time that did not correspond to any asymmetry in the real world (the phenomenon). We can measure current, but the theory at the time seemed to do a bad job of explaining why, and Einstein was criticizing it for that.
- I would advise against reading this article, whatever it is, because it looks like bad philosophy. It seems to imply that "absolute velocity is not measurable" is different from "absolute velocity [...] cannot [...] be computed from measured quantities"—which is nonsense and certainly not something that Einstein believed—and it implies that "absolute velocity is not measurable" is an obviously true statement a priori; see Section 16–1 of the Feynman Lectures on Physics for a response to that. -- BenRG (talk) 11:17, 6 August 2009 (UTC)
thank you all. i can't understand why when you swaping the objects the result shuold be a-symmetric. why the frame of reference imoprtant when i (the observer) don't move and one time i take the counducter rotate it around the magent and the other time, the smae places but the magnet is rotating. each object was one time static and the second time mobile - soo it should'nt change the current result. it is the way you look at that, one can say both pbjects moving but one is faster (the frame reference is earth). if we will think about a person seeing a child throw an apple on a moving train (a magnet make electron move) is the equivalent for the child to see the man outside throwing an apple. [not connected to the fact the apple will move slower for the an outside) - i really hope i didn't make it much to confusing
the speed of light is absolut, so there is an absolute speed or not?
about the article itself is michael r. garder - realism and instrumentalism in 19th century atomism
if i may,the lorentz transformation article is complicated, is there intuative explantion?
thank you —Preceding unsigned comment added by מני111 (talk • contribs) 12:34, 6 August 2009 (UTC)
Einstein was pointing out a problem : in reality, the result is symmetric. Science in Einstein's time suggested it should be asymmetric.
The Maxwell equations allow you to calculate the speed of light without specifying an observer who measures this speed. In classical mechanics on the other hand, all calculated speeds are relative to a (usually implicitly specified) observer : if you calculate the speed of an apple that falls from a tree, your result will be relative to an observer who saw the apple at rest in the tree.
Thus, consider a light in a train with speed v. According to the Maxwell equations, an observer in the station sees the light moving with a speed c. According to gallilean relativity, an observer in the train will see the light moving with a speed c-v, but according to the Maxwell equations, he will also see it moving with a speed c. This is a problem! In reality, both observers will observe the light moving with speed c, but that is not what Einstein's contemporaries thought.
Einstein (and Lorentz) discovered that you must not only transform velocities, but also lengths and durations, when calculating what a moving observer sees. This is known as the Lorentz transform.
The above example involves the speed of light. Light is an electromagnetic phenomenon, and comparable examples can be found for other electromagnetic phenomena, including moving magnets.
---
- If you read the whole paragraph (the first one) in Einstein's paper it is clear what he meant by the asymmetries. The problem is that theory of the day gave a different physical explanation for what happened when you had a moving magnet and a stationary coil, and when you had a moving coil and a stationary magnet (this is a problem relating to a unipolar dynamo, if one is interested). Einstein says that this is silly and means something is fundamentally wrong: you can't distinguish between the two if you don't believe in absolute rest (which he doesn't). It's an introduction to a general attack on the theoretical framework of the day (a clever one, but not necessarily a convincing one in its time, more of a logical attack than an empirical attack), and gives some indication of Einstein's characteristic style in attacking problems (Machian, axiomatic, concerned with basic concepts and definitions, not specific experimental results). Asymmetries are a huge deal to Einstein—he says if you can't possibly distinguish between two different states, then you shouldn't have a separate set of physical explanations (or physics) for them. It's a philosophical critique, though one that led him to some rather fruitful revisions of the theories. The specific criticism is something of a throwaway introduction—here's one of many things that indicate something is wrong, so let me try and rework this from the ground up without assuming the existence of concepts (e.g. absolute rest) that we can't measure. --98.217.14.211 (talk) 16:39, 6 August 2009 (UTC)
thank you very much, that is very intersting, why the physicists at that time thought those are completly different phenomena? can you give me a link please to those different explanations? —Preceding unsigned comment added by מני111 (talk • contribs) 22:38, 6 August 2009 (UTC)
- Click the link—he discusses it. It isn't very interesting in and of itself. I don't remember the details of it, but it's basically that in both cases you get an electric current. One explanation was used to say that this came from some sort of interaction with an electric field. The other said there was an electromotive force involved. These are two rather different types of answers and Einstein said, "you can't have both of them." this (from "Electric Dynamos" on) gives a good discussion of the technical aspects of what Einstein was talking about. --98.217.14.211 (talk) 14:12, 7 August 2009 (UTC)
thanks (meni) —Preceding unsigned comment added by מני111 (talk • contribs) 11:37, 8 August 2009 (UTC)
METHANE ON MARS
An article on BBC News Science page says there is methane being produced on Mars it says this could be geological in origin or biological, Geological would be Active volcano not heard of this before on Mars 1.is there known active Volcanoes? or by a process of Serpentinisation - which is a process involving water. 2.is water in suffcient quantity to produce this effect would it need to be free flowing or in a solid form Ice maybe? and last but not least Biological activity which i assume they mean life in some form? —Preceding unsigned comment added by Chromagnum (talk • contribs) 07:30, 6 August 2009 (UTC)
- Our article Atmosphere_of_Mars has an extensive section on Methane that explains the current theories rather well. We also have a section in Life on Mars that talks about how Methane might imply the presence of life on that planet. SteveBaker (talk) 12:46, 6 August 2009 (UTC)
- Maybe the methane is being produced because the Martians are drilling for natural gas! :-D 98.234.126.251 (talk) 23:12, 6 August 2009 (UTC)
Thanks SteveChromagnum (talk) 08:39, 8 August 2009 (UTC)
Ipod
(moved from Miscellaneous reference desk)
How can I connect my Ipod to my Sony (maybe Panasonic) Hi Fi? It has a usb port on the front, but when I put my Ipod, or a flsh drive in there it says Unsupport In fact what can I plug into this port to make it work. I have put about 3 months worth of music on my Ipod, but some times I want to listen to music through proper speakers, eg a party. Any help in this would be greatly appreciated. Thank you. ~~Zionist —Preceding unsigned comment added by 62.172.58.82 (talk) 06:58, 6 August 2009 (UTC)
- I don't know about your specific hi-fi, but I would connect the two via audio cable. On the back of your Hi-fi there is likely an auxilliary input (probably an RCA connector, but check). You can buy a cable that plugs into the headphone jack on your iPod at one end and into the auxilliary input on your Hifi on the other. That way, your Hifi is acting as speakers for your iPod. — QuantumEleven 08:39, 6 August 2009 (UTC)
- This is exactly what I did for an outdoor party at my house. Dismas|(talk) 20:21, 6 August 2009 (UTC)
Your Ipod doesn't support the USB mass storage device class (MSC) interface the way that a pen drive or some other mp3 players do. You have to get an ipod docking adapter that connects to the docking socket on the bottom of the ipod, or else use an analog audio cable as Dismas suggested. There are also lots of boom boxes with dedicated ipod ports these days. 70.90.174.101 (talk) 07:18, 7 August 2009 (UTC)
- Another option is to buy a transmitter for your iPod which will transmit what your iPod is playing over an FM radio frequency, and tune your hi-fi to this. These are predominantly used in cars without iPod inputs to the stereo, but I have used mine with my home stereo and it works fine. --jjron (talk) 08:34, 7 August 2009 (UTC)
- Presuming your hifi still has a tape deck, buying a tape one may be a better option to reduce the chance of interference etc Nil Einne (talk) 09:28, 7 August 2009 (UTC)
- But the solution suggested by QuantumEleven above is by far the simplest and cheapest. The connector (with a 3.5 mm stereo jack at one end and two phono plugs at the other end) costs only a few dollars/pounds, and most amplifier phono inputs are happy with an earphone output, though you can add an impedance matching circuit if you are concerned about highest quality of sound. The next cheapest is the tape adaptor (a mock cassette tape at one end and a 3.5 mm stereo jack at the other end). Dbfirs 19:33, 7 August 2009 (UTC)
If you store them as files (ie just copy the songs onto the hard drive when you plug it into your computer, don't use iTunes) does it work then? Your iPod wouldn't be able to play the files, but I think it would just work as a USB drive which your Sony could play from. TastyCakes (talk) 20:24, 11 August 2009 (UTC)
Fast poison
I've recently read a novel (Joe_Abercrombie-Best Served Cold) in which one of the characters poisons a number of his victims via fast acting poisons. Specifically;
- Using a needle/pin dipped in a vial of poison to prick his victim.
- Brushing a solution of poison onto books/metal and allowing the victim to touch the poison.
My questions;
- Assuming that the pin prick allows direct transfer to the blood, do such poisons exist that work fast at such a small dosage (the amount that would be left after the pin is pushed through the skin)?
- Do fast acting poisons exist that could be transmitted by touch?
- Lastly, how many of these would be available to medieval level technology?
Thanks, Kellhus (talk) 11:22, 6 August 2009 (UTC)
- A lot of poisons work at such small dosages (see LD50 for some good examples) -- botox seems like a good candidate. John Riemann Soong (talk) 12:28, 6 August 2009 (UTC)
- There was that case of Georgi Markov - a Bulgarian dissident working for the BBC who was killed in London by a soviet agent using a poison-tipped umbrella. I know that sounds like the plot of a James Bond movie...but it's true. The poison use in that case was Ricin. The lethal dose is 500 micrograms...which could certainly be administered with a pinprick. The stuff is found in the beans of the Castor Oil plant - it's easy to extract and separate - so I presume it would have been possible to make it in medieval times. (Oh - but that's no good - you wanted "Fast acting". Markov died 3 days later.) SteveBaker (talk) 12:36, 6 August 2009 (UTC)
- Not strictly a poison, but in medieval times, perhaps venom from a viper or something could fill the bill. Googlemeister (talk) 14:04, 6 August 2009 (UTC)
- These are are excellent examples of injected poisons. As for touch, the only thing I can think of off the top of my head is some variation of organophosphate nerve agent, like sarin or VX, which will soak right through the skin. Most of the really nasty ones, though, are also pretty volatile since they were designed to be employed as gas weapons. – ClockworkSoul 17:55, 6 August 2009 (UTC)
- This fits number 1, but not so much for 2 and 3. From our article on Suicide pill, The Central Intelligence Agency began experimenting with saxitoxin, an extremely powerful neurotoxin during the 1950s. It was rumored that they issued a tiny, saxitoxin-impregnated needle (hidden inside a fake silver dollar) to each American U-2 pilot, with instructions to stab themselves with it if shot down over the USSR.. Vespine (talk) 23:11, 6 August 2009 (UTC)
- Maaan -- from the article it sounds like that's one of those things where you're completely conscious, but can't breathe. I wouldn't do it. I'd take my chances with the Reds. --Trovatore (talk) 23:27, 6 August 2009 (UTC)
- A pin dipped in cyanide would work pretty quick too. As for medieval stuff -- well, Black widow venom might be a good candidate (better even than snake venom, which is not that fast-acting at any rate). FWiW 98.234.126.251 (talk) 23:17, 6 August 2009 (UTC)
- Not strictly a poison, but in medieval times, perhaps venom from a viper or something could fill the bill. Googlemeister (talk) 14:04, 6 August 2009 (UTC)
- I remember reading a story where an assassin used Dimethyl sulfoxide to carry a poison through the skin and into the target's bloodstream. It turns out that is actually possible. 152.16.59.102 (talk) 10:42, 8 August 2009 (UTC)
Frying an egg with a nuclear weapon.
I have found the figure 4.184×1017 J for a potential nuclear weapon yield, and 17500 Joules to fry an egg. I can't, however, find or understand any figures on the dissipation of energy per unit of distance from ground zero. Does anyone have these figures? I want to work out how far from a nuclear blast I need to be to fry an egg, my cooker is broken. SGGH ping! 11:52, 6 August 2009 (UTC)
- Not sure, but you need to make sure you can actually touch it afterward. :-) I'd suggest using the rule of thumb Johnny Carson suggested once for turkeys, and extrapolate down for eggs. He noted that if you heat your oven to 10,000 degrees, you can cook your turkey in 2 seconds - but you won't be able to touch it for six years. :-)Somebody or his brother (talk) 12:05, 6 August 2009 (UTC)
- That would be: 4x1017J, not 4.184x1017J...anyway: The big question here is how the energy dissipates over distance - and that's a real tough one because we don't know what's between you and the bomb. We also don't know what percentage of that energy is heat, radiation, the pressure wave, etc. But we also don't know what percentage of the energy impinging on the surface of the egg will be absorbed by it and turned into heat...an egg isn't like a 3" thick slab of solid lead...it doesn't stop all of the energy passing through it. Furthermore, it's very likely that if you throw enough radiant energy at an egg to cook it - but you do it all within a couple of milliseconds - then it's pretty safe to assume that a lot of that energy would actually go into splattering the egg all over surrounding landscape rather than cooking it. In short, I think the error bars on this thought experiment are so huge that the answer could be incorrect by several orders of magnitude. In other words, we might calculate the answer at 1 mile - when in fact it's something like 100 yards or perhaps 10 miles. Bottom line - we don't know. SteveBaker (talk) 12:31, 6 August 2009 (UTC)
- ... or indeed 93 million miles - see solar cooker. Gandalf61 (talk) 12:38, 6 August 2009 (UTC)
- If you wanted to do it "safely," use a parabolic mirror from a safe distance to focus the heat energy. Ted Taylor did this to light a cigarette with a nuclear bomb, once, if I recall from The Curve of Binding Energy. --98.217.14.211 (talk) 15:23, 6 August 2009 (UTC)
- Do NOT try this at home because cigarettes are unhealthy. Cuddlyable3 (talk) 20:03, 6 August 2009 (UTC)
- That's officially the coolest thing I've ever heard. I bet he told that story to girls for years. – ClockworkSoul 17:51, 6 August 2009 (UTC)
- If I recall correctly, there are places in the world where you can cook an egg just by dropping it on a rock. Probably somewhere with a lot of geothermal energy. Iceland? Jeremy Clarkson did it. Vimescarrot (talk) 18:23, 6 August 2009 (UTC)
- I'm sure you can do this on some of the rocks in Rotorua at least some of the time. However your eggs may also start to smell rotten if you leave them too long Nil Einne (talk) 20:33, 6 August 2009 (UTC)
- Cooking eggs in hot springs is a feature of Icelandic cuisine where the sulfur is thought to add to the flavor. Rmhermen (talk) 22:11, 6 August 2009 (UTC)
- I'm sure you can do this on some of the rocks in Rotorua at least some of the time. However your eggs may also start to smell rotten if you leave them too long Nil Einne (talk) 20:33, 6 August 2009 (UTC)
- If I recall correctly, there are places in the world where you can cook an egg just by dropping it on a rock. Probably somewhere with a lot of geothermal energy. Iceland? Jeremy Clarkson did it. Vimescarrot (talk) 18:23, 6 August 2009 (UTC)
- That's officially the coolest thing I've ever heard. I bet he told that story to girls for years. – ClockworkSoul 17:51, 6 August 2009 (UTC)
- From accounts of the effects on people and structures at the Hiroshima and Nagasaki bombings, along with U.S. tests, it should be possible to determine what distance from a blast of what magnitude would produce the desired effect. If your skillet is too close, the blast effect would scramble the egg and send it flying. But farther from ground zero the thermal effects might provide enough heat energy to do the trick, without excessive radiation or blast. Edison (talk) 18:43, 6 August 2009 (UTC)
- According to the article Effects of nuclear explosions, the thermal radiation would be enough to cause second-degree burns (and also to fry an egg) at about 3.2 kilometers from ground zero for a 20-kiloton bomb detonated at 1800 feet. Unfortunately, at that range the blast wave would be powerful enough to splatter the egg all over the place and also send the pan flying through the air at Mach speed. Also, the thermal radiation will have the effect of cooking the OP's skin, not just the egg, and it will hurt like you wouldn't believe. "Prompt" radiation will be negligible at that range, but radioactive fallout could be a concern if the nuclear explosion is upwind of the frying pan. FWiW 98.234.126.251 (talk) 00:18, 7 August 2009 (UTC)
- Ah, but the radiation travels at the speed of light whereas the blast wave is only somewhat faster than ordinary sound. I'm not sure exactly how much faster, but supposing it is at Mach 1.1, say, what you need to do is cook the egg for 8 seconds and then you have 1 second to cover the pan. --Anonymous, 01:28 UTC, August 7, 2009.
- But then you'll still have your (covered) pan flying through the air at Mach 1.1... 98.234.126.251 (talk) 02:27, 7 August 2009 (UTC)
- The trick is to use a sufficiently heavy lid. --Anon, 22:06 UTC, August 7, 2009.
- Wouldn't it squash the eggs and the pan as well? 98.234.126.251 (talk) 00:37, 8 August 2009 (UTC)
- So you also need a sufficiently strong pan, that's all. --Anon, 23:41 UTC, August 8, 2009.
- Wouldn't it squash the eggs and the pan as well? 98.234.126.251 (talk) 00:37, 8 August 2009 (UTC)
- The trick is to use a sufficiently heavy lid. --Anon, 22:06 UTC, August 7, 2009.
- But then you'll still have your (covered) pan flying through the air at Mach 1.1... 98.234.126.251 (talk) 02:27, 7 August 2009 (UTC)
- Ah, but the radiation travels at the speed of light whereas the blast wave is only somewhat faster than ordinary sound. I'm not sure exactly how much faster, but supposing it is at Mach 1.1, say, what you need to do is cook the egg for 8 seconds and then you have 1 second to cover the pan. --Anonymous, 01:28 UTC, August 7, 2009.
- According to the article Effects of nuclear explosions, the thermal radiation would be enough to cause second-degree burns (and also to fry an egg) at about 3.2 kilometers from ground zero for a 20-kiloton bomb detonated at 1800 feet. Unfortunately, at that range the blast wave would be powerful enough to splatter the egg all over the place and also send the pan flying through the air at Mach speed. Also, the thermal radiation will have the effect of cooking the OP's skin, not just the egg, and it will hurt like you wouldn't believe. "Prompt" radiation will be negligible at that range, but radioactive fallout could be a concern if the nuclear explosion is upwind of the frying pan. FWiW 98.234.126.251 (talk) 00:18, 7 August 2009 (UTC)
- What you do is cook it inside a supersonic vehicle. Just outrun the blast wave. — DanielLC 05:02, 7 August 2009 (UTC)
- You could also melt some Velveeta over it during your supersonic trip, allowing you to make a nice Mach & cheese. DMacks (talk) 05:37, 7 August 2009 (UTC)
- Supersonic flight tends to heat up the skin of aircraft fairly well. When Concorde was fliying, it would be at 100C on the outside surface of the aircraft. Fry the egg on that. Googlemeister (talk) 13:34, 7 August 2009 (UTC)
- Just make sure you hold on tight so the shockwaves don't carry you (or the pan) away. 98.234.126.251 (talk) 00:26, 8 August 2009 (UTC)
- Supersonic flight tends to heat up the skin of aircraft fairly well. When Concorde was fliying, it would be at 100C on the outside surface of the aircraft. Fry the egg on that. Googlemeister (talk) 13:34, 7 August 2009 (UTC)
- You could also melt some Velveeta over it during your supersonic trip, allowing you to make a nice Mach & cheese. DMacks (talk) 05:37, 7 August 2009 (UTC)
- What you do is cook it inside a supersonic vehicle. Just outrun the blast wave. — DanielLC 05:02, 7 August 2009 (UTC)
Please clarify the question: 1) Which egg? 2) Can the egg be still inside the bird ? 3) Is it a requirement that the OP survive to eat the egg ? 4) Is this method of frying to be used regularly until the OP's cooker can be fixed ? Cuddlyable3 (talk) 20:20, 6 August 2009 (UTC)
- You forgot point 4. Will the egg be radioactive enough to kill you if you eat it. Maybe not the egg, but I bet the pan would be pretty nasty to be around. Googlemeister (talk) 20:36, 6 August 2009 (UTC)
- Mmmm... probably not. Thermal radiation extends much further than the ionizing radiation does in most scenarios (neutron bombs excepted). Mildly radioactive eggs will probably not kill you. Trivia: During Operation Teapot, they did test the effects of nuclear tests on varying types of food products, including beer. Radioactive beer apparently is somewhat stale tasting but definitely potable. --98.217.14.211 (talk) 01:20, 7 August 2009 (UTC)
- How do you know what it tastes like -- were you the brave soul who tasted it? ;-) 98.234.126.251 (talk) 02:24, 7 August 2009 (UTC)
- The reports actually discuss the results of their atomic taste testing... --98.217.14.211 (talk) 14:06, 7 August 2009 (UTC)
- How do you know what it tastes like -- were you the brave soul who tasted it? ;-) 98.234.126.251 (talk) 02:24, 7 August 2009 (UTC)
- Mmmm... probably not. Thermal radiation extends much further than the ionizing radiation does in most scenarios (neutron bombs excepted). Mildly radioactive eggs will probably not kill you. Trivia: During Operation Teapot, they did test the effects of nuclear tests on varying types of food products, including beer. Radioactive beer apparently is somewhat stale tasting but definitely potable. --98.217.14.211 (talk) 01:20, 7 August 2009 (UTC)
- The U.S. also exposed live pigs to nuclear blasts (in lieu of U.S. servicemen) at close enough distances to produce 1st, 2nd and 3rd degree thermal burns. So you could have ham or bacon with your hypothetical eggs. Wear oven mitt or welding gloves, and hold the skillet out the window for a few seconds until the ham and eggs are cooked to taste, then bring in and cover before the blast wave and fallout arrive. Edison (talk) 05:30, 7 August 2009 (UTC)
- Would that be Operation Crossroads? ~AH1(TCU) 02:10, 8 August 2009 (UTC)
From Tsipis, K. "Arsenal" c. 1983 App. D: (brokenly translated by me to an ASCII approximation of the math):
- Energy of a nuclear explosion = 4.2x10E15 joule / megaton
- Neutron flux in rads = 5x10E13 * (Y/R**2)*exp(-R*rho/780)
- Y is yield in megatons henceforth, rho is density of air = 1.1 g/l, exp is
the natural logarithmthe exponent of Euler's number, the inverse of the natural logarithm oops, I meant what a calculator does when you push the e**x key!, R is distance in feet (bear with me, the guy was writing for an American audience) - And neutron flux and the forthcoming peak overpressure are important. Presumably you will be persuading someone else to hold the frypan out there, preferably swinging the pan face on into the blast at just the right time, otherwise I don't think the egg will get properly cooked. You don't want the egg to be overdone on one edge, right?
- Y is yield in megatons henceforth, rho is density of air = 1.1 g/l, exp is
- Thermal radiation for an airburst is Q = 1.8X10E3*(Y/R**2)*t
- Q is cal/cm**2, R is in km, t is the transmission factor through the atmosphere, ~= 0.8 at 1km
- Groundburst, use 1.15 instead of 1.8
- And the big question which sunk my ship is whether these are "big" calories or "little" calories. Comments later.
- Also from Tsipis: there are two thermal pulses, the "prompt" pulse is 0.1% of the total energy before the fireball becomes opaque (the double-flash) and the following emission when the fireball has cooled enough, for a 1-megaton device this lasts about 10 sec and emits approx. 1/3 of the total energy.
- Airblast, as in peak overpressure: P-naught = 3300*(Y/R**3) + 192 * (Y/R**3)**-2
- P-naught is in psi, R is in "kilofeet"
- Again, this will be important for whoever you persuaded to cook your eggs. The peak overpressure at your location will determine whether their arm gets blown off. (Or whether the tank gets pushed away)
- So I've calculated so far that by analogy to my stove burner, you could cook a good egg at 4.5 km distance from a 500 kiloton detonation, or 0.9 km from a 20 kiloton Hiroshima-style egg cooker. I need to look at those peak overpressures though, to find out if 1 psi overpressure exceeds the sonic velocity needed to push that tank off of the frypan. :) Franamax (talk) 05:01, 8 August 2009 (UTC)
- At 0.9 km from a 20-kt "egg cooker", the blast wave is strong enough to throw a train off the tracks. 98.234.126.251 (talk) 11:01, 8 August 2009 (UTC)
Julia Set
Does the Julia Set happen in nature? If so, where? --Reticuli88 (talk) 12:48, 6 August 2009 (UTC)
- In a literal sense, no, because a (typical) Julia set has complexity (lack of smoothness) on every scale, whereas the complexity of natural objects is limited ultimately by the size of atoms and molecules. However, it is possible to use Julia sets and other fractals to model or approximate natural objects - see fractal landscape for an example. Gandalf61 (talk) 13:04, 6 August 2009 (UTC)

Romanesco broccoli
- Idealised, perfect, fractals are impossible in the real world - but things like clouds, landscapes, trees, coastlines, (broccoli!)...those are all fractal to a point...they can't be fractal down to the smallest scales of atoms because the laws of nature are very different down at those sizes. Coastlines can't be fractal above the scale of a continent because the earth isn't flat. Clouds can't be fractal on scales larger than a few miles because the weather patterns that form them aren't fractal - and around the size of a single water droplet, the cloud is dominated by the 'new' force of surface tension that's not relevant when it's hundreds of feet across. But within the range of a couple of inches to a mile or so, clouds are pretty decent fractals. You can even calculate things like their "fractal dimension".
- So if, for example, you measure the angle between the trunk of a tree and the first two main branches - you'll find that the angles are pretty much the same as between the main branches and the secondary branches, and between the secondary branches and the tertiary branches - and so on down to the smallest twigs. The ratio of the diameter of the trunk and that of the main branches follows a similar rule. One consequence of this is that if you cut a main branch off of a tree and stick it upright into the ground...it looks more or less like a little tree. This 'self-similarity' is the same kind of thing you're seeing in the mathematical fractals like the Mandelbrot and Julia sets - and it's at the heart of fractal mathematics. It's interesting to note that humans find both natural and mathematical fractals beautiful. We've evolved to enjoy things like mountainscapes, trees and clouds - and that's probably at the heart of the reason that we enjoy mathematical fractals. SteveBaker (talk) 13:59, 6 August 2009 (UTC)

- And we enjoy Romanesco broccoli because it's not only delicious and nutritious, but eating it makes you smarter and better at math. That's a scientific fact! ;-) —Scheinwerfermann T·C17:56, 6 August 2009 (UTC)
- [citation needed] Nil Einne (talk) 20:22, 6 August 2009 (UTC)
- Yeah - indeed, I find it very hard to believe that anyone actually eats broccoli without being forced to by their moms. We need references for any claim that someone actually does that! SteveBaker (talk) 00:31, 7 August 2009 (UTC)
- Just steam them a little so then soften up! Or put them in the oven with a little bit of olive oil and something slightly spicy. Mother will be soooo happy with you. --98.217.14.211 (talk) 01:09, 7 August 2009 (UTC)
- [citation needed] SteveBaker (talk) 01:34, 7 August 2009 (UTC)
- I like broccoli. This is unpublished work. --Trovatore (talk) 01:36, 7 August 2009 (UTC)
- So do I. Broccoli is awesome (though easy to ruin if you don't know what you're doing). -- Captain Disdain (talk) 11:54, 7 August 2009 (UTC)
- WP:NOR. SteveBaker (talk) 18:15, 7 August 2009 (UTC)
- It's not my fault you're not bold enough to love broccoli, Steve. -- Captain Disdain (talk) 17:47, 9 August 2009 (UTC)
- WP:NOR. SteveBaker (talk) 18:15, 7 August 2009 (UTC)
- So do I. Broccoli is awesome (though easy to ruin if you don't know what you're doing). -- Captain Disdain (talk) 11:54, 7 August 2009 (UTC)
- I like broccoli. This is unpublished work. --Trovatore (talk) 01:36, 7 August 2009 (UTC)
- [citation needed] SteveBaker (talk) 01:34, 7 August 2009 (UTC)
- Just steam them a little so then soften up! Or put them in the oven with a little bit of olive oil and something slightly spicy. Mother will be soooo happy with you. --98.217.14.211 (talk) 01:09, 7 August 2009 (UTC)
- Yeah - indeed, I find it very hard to believe that anyone actually eats broccoli without being forced to by their moms. We need references for any claim that someone actually does that! SteveBaker (talk) 00:31, 7 August 2009 (UTC)
- [citation needed] Nil Einne (talk) 20:22, 6 August 2009 (UTC)
- And we enjoy Romanesco broccoli because it's not only delicious and nutritious, but eating it makes you smarter and better at math. That's a scientific fact! ;-) —Scheinwerfermann T·C17:56, 6 August 2009 (UTC)
Omg! I thought that type of broccoli was fake. It's real!--Reticuli88 (talk) 18:32, 7 August 2009 (UTC)
- Frightening, horrifyingly, gutwrenchingly real. SteveBaker (talk) 22:56, 7 August 2009 (UTC)
- You forgot 'eldritch'. —Tamfang (talk) 03:35, 11 August 2009 (UTC)
Jubilee Clips?
Where did the name 'Jubilee Clip' come from? Why are they called this?57.86.133.98 (talk) 14:29, 6 August 2009 (UTC)
- Jubilee Clip states that the producing companies were named "Jubilee Components Ltd and Jubilee Clips Ltd", having been invented by Royal Naval Commander, Lumley Robinson, in 1921. 1921 was not a Christian jubilee, nor was in a jubilee of George V of the United Kingdom. So, I am at a loss as to why. SGGH ping! 14:49, 6 August 2009 (UTC)
- Perhaps Lumley Robinson celebrated his own golden jubilee in 1921. Cuddlyable3 (talk) 18:24, 6 August 2009 (UTC) (updated)
- Possible - I found that he died in 1939 [6], if he was 50 in 1921, then he would have been 68 in 1939 - probably typical life span for that time.
- Robinson was born in 1877. MilborneOne (talk) 22:18, 6 August 2009 (UTC)
- Possible - I found that he died in 1939 [6], if he was 50 in 1921, then he would have been 68 in 1939 - probably typical life span for that time.
- Perhaps Lumley Robinson celebrated his own golden jubilee in 1921. Cuddlyable3 (talk) 18:24, 6 August 2009 (UTC) (updated)
- Jubilee Clip states that the producing companies were named "Jubilee Components Ltd and Jubilee Clips Ltd", having been invented by Royal Naval Commander, Lumley Robinson, in 1921. 1921 was not a Christian jubilee, nor was in a jubilee of George V of the United Kingdom. So, I am at a loss as to why. SGGH ping! 14:49, 6 August 2009 (UTC)
Poisoning the sea
If you took a cupful of something like, say, Botulinum toxin, and poured it into the sea...how big would the effect be? Vimescarrot (talk) 18:27, 6 August 2009 (UTC)
- It would be time related. At the moment of pouring the concentration would be high, but the toxin will start to diffuse away, and some time later, depending on the speed of the wave and tide actions, the concentration will fall below a "no effect level". Ronhjones (Talk) 18:56, 6 August 2009 (UTC)
- What kind of radius are we talking before that happens? Vimescarrot (talk) 19:27, 6 August 2009 (UTC)
- Probably not too big - no more than a thousand feet I'd say. A cup is 236 cm3, and that in a cubic kilometer of water is a concentration of 2 × 10-13 by volume. Botulinum toxin has a toxicity of 1 ng/kg, but only when administered intravenously. Its effect would dissipate quite rapidly. On the other hand, if you spill a tanker full of oil into the sea, the entire area can be quite severely affected, not least because oil floats on the surface. Dcoetzee 19:40, 6 August 2009 (UTC)
- What kind of radius are we talking before that happens? Vimescarrot (talk) 19:27, 6 August 2009 (UTC)
The effects in order of increasing radius (dilution) would be: (i) kills everything, (ii) kills some things and paralyses others, (iii) paralyses some things only, (iv) temporarily hides wrinkles on a mermaid, (v) does nothing and can't be detected, and (vi) can be marketed as a homeopathic remedy for literally anything. Cuddlyable3 (talk) 19:58, 6 August 2009 (UTC)
- One old saw that I always thought was unfortunate was, "The answer to pollution is dilution." Tempshill (talk) 20:05, 6 August 2009 (UTC)
- I've heard that one as "The solution to pollution is dilution." More rhyming makes Mother Nature smile while we poison her. DMacks (talk) 22:20, 6 August 2009 (UTC)
- Diluting a toxin until it has negligible effect has more basis in scientific reason than the concept of "mother nature". This is not to suggest that I enjoy polluting lakes, rivers, or oceans - but a thorough, unbiased analysis is necessary to determine if a particular action is environmentally harmful, or merely "repugnant". Nimur (talk) 23:44, 6 August 2009 (UTC)
- I've heard that one as "The solution to pollution is dilution." More rhyming makes Mother Nature smile while we poison her. DMacks (talk) 22:20, 6 August 2009 (UTC)
- Of note is that we (in which "we" can mean I think any of the five "nuclear weapons states," among others) have, in fact, dumped tons and tons of radioactive wastes into deep sea trenches. The idea here is that the buffer of the ocean would generally keep it in one place and if it got spread out a bit, no harm, no foul (unless you are some sort of deep sea creature who is unfortunate enough to live near it). --98.217.14.211 (talk) 01:05, 7 August 2009 (UTC)
- By "we", do you mean the former Soviet Union? 98.234.126.251 (talk) 02:29, 7 August 2009 (UTC)
- I specified who I meant. The US, the USSR, the UK, France—all of them did ocean dumping of radioactive wastes. Look it up! (I don't know about China, but I would be surprised if they didn't.) --98.217.14.211 (talk) 13:07, 7 August 2009 (UTC)
- I'm absolutely sure the USSR and China did the most radioactive dumping of all (easy enough for them, cause they don't let anyone know what they're doing); France and the US did some "dumping" of radioactives (mainly through atmospheric nuclear weapons testing), but not nearly as much. I don't think the UK did any, though -- why don't you look it up? 98.234.126.251 (talk) 22:25, 7 August 2009 (UTC)
- I specified who I meant. The US, the USSR, the UK, France—all of them did ocean dumping of radioactive wastes. Look it up! (I don't know about China, but I would be surprised if they didn't.) --98.217.14.211 (talk) 13:07, 7 August 2009 (UTC)
- By "we", do you mean the former Soviet Union? 98.234.126.251 (talk) 02:29, 7 August 2009 (UTC)
- The Convention for the Protection of the Marine Environment of the North-East Atlantic says the UK has dumped 200kg of plutonium into the Irish Sea, per the Sellafield article. -- Finlay McWalter • Talk 22:29, 7 August 2009 (UTC)
- Only 200 kg? The Soviets used to dump nuclear waste in the Arctic Ocean by the shipload. BTW, the claim in the Sellafield article that you're referring to has a "citation needed" tag. FWiW 98.234.126.251 (talk) 22:41, 7 August 2009 (UTC)
- There is a significant difference between "nuclear waste" - which can be things as (relatively) harmless as the disposable paper coveralls used by workers in nuclear power plants...and "plutonium". The lethality of plutonium is not just due to the radioactivity - but it's also poisonous in a conventional sense...however, a lot depends on how big the chunks were that they dumped - what it was encased in - and (especially) what mix of isotopes it contained. If it were Plutonium 241 - encased in a good amount of lead and concrete and in small chunks, then after 50 years, there would only be 1/16th as much of the stuff as they dumped - most of it having decayed to something less lethal. SteveBaker (talk) 22:55, 7 August 2009 (UTC)
- By "nuclear waste", I specifically meant high-level nuclear waste, like plutonium, fission products, etc. As for what the Soviets used to dump in the ocean: they used to dump many tons of spent nuclear fuel, which is even worse than plutonium. 98.234.126.251 (talk) 23:12, 7 August 2009 (UTC)
- There is a significant difference between "nuclear waste" - which can be things as (relatively) harmless as the disposable paper coveralls used by workers in nuclear power plants...and "plutonium". The lethality of plutonium is not just due to the radioactivity - but it's also poisonous in a conventional sense...however, a lot depends on how big the chunks were that they dumped - what it was encased in - and (especially) what mix of isotopes it contained. If it were Plutonium 241 - encased in a good amount of lead and concrete and in small chunks, then after 50 years, there would only be 1/16th as much of the stuff as they dumped - most of it having decayed to something less lethal. SteveBaker (talk) 22:55, 7 August 2009 (UTC)
- Only 200 kg? The Soviets used to dump nuclear waste in the Arctic Ocean by the shipload. BTW, the claim in the Sellafield article that you're referring to has a "citation needed" tag. FWiW 98.234.126.251 (talk) 22:41, 7 August 2009 (UTC)
- The Convention for the Protection of the Marine Environment of the North-East Atlantic says the UK has dumped 200kg of plutonium into the Irish Sea, per the Sellafield article. -- Finlay McWalter • Talk 22:29, 7 August 2009 (UTC)
If they are able to carry and transmit energy, does that mean that they contribute to the stress energy tensor as Einstein's continuity equation seems to indicate? 70.24.37.78 (talk) 18:46, 6 August 2009 (UTC)
- Yes, I believe so. The stress energy tensor includes everything in the universe, as far as I know. --Tango (talk) 21:07, 6 August 2009 (UTC)
- Yes, absolutelly, and that contribution will also have to be included in the Einstein's field equations which will feed back as a gravity source. That's the main reason why Einstein's field equations are not linear. Dauto (talk) 21:49, 6 August 2009 (UTC)
- Actually, gravitational stress-energy cannot be expressed as a nonzero tensor. It can, however, be expressed as a pseudotensor. See Stress-energy tensor#Gravitational stress-energy and Stress-energy-momentum pseudotensor. Red Act (talk) 01:54, 7 August 2009 (UTC)
- Gravitational waves do not contribute to the stress-energy tensor. In weak-field gravity they clearly carry energy, as seen in PSR B1913+16, so doesn't express conservation of energy even though it looks like it ought to. As Red Act said, you can define a "pseudotensor" that includes gravitational energy, but that breaks general covariance. In general spacetimes it's far from clear what energy even is. See this Physics FAQ entry and also §19.4 of MTW ("Mass and Angular Momentum of a Closed Universe"). -- BenRG (talk) 04:47, 7 August 2009 (UTC)
Jumping Americans cause earthquake in China
Is this possible or just a myth? If most of the population of the USA co-ordinated jumping in the air at the same time, would this cause an earthquake in China? Or vice-versa - jumping Chinese cause an earthquake in the US? 92.26.30.9 (talk) 21:21, 6 August 2009 (UTC)
- Seems to me I read this in one of Cecil Adams books, or antoher like that. the short answer - it is a myth. If 300 million people exert enough force, coming down at the same time, and each is an average of a little over 100 pounds, that's only 30 billion pounds of force, and when cmopared witht he mass of the earth (not sure the exact number, but I'm sure more than what, 30 million tons?) it wouldn't be nearly enough. it's like saying "what's the effect if a few thousand people tried to push a WW II battleship." You also have the problem of the cushioning of the erth's crust, too.Somebody or his brother (talk) 21:57, 6 August 2009 (UTC)
- As an aside, I'm pretty sure it's come up here before that one person can push a battleship (actually a destroyer IIRC, but same sort of thing) when it's in still water enough that it moves. 91.143.188.103 (talk) 17:29, 8 August 2009 (UTC)
- Earth's mass is 5.9742 × 1024 kg, according to Wikipedia. Rmhermen (talk) 22:12, 6 August 2009 (UTC)
- There would also be the counteracting force of the gravitational pull of the 30,000 airborne people just above the surface of the planet Earth. That too should probably be factored into any total calculation. Bus stop (talk) 22:10, 6 August 2009 (UTC)
- Uncle Cecil answered this question, sort of, in 1984, starting off with one of his better intros, "Believe it or not, I'm actually going to answer this ridiculous question." Tempshill (talk) 22:15, 6 August 2009 (UTC)
- It certainly isn't going to throw "earth of its axis". Jumping or not the centre of gravity of "earth+humans" stays the same. 1 human (75kg) jumping to 0.5m high against gravity at 10m/s^2 has an energy of 375J. If 1.000.000.000 people do that its 375GJ or about 80t TNT. The Hiroshima bomb was about 15000t TNT. There was no earthquake in the USA after that. I always thought it is more interesting if by coordinated repeated jumping you could somehow trigger some resonance frequency of the earth/earth's crust. But I would guess the calculation above suggests that the energies involved are far too small - apart from the considerable synchronization problem195.128.250.173 (talk) 22:41, 6 August 2009 (UTC)
- The part of this that most people forget is that the total momentum of the earth/people system never changes as they all jump. So when they push off - they make the earth move a little bit in one direction - but the force of gravity pulling them back down again accelerates the earth towards them. When they land, all of those forces exactly cancel out and the earth is exactly where it was before they jumped. That's not to say that there wouldn't be some vibration and such - but you can't knock the earth out of orbit that way. SteveBaker (talk) 01:33, 7 August 2009 (UTC)
- If they have any effect at all, your jumping Americans are probably more likely to trigger an earthquake along the San Andreas fault (but only because it is going to happen soon anyway). Dbfirs 02:07, 7 August 2009 (UTC)
- Ah. but what if Nikola Tesla had determined the sequencing and used radio to direct the repeated jumping, so as to establish a resonance which focussed the reflected disturbance at a particular spot in China? The amplitude of motion of the earth there should be huge. Edison (talk) 05:24, 7 August 2009 (UTC)
- Tesla was a clever guy - but he was also, in many ways a typical clueless nut-job! This is annoying because many of the full-time nut-jobs out there latch on to every word he said and insist that it must all be true (I'm not putting User:Edison into that category of course!). In truth, maybe 10% of the things Tesla worked on were sheer first-class brilliance - the other 90% were WAY out in la-la-land. His theories on resonance mostly fall into the 90% category. He extrapolated his small scale experiments into gigantic thought-experiments without really thinking about what he was saying - and came up with some ridiculous claims as a result. The Mythbusters attacked some of those in one of their shows - and while the results they had were somewhat surprising, they all fell FAR short of Tesla's claims. Notably, his ideas about resonance...that if a system oscillates - and you add just a tiny bit of energy into each oscillation at just the right moment - then the energy and the size of the oscillation will build up without limit until the object disintigrates. Sure, there are some kinds of resonance that can destroy things in impressive ways...opera singers breaking wine glasses, the spectacular failure of the Tacoma Narrows Bridge (1940), etc. But unaccountably (for such a smart guy) he completely failed to realise that if the amount of mechanical energy the system dissipates due to internal friction, heating, etc during the course of one oscillation exceeds the amount of energy you are adding with each oscillation - then the amount of oscillation won't increase at all. And that's why your idea fails...sure, all of the people jump - and the earth oscillates a little bit. However, the planet is so big that all of the energy from that jumping has been absorbed LONG before it's time to jump again...so no resonance will build up. SteveBaker (talk) 18:01, 7 August 2009 (UTC)
- Indeed I wonder if this idea is based in part on the myth that China is exactly on the oppposite side of the planet of the US (common in cartoons for example). This isn't true, that's the Indian Ocean [7] [8] Nil Einne (talk) 09:25, 7 August 2009 (UTC)
- It's quite impressive how little of the inhabited land surface of the Earth has an antipodal point that's also on land and inhabited.
- The closest noticeable land to my antipodes is some little dot in the French Southern and Antarctic Lands. But even that is several hundred miles from the right spot. --Trovatore (talk) 23:09, 7 August 2009 (UTC)
- We actually have an article about this which I partially remembered but didn't find at the time but have now, it's Antipodes. These maps are perhaps better for finding them in general terms and for visualising them File:Antipodes LAEA.png & File:Antipodes rect2160.png. Parts of China do have antipodes but they're in South America of course not north. Nil Einne (talk) 09:51, 8 August 2009 (UTC)
- At the very least, the Americans would all get a little bit of exercise, which is not a bad deal. Googlemeister (talk) 13:30, 7 August 2009 (UTC)
- Indeed I wonder if this idea is based in part on the myth that China is exactly on the oppposite side of the planet of the US (common in cartoons for example). This isn't true, that's the Indian Ocean [7] [8] Nil Einne (talk) 09:25, 7 August 2009 (UTC)
August 7
UV filters
I'm interesting in finding out if some sunglasses and a camera filter that I have are actually doing a good job of blocking UV light. Is there any easy or cheap way to measure the amount blocked? Any way to tell if cheap sunglasses are actually helping or just hurting or to see if my UV camera filter is doing anything put protecting the lens? Tobyc75 (talk) 02:38, 7 August 2009 (UTC)
- Sunglasses in some countries are standards tested and rated, see Sunglasses#Standards, but may depend where you live, and don't know how you'd do this yourself if you live somewhere where they're not. In general, in Australia, the rule of thumb is don't buy them if they're so cheap as not to be standards approved. Re the camera what are you actually wanting the UV filter to do? Try the identical shot with and without the filter on and compare the photos down to a pixel level - but many people (myself included) chiefly use the UV filter to protect the lens itself rather than for any real effect. See UV filter and Photographic_filter#Clear_and_ultraviolet. --jjron (talk) 03:10, 7 August 2009 (UTC)
- I bought a Hoya Super HMC UV filter for a camera lens, and inside the package was a paper card with a specially treated dot. The dot changes color when exposed to UV light. Outdoors in sunlight, the dot would change color, and when I put the filter in front of it, it would go back to white. I don't know what the dot was made of, or its wavelength response, or sensitivity, or anything else that would make it a quantitative test. -- Coneslayer (talk) 12:42, 7 August 2009 (UTC)
- A fluorescent thing would help - but only proves that a narrow band of uv is being blocked/transmitted in general. A better solution would be a photodiode (light meter) and a filter that you know only allows uv (but not visible)83.100.250.79 (talk) 13:00, 7 August 2009 (UTC)
- I don't know that it matters to you - but I recently got a new set of eye glasses - specifically for driving. I asked about photochromic lenses (the kind that change color) and asked if they were able to block UV efficiently. I was surprised to hear that pretty much all normal eye glasses sold through mainstream opticians in the US now have UV-protective coatings as a matter of course...even the glasses that are completely clear! So you don't even need tinted glass - a completely clear lens can be an efficient UV blocker if it has the right coatings. SteveBaker (talk) 17:46, 7 August 2009 (UTC)
- In fact you often don't even need coatings - CR-39 which is (probably) the most popular lens material blocks almost all uv itself. It's quite difficult to get a glass or plastic lens that doesn't cut out practically all uv.83.100.250.79 (talk) 23:24, 7 August 2009 (UTC)
- UV filters are essential for outdoor photography using film, since film is more sensitive to UV than to visible light, and even the near-opacity of glass to UV isn't enough. Digital cameras are the other way around: they're more sensitive to visible than to UV, and the glass that makes up the lenses is sufficient to filter out the rest. --Carnildo (talk) 23:00, 7 August 2009 (UTC)
- Thanks for the responses. Re: the sunglasses, I was actually interested in whether the cheap children's sunglasses did more harm than good, and I'm in the US, so I'm not sure if I've seen any government testing stickers on them. I'd guess that, yes the cheap ones most likely are pretty bad. As for the camera filter, I mostly just use it to protect the lens. It's on a DSLR and I can't see any difference between with and without the filter. Tobyc75 (talk) 23:19, 7 August 2009 (UTC)
- As I mentioned above in response to SteveBaker - most optical materials block uv anyway, so your uv filter is probably only protecting the lens (which is a good thing anyway). Don't know about sunglasses - you're probably right. 83.100.250.79 (talk) 23:43, 7 August 2009 (UTC)
- The trouble with sunglasses is that the darkened lenses fool people into looking at brighter objects without squinting and the reduction in visible light causes your irises to open wider to allow in more light. So if the sunglasses aren't really good a blocking UV, the result is much more UV entering the eye than would normally be the case if you weren't wearing them. However, at least in Europe, those kinds of sunglasses are supposedly banned. SteveBaker (talk) 03:05, 8 August 2009 (UTC)
- As I mentioned above in response to SteveBaker - most optical materials block uv anyway, so your uv filter is probably only protecting the lens (which is a good thing anyway). Don't know about sunglasses - you're probably right. 83.100.250.79 (talk) 23:43, 7 August 2009 (UTC)
- Thanks for the responses. Re: the sunglasses, I was actually interested in whether the cheap children's sunglasses did more harm than good, and I'm in the US, so I'm not sure if I've seen any government testing stickers on them. I'd guess that, yes the cheap ones most likely are pretty bad. As for the camera filter, I mostly just use it to protect the lens. It's on a DSLR and I can't see any difference between with and without the filter. Tobyc75 (talk) 23:19, 7 August 2009 (UTC)
Bike gearing
This came up in a discussion about motorbike gearing, but probably easier to ask in the context of bicycle gearing and in a simplified form.
Consider a multi-speed bike with typical front and rear gears, and for convenience express the ratios as FR:RR. Now lets take two gear ratios, say 28 teeth front and 14 teeth on the rear, i.e., 28:14, and 48 front & 24 rear, i.e., 48:24. Mathematically both these ratios simplify to 2:1, which is the easy bit.
For a perhaps less 'artificial' or simplistic example consider ratios of 38:16 and 48:20, which come out to 2.38 & 2.40, which again is mathematically essentially the same.
The question is that while these ratios are basically the same mathematically, physically is there a difference between changing the gearing on the 'bigger' chainring or the 'smaller' chainring, i.e., would these gear ratios require say an identical amount of energy to push them?
I'm thinking in terms of effects due to torque, angular velocity or momentum due to the different sized rings, or other relevant things. Anyone with better knowledge of this than me... Thanks in advance. --jjron (talk) 02:58, 7 August 2009 (UTC)
- Torque (rotational force) depends on radius. What is the relationship between number of teeth and the circumference of the gear? What is the relationship between circumference and radius? Based on those answers, you can determine if tooth-ratio is equivalent to torque-ratio. Once you've figured it out yourself, you can check with our gear ratio article for more information. DMacks (talk) 03:24, 7 August 2009 (UTC)
- Yes, you are right. what matters here is the ratio so, as you pointed out, 28:14 and 48:24 will lead to essentially the same torque, assuming that other factors such as friction can be considered negligible. Dauto (talk) 04:36, 7 August 2009 (UTC)
From Surely You're Joking, Mr. Feynman!:
| “ | Near the end of the summer I was given my first real design job: a machine that would make a continuous curve out of a set of points--one point coming in every fifteen seconds--from a new invention developed in England for tracking airplanes, called "radar." It was the first time I had ever done any mechanical designing, so I was a little bit frightened. I went over to one of the other guys and said, "You're a mechanical engineer; I don't know how to do any mechanical engineering, and I just got this job. "There's nothin' to it," he said. "Look, I'll show you. There's two rules you need to know to design these machines. First, the friction in every bearing is so-and-so much, and in every gear junction, so-and-so much. From that, you can figure out how much force you need to drive the thing. Second, when you have a gear ratio, say 2 to 1, and you are wondering whether you should make it 10 to 5 or 24 to 12 or 48 to 24, here's how to decide: You look in the Boston Gear Catalogue, and select those gears that are in the middle of the list. The ones at the high end have so many teeth they're hard to make. If they could make gears with even finer teeth, they'd have made the list go even higher. The gears at the low end of the list have so few teeth they break easy. So the best design uses gears from the middle of the list." I had a lot of fun designing that machine. By simply selecting the gears from the middle of the list and adding up the little torques with the two numbers he gave me, I could be a mechanical engineer! |
” |
70.90.174.101 (talk) 07:52, 7 August 2009 (UTC)
- And the parts in the middle of the spec range are most likely to be available on short turnaround. As I mentioned above, there's a difference between "it's in the catalog" and "you can order it and receive it in a reasonable time". This is what's mean by the "OTS" in "COTS." Nimur (talk) 17:21, 7 August 2009 (UTC)
- Naturally, if we neglect friction, the torque onto the pedals of a bicycle needed to drive it forward with a specified force is dependent on just the gear ratio. However, the torque is transferred to tension in the chain and that is inversely proportional to the radius of a gear. Hence, to save the chain it's best to use preferably large cogwheels, as then both the tension and the bending of the chain are minimum. — Pt (T) 02:39, 8 August 2009 (UTC)
- The actual diameters also matter if you're dealing with shifting Derailleur gears. This shift is a change of absolute radius and chain-slack on one gear, not a ratio between two gears, but the goal is a shift of relative ratio. That means if the gear has a large radius, a relatively large change is needed to accomplish a shift of ratio. That is, if you are doing a ratio shift 3:1→2:1, changing gears 30:10→20:10 is a change of 10 whereas 60:20→40:20 is a change of 20. WP:OR says derailleurs and chains are more susceptible to jamming with very large changes. Also, the derailleur needs to take up all the slack, so the larger the change, the larger the derailleur cage needs to be. At the other extreme (small gears), a small change in radius represents a more significant change in ratio, so they need to be manufactured more accurately and precisely. DMacks (talk) 05:09, 8 August 2009 (UTC)
- Naturally, if we neglect friction, the torque onto the pedals of a bicycle needed to drive it forward with a specified force is dependent on just the gear ratio. However, the torque is transferred to tension in the chain and that is inversely proportional to the radius of a gear. Hence, to save the chain it's best to use preferably large cogwheels, as then both the tension and the bending of the chain are minimum. — Pt (T) 02:39, 8 August 2009 (UTC)
- Friction, however, does play a small but noticeable role at the margins, a percentage point or two according to the books I've read on the topic. IN a nutshell, the straighter your chain, the lower the friction; not only does this marginally reduce your pedaling effort, but it also reduces the wear on your chain.--Robert Merkel (talk) 23:09, 10 August 2009 (UTC)
conservation of momentum and heat
Does a system gain momentum when it absorbs blackbody radiation, or when it emits it? Thus even though photons are massless, are they potentially carriers of momentum? (This was never covered in AP Physics really...) John Riemann Soong (talk) 08:41, 7 August 2009 (UTC)
Yes, photons carry momentum (despite their rest mass being exactely zero, the classical formula p=m*v does not hold here). Yes, systems absorbing or emitting electromagnetic radiation in a non-uniform way will gain or lose momentum.
See http://en.wikipedia.org/wiki/Photon#Physical_properties
- See also radiation pressure and the Nichols radiometer, which can measure the force imparted by a light beam. AlmostReadytoFly (talk) 10:16, 7 August 2009 (UTC)
- Doesn't this mean the Earth is actually gaining and losing momentum just through radiation alone...? Or is it assumed that global net radiation flux (Rnet?) is zero? John Riemann Soong (talk) 11:12, 7 August 2009 (UTC)
- It's pretty close to zero, yes. If it were not, it's difficult to see how the Earth could remain habitable over millions of years. Although photons don't have rest mass, they do carry energy. The formula gives the relationship between these quantities, where is momentum.--Leon (talk) 11:27, 7 August 2009 (UTC)
- Well, I mean, does Rnet account for photosynthesis? So all those plant sugars and fossil fuels ... are actually carriers of momentum? It's strange cuz in high school (AP Physics C) they always told me, that excepting tiny particles colliding with the Earth, or asteroids, that as far as momentum was concerned, we could treat Earth as a closed system, with no mention of light as a bearer of momentum.
- Momentum ain't energy! Momentum isn't really "stored" per se, and whilst the momentum of the Earth would be changed by this, the momentum of the Earth--bomb system would be constant. But you would be converting energy from one form to another. --Leon (talk) 12:31, 7 August 2009 (UTC)
- I also remember solving a few problems about conservation of momentum on a system composed of bound protons and neutrons...(yeah a nucleus). The nucleus underwent nuclear decay, ejecting various daughter nuclei. Since the original nucleus was at rest to begin with, we used this condition to solve for the velocities and masses of some of the daughter nuclei (being given the mass of the original nucleus and the masses and velocities of some of the other nuclei, etc.). Thankfully, the problem was in 2D ... I don't think I could have done it in 3 dimensions without some serious linear algebra. Anyway, I'm wondering whether the methodology is necessarily valid, since 1) gamma radiation is likely to be emitted nonuniformly 2) since fission energy is being converted into kinetic energy, why should the total momentum of the system be still zero? John Riemann Soong (talk) 12:02, 7 August 2009 (UTC)
- It's a law of physics that momentum is always conserved. However, kinetic energy needn't be. If bombs are detonated as you describe, the kinetic energy of component parts is increased, but the momentum isn't. Remember, momentum is a vector quantity and if you add up those vectors the result doesn't change. Kinetic energy is scalar, and is proportional to v squared not v. --Leon (talk) 12:31, 7 August 2009 (UTC)
- Wait a minute - if light is converted to stored energy through photosynthesis there's no impulse (change of momentum) on the body (tree/plant) it acts on - if there was then the energy would be dissapated as kinetic energy - but it's not - it's stored as chemical energy. - a momentum change would need the light to be reflected.
- As for the bomb - yes, think so.83.100.250.79 (talk) 12:08, 7 August 2009 (UTC)
- No, a momentum change does not need light to be reflected. Though a certain amount of reflection does occur, incidentally. And there is some impulse on the plant! --Leon (talk) 12:31, 7 August 2009 (UTC)
- Well yeah, it's the electrons that gain energy, with the potential to transfer momentum later on? John Riemann Soong (talk) 12:22, 7 August 2009 (UTC)
- Yes
- (edit conflict)The force on the earth due to light reflection is ~ 50 mega newtons (assuming intensity of 920W/m2 [[9]], albedo of 30%, radius of earth = 3x106m , p=E/c , F=dp/dt )83.100.250.79 (talk) 12:29, 7 August 2009 (UTC)
- That's the same as 100 British Rail Class 59 trains pulling together.
- Mass of earth ~ 5x10^24kg , F=ma so a= F/m a=5x10^7N / 5x10^24kg = 10^-17 m/s2
- change in velocity due to acceleration =at . 1year = ~3x10^7 seconds
- So increase in outward velocity of earth in 1 year would be 3x10^7 x 10^-17 = 3x10^-10m/s - quite small..83.100.250.79 (talk) 12:35, 7 August 2009 (UTC)
- Hmm, but now take billions of years of cyanobacteria and fossil fuel formation ... also I ask about photosynthesis because it's basically a sort of energy sink, allowing Earth to reach thermal equilibrium while actually stockpiling energy. Thus even if no reflection occurs, the system of Earth's atoms has arguably gained momentum, hasn't it? Also, I assume things like angular momentum, axial tilt, and other planetary parameters could be more sensitive? (Though still not very sensitive to anything man-made ... but I am wondering about variation over long periods of years, after accounting for things like tidal lock effects and such.) John Riemann Soong (talk) 12:45, 7 August 2009 (UTC)
- Not really momentum - they have gained energy. If you say convert carbon dioxide and water into sugar (increase in energy) there's no real increase in classical momentum.83.100.250.79 (talk) 12:57, 7 August 2009 (UTC)
- Indeed, the earth absorbs more momentum by reflecting and/or absorbing photons on the dayside than is radiated away by thermal radiation on the nightside. The photons exert a tiny net force away from the sun. Gravity exerts a force towards the sun. The net effect is a tiny decrease of the force keeping the earth in its orbit, as if the sun were slightly less massive than it actually is. For orbiting dust particles and solar sails, the radiation pressure is comparable to or larger than the gravitational force due to the sun, and the orbits of these things are significantly different from purely gravitational orbits.
- Hmm, but now take billions of years of cyanobacteria and fossil fuel formation ... also I ask about photosynthesis because it's basically a sort of energy sink, allowing Earth to reach thermal equilibrium while actually stockpiling energy. Thus even if no reflection occurs, the system of Earth's atoms has arguably gained momentum, hasn't it? Also, I assume things like angular momentum, axial tilt, and other planetary parameters could be more sensitive? (Though still not very sensitive to anything man-made ... but I am wondering about variation over long periods of years, after accounting for things like tidal lock effects and such.) John Riemann Soong (talk) 12:45, 7 August 2009 (UTC)
Bullets fired in the air returning to ground
When live bullets are fired into the air (9 gun salute e.g.) are the falling bullets just as deadly as usual, or do they burn up or something? Are blanks used in these kind of displays? —Stanstaple (talk) 17:45, 7 August 2009 (UTC)
- It depends on the trajectory of the bullets. If fired at an angle, the bullets can maintain their spin and lethality -- there are many documented cases. If fired strictly vertically, the bullets lose their spin and fall at a much slower speed. The MythBusters covered this in some detail a few years back. As for whether blanks are used in x-gun salutes: it depends on who's doing it. — Lomn 17:52, 7 August 2009 (UTC)
- Depends how massive the bullets are. If you use cannon, you might want blanks. If you're dealing with a lighter object like a bullet, you should be familiar with the concept of terminal velocity because of air resistance. Bulkier objects tend to have a larger mass / surface area ratio (due to geometric trends). John Riemann Soong (talk) 17:53, 7 August 2009 (UTC)
- Of course! For some reason i'd never figured in the effects of wind resistance- Firing a bullet into the air would be akin to droping the bullet from the apex- makes sense when you think about it- thanks again. —Preceding unsigned comment added by Stanstaple (talk • contribs) 18:59, 7 August 2009 (UTC)
- Not quite. If you read the article careful enough, it all depends on whether the bullet keeps its spin or not. If it does, just the terminal velocity after the fall can be fatal. If, however, it starts tumbling on the way down, it will fall to earth harmlessly. Seems whether it will tumble or not is very hard to predict.195.128.250.139 (talk) 21:18, 7 August 2009 (UTC)
- Of course! For some reason i'd never figured in the effects of wind resistance- Firing a bullet into the air would be akin to droping the bullet from the apex- makes sense when you think about it- thanks again. —Preceding unsigned comment added by Stanstaple (talk • contribs) 18:59, 7 August 2009 (UTC)
- There's a saying in Russian (and in Serbo-Croatian too), "Even when you shoot into the air, the bullets still fall on someone's head". 98.234.126.251 (talk) 22:32, 7 August 2009 (UTC)
- I like the "Bullets are not greeting cards. Celebrate without firearms" slogan from Macedonia. I wonder - are the people who fire off their guns in joy the same kind of people who shoot their TV set when their football team loses, or shoot their monitor when their PC crashes? --Kurt Shaped Box (talk) 23:00, 7 August 2009 (UTC)
- No, no, no! Be VERY careful how you answer this question. Shooting a gun into the air is lethally dangerous - period. Unless you shoot it pretty much exactly vertically - it will have considerably more velocity when it finally comes to earth than if you dropped it from the maximum height it achieves. You can imagine the horizontal and vertical velocities of the bullet as entirely separate things. If you shoot a bullet horizontally (eg if there was no gravity) then only air resistance slows it down - and not by very much...the bullet is (predictably) lethal. When you fire it vertically, it travels to the apex, gradually slowing down because of gravity and wind resistance - and then stops - and falls back to earth with gravity accellerating it and air resistance slowing it down. Because of the air resistance, the bullet is travelling more slowly when it hits the ground than when you fired it. The terminal velocity of a spinning bullet is pretty fast - because they are fairly aerodynamic - and quite heavy for their cross-sectional area...but when the bullet is fired vertically, it slows to a stop and has to flip over...it loses the spin that keeps it stable in flight - and one the way back down again it's tumbling randomly - which introduces a lot more drag and slows it down quite a bit. A bullet that fell back on you would hurt quite a bit - but it wouldn't kill you. Mythbusters found them buried in a couple of inches of dirt. But what gets complicated (and VERY dangerous) is when you fire the bullet at an angle - not quite vertical. The horizontal component of the motion isn't slowed down by gravity at all - and that allows the bullet to keep its spin - so as it goes over the top of the arc, it stays nicely pointed into the airflow - wind resistance hardly slows it down at all and it remains completely lethal - exactly as lethal as if you'd fired it in a straight line over the same total distance. The precise angle at which the spin of the bullet turns into a tumble and it becomes much safer is unknown and would certainly depend on the initial muzzle velocity, the precise shape of the bullet and a whole bunch of aerodynamic complications. However, it is very safe to say that if you fire a bullet into the air - you could quite easily kill someone. There are PLENTY of well-documented cases of innocent people who have been killed and injured by stray bullets at celebrations where people shoot guns into the air. Please don't let anyone convince you otherwise...most of them are oversimplifying a complicated aerodynamic situation. They simply don't understand what's going on in enough detail to explain the problem - so they claim to "know" that it's safe - which it patent nonsense given the number of documented cases of people dying.
- Shooting blanks is obviously safer - but even so, there have been people killed by blank rounds too - blank rounds are far from "safe".
- Paradoxically, if you were going to fire off your AK-47 in celebration of <whatever>, aiming it straight up in the air would seem like one of the most stupid things that anyone could possibly do... --Kurt Shaped Box (talk) 22:56, 7 August 2009 (UTC)
- Yep - ironically, perfectly vertically is the safest...(well, safest for humanity in general...perhaps not safest for you personally!) But that requires much more faith in aerodynamics than most people have! The Mythbusters tried quite hard to fire their guns perfectly vertically - but the bullets still landed hundreds of feet away. SteveBaker (talk) 02:59, 8 August 2009 (UTC)
Space-filling models software
Hello, I am looking for free software that allows me to generate atomic and molecular space-filling models. Do you know of any? Thank you. Leptictidium (mt) 20:33, 7 August 2009 (UTC)
- try browsing Category:Molecular modelling software for free ones that do what you want.83.100.250.79 (talk) 21:26, 7 August 2009 (UTC)
Nipping out buds of tomatoes
I'm growing some tomato plants. I've read a few times that it helps to remove some of the buds on the plant - could anyone clarify what you are supposed to do please? And is doing this truelly worthwile, or just a myth? I happened to see on tv about farmers growing thousands of tomatoes in a big field. I doubt they do any bud nipping, but also they do not support the plants at all - is supporting the plants and trusses necessary? 78.146.176.224 (talk) 21:09, 7 August 2009 (UTC)
- The process of removing the buds/side shoots of tomatoes is called "suckering" (at least that's what I learned it as). The thought is to remove growth that will never amount to much, and get the plant to put that energy into flowering and fruiting. The Wikipedia article isn't too applicable, but if you search the web for "suckering tomatoes" (or similar), you should get a number of sites which discuss it. The reason you stake/cadge tomatoes is to keep the fruit off the ground, and to allow it to dry out more thoroughly. Tomatoes are particularly susceptible to diseases especially fungal ones, such as verticillium wilt and fusarium wilt. Commercial farmers don't prune or stake because it's very labor intensive, and labor is costly. It's cheaper/more efficient to just grow more plants, spray with fungicides, and hope for the best. The analysis is different with a backyard gardener, though. The labor expenditure is not as high with just half a dozen plants, and you'll get a much larger crop. -- 76.201.158.47 (talk) 14:57, 8 August 2009 (UTC)
power leakage
Is it true that power leaks away when, for example, a phone charger is left plugged in without the phone? How does this happen? How much energy is wasted? --Halcatalyst (talk) 22:10, 7 August 2009 (UTC)
- Even the best phone chargers waste some energy - even if they are not connected to anything - they're mostly better than 90% efficient, and probably waste even less when not charging.
- Basically they are Switched-mode power supplys - a type of electronic device - they need some electrical energy to keep ticking over, about the same as an electric clock.83.100.250.79 (talk) 22:23, 7 August 2009 (UTC)
- Well, the power doesn't "leak" out of the end of the wire...that's a common fallacy. (If it did, every power socket in your house without something plugged into it would be "leaking" too!) However, because a charger is a transformer - which has a coil of wire on the wall-socket side of things, there could be a VERY tiny amount of current consumed - which would appear as a small amount of heat being emitted by the charger. But unless the charger has an LED on it that's lit up to show that it's plugged in - I think the amount of energy wasted is very small indeed. If you are looking for places where electricity is being uselessly consumed, consider things like TV sets which can be "turned on" with a remote. Anything that can be turned on and off with a remote has to have some circuitry running in order to detect the signal coming from the remote. So when you turn off your TV/DVD/Satellitebox/etc - it's not REALLY being turned off - it's just turning off most of it's functions and hopefully saving energy. However, nothing short of unplugging such devices will truly get them "turned off" completely. SteveBaker (talk) 22:24, 7 August 2009 (UTC)
- I've heard wall warts (transformers) tend to take significant amounts of energy even if nothings plugged in. I think the good ones have a way of telling if something is plugged in, and disconnect the circuit nothing is. I know from experience that I have at least one wall wart that tends to get warm if you leave it plugged in, even if the device it's connected to is off. — DanielLC 04:16, 8 August 2009 (UTC)
- Cecil Adams did a column on this here. He found the the idea that wall-warts wasted huge amounts of energy was mostly a myth. APL (talk) 04:47, 8 August 2009 (UTC)
- Since the wasted electricity is converted to heat, you can get a good qualitative idea of the wastage by simply placing you hand on the charger. For chargers of the same physical size, a warmer charger is wasting more heat. A power dissipation as low as one watt will make a charger-sized object quite warm. My two-watt Sheevaplug feels much warmer than the (much smaller) telephone chargers sitting next to it, so I infer that they are each consuming very much less than one watt. I can save more energy by turning off my computer monitor for five minutes than by turning off all my chargers for one day. -Arch dude (talk) 15:30, 8 August 2009 (UTC)
Surgery recovery time
Suppose someone has had surgery for eye injuries due to a shotgun backfire (see the science ref desk archives section "Shotgun backfiring"), and also for multiple bone fractures and lacerations due to being mauled by a bear. How long must the patient stay in one place before he can be evacuated by plane? Also, how likely is he to have complications that would require follow-up surgery? (No, this ain't no medical advice, it's for my story again.) Thanks in advance! 98.234.126.251 (talk) 22:59, 7 August 2009 (UTC)
- Too many unknowns to make a definitive decision. The main fulcrum of the decision to move the patient would be the need for improved care against the possibility of damage caused by the evacuation. Helicopters are frequently used to move seriously injured individuals. What sort of plane had you in mind? A small single engined plane that did not ascend to altitude would OK for early evacuation. IMHO. 86.4.181.14 (talk) 07:24, 8 August 2009 (UTC)
- The plane I had in mind was that same Queen Air that had to land in a 15-knot crosswind (see the section "Crosswind landing"), but the pilot knows that there's a surgery patient on board, so she flies low and does her level best to avoid any turbulence. What I wanted to know is, how soon before it's safe to even move the patient? Assume that he had to have (1) the splinters taken out of the scleras and corneas of both his eyes, (2) small tears in both irises repaired, (3) blood drained from inside both eyeballs, (4) a SMALL part of the vitreous humor taken out to remove dried blood, (5) compound-comminuted fractures in both forearms cleaned, aligned, and fixed in position, (6) simple fractures of the ribs repaired, and (7) lacerations sutured on face and body. (Quite a list, huh?) 98.234.126.251 (talk) 10:13, 8 August 2009 (UTC)
- That bear must have been fairly close to a largish city, for that level of surgical help to be available to your victim. So why does he have to be moved? If it happens in the back of nowhere, then he'd just have pressure bandages applied to stop bleeding, splints applied to stop the bones moving (possibly those inflatable splints carried by ambulances) and then he'd be evacuated to a place with a large enough hospital to do the actual surgery.
- Once he's had the surgery he could be safely moved immediately, by road or air transport, as long as his IV drips go with him. It would be no different than being wheeled through 2 hospital buildings to get from the operating theatre to his ward. But if he had the full surgery close to where the incident happened, why move him?
- If moving him is a necessary part of the story, you either need a compelling non-medical reason for moving him, or he'd only be able to get basic first-aid at the scene and need evacuation to be able to get the surgery.- KoolerStill (talk) 14:28, 8 August 2009 (UTC)
- Ah, but that's not how the story goes. The story is, at the time that he's got hurt, he's trapped on an island in the Canadian Arctic so they can't evacuate him to a hospital. They can't get to the island by sea or over the ice because the ice is just starting to break up, and the weather is so bad that the Canadian chopper pilots are afraid to fly to the island. The Americans are willing to send help, though, but the island is out of range of American choppers, so they can only get there by plane (the aforementioned Queen Air). Unfortunately the island ain't got no airstrip (only a helipad) and landing on the ice is impossible due to the ice breaking up. And obviously with these kinds of injuries the poor guy needs surgery (not just splints and pressure bandages) within 24 hours at the very most or he could very well be maimed for the rest of his life. So what they do is they drop the surgeon on the island by parachute so he can do the surgery in the field, and then later on when the ice breaks up completely they evacuate him by boat and then by plane so he can get follow-up surgery if necessary. As a matter of fact, I got the story line from the poem "Ice Island" by the Russian poet Samuel Marshak, which was written back in the late '40s, before choppers were in widespread use, so I had to take some creative latitude to make sure that the new technologies are unavailable (I also moved the setting from the Russian Arctic to the Canadian Arctic, and added a love story between the surgeon and the pilot). By the way, thanks for answering my question about how soon it's safe to move the patient -- I see now that I won't have to rewrite that part at all. 98.234.126.251 (talk) 21:13, 8 August 2009 (UTC)
- Okay, that surgeon is not going to have an operating theatre at his disposal, nor x-rays to see the broken bones, nor an anesthetist, nor proper aseptic conditions. So he can stitch the worst surface wounds where there are no underlying fractures, remove the shrapnel from the eyes to save the victim's sight (to stop further damage from the eyes moving); then splint him up for evacuation. The compound fractures would take many hours of surgery, pins etc to set, which he won't have the equipment for. And which he'd normally have 3 or 4 assistants for, at least. Those would need to be done at a proper hospital. He'd be okay for several days without the bones being set, as long as he is splinted (to prevent painful movement) and has painkillers and an IV going (for hydration and anti-biotics). Seeing you have a lot of ice, consider using a sled rather than a boat. How does this guy call for help in the first place? - KoolerStill (talk) 22:50, 8 August 2009 (UTC)
- Ah, but that's not how the story goes. The story is, at the time that he's got hurt, he's trapped on an island in the Canadian Arctic so they can't evacuate him to a hospital. They can't get to the island by sea or over the ice because the ice is just starting to break up, and the weather is so bad that the Canadian chopper pilots are afraid to fly to the island. The Americans are willing to send help, though, but the island is out of range of American choppers, so they can only get there by plane (the aforementioned Queen Air). Unfortunately the island ain't got no airstrip (only a helipad) and landing on the ice is impossible due to the ice breaking up. And obviously with these kinds of injuries the poor guy needs surgery (not just splints and pressure bandages) within 24 hours at the very most or he could very well be maimed for the rest of his life. So what they do is they drop the surgeon on the island by parachute so he can do the surgery in the field, and then later on when the ice breaks up completely they evacuate him by boat and then by plane so he can get follow-up surgery if necessary. As a matter of fact, I got the story line from the poem "Ice Island" by the Russian poet Samuel Marshak, which was written back in the late '40s, before choppers were in widespread use, so I had to take some creative latitude to make sure that the new technologies are unavailable (I also moved the setting from the Russian Arctic to the Canadian Arctic, and added a love story between the surgeon and the pilot). By the way, thanks for answering my question about how soon it's safe to move the patient -- I see now that I won't have to rewrite that part at all. 98.234.126.251 (talk) 21:13, 8 August 2009 (UTC)
How can I tell if a martial arts school is crap?
I know to avoid the ones that guarantee that you'll become a black belt in n years (as long as you keep paying every month), or don't actually do any real full-contact sparring against a resisting opponent, tell you that they'll teach you to be able to beat up six guys at once - every time, or claim ninja movie rubbish like that they have 'ancient secret high-level techniques' that will allow you to kill people with one punch, or levitate/teleport/become invisible using chi magic, or throw fireballs, or move fast enough to catch bullets, etc.
But what else should I look out for, as a newb, before I sign up for training that might be generally poor and ineffective? Sorry if this is in the wrong place, but I think that MA is a sort of science. Wasn't exactly sure where to put my Q. --84.69.218.212 (talk) 23:25, 7 August 2009 (UTC)
It's not really a 'science' in the usual sense of what questions are posted here, but here goes: Word of mouth is wonderful for things such as these, similarly if you can find more about local tournaments then you'll maybe find which 'schools' teach the top performers in your area (a-la Karate Kid). Perhaps find a few local ones and ask if they'd let you come and watch in on a session - most will be receptive if they know you're seriously considering joining. ny156uk (talk) 23:35, 7 August 2009 (UTC)
Well, as a karate brown belt, I'd say that you should seek out a martial arts school that first puts an emphasis on teaching the right moves so that you do them right every time, and then, once you learn the techniques, incorporate them into one-on-one full-contact sparring with your fellow students. It's also highly desirable that the instructor should take the time to personally demonstrate the martial arts techniques to the students, and explain or demonstrate what (if anything) they're doing wrong. It's also generally (but not always) true that small MA schools offer better quality training than bigger ones. And last but not least, if within a reasonable amount of time (say a year or so) you don't see your technique improving in perceptible terms, then you should consider changing your MA school. There might be other considerations too, but I can't think of them off the top of my head. 98.234.126.251 (talk) 23:44, 7 August 2009 (UTC)
- It's also a good idea to talk to your teacher and ask him who he trained under - then look him/them up. Beware of the guys who claim to be the only non Chinese/Japanese/Korean/Tibetan/etc. person to ever train in the style (which he learnt after living and fighting alongside Far-Eastern monks/ninjas/mobsters/etc.) - which, of course, no-one else will have ever heard of, because it's so secret and deadly. More than likely, he trained to the point of 'not being rubbish' in something else, watched some instructional DVDs in a couple more MAs, then founded his own made-up MA using all the coolest-looking moves and made himself grandmaster. Likewise, avoid the 'former SAS/MI5/black ops guy' - people who've done that don't talk about it, especially not to sell 'how to kill people on the street with your bare hands' classes out of their garages to teenagers. It should be common sense - but for some reason, a lot of people seem to fall for this Walter Mitty crap when it comes to MAs. There was a guy putting flyers about in my town a couple of years ago, claiming SAS (secret, BATTLEFIELD-PROVEN commando techniques FAR TOO DEADLY to be taught at regular MA classes, etc.) - with the caveat "HARDCORE people ONLY! No women, children or time wasters!" at the bottom. You can guarantee that some people saw those and thought "FUCKIN A! I'M THERE!" --Kurt Shaped Box (talk) 18:59, 8 August 2009 (UTC)
Why not invite the instructors to a no holds barred fighting competition? The winner will be the best fighter and likely the best instructor. Marcus Greene (talk) 23:49, 7 August 2009 (UTC)
- I find your second statement unlikely. Many people who are good at something are not great teachers. Many great teachers are not the best at their subject. While I'm primarily thinking of academic teachers here, I don't see why this won't apply to martial arts as well. Indeed if we consider sports, the best coaches were often not the best players, and the best players often fail as coaches. Of course a more significant fact is that I don't see any evidence the OP is particularly interested in learning no hold barred fighting and most importantly, unless you happen to have a large amount of money, the chance you a prospective student will convince many instructors to get involved in such a thing is slim Nil Einne (talk) 09:43, 8 August 2009 (UTC)
We have an article about a bogo-martial-art debunking website, bullshido.net. 70.90.174.101 (talk) 01:26, 8 August 2009 (UTC)
In my 30+ years of martial arts (several art forms to keep me well rounded), I would suggest that you watch the instructor(s) and see how they teach. Are they respectful to the students? Do they bully them? Are they good with both children and adults? What are their credentials (e.g. one of my Senseis is a member of Karate Ontario as well as associations in Japan and the US). One can easily check the vaildity of any Association (like Karate Ontario) via the web and then check out the instructor via the Association). I have had instructors who were very good fighters in tournaments but on the street were useless. I have had instructors who were good all around fighters but could not teach if their life depended on it! A few years ago, my instructor asked me to teach the children's class because he didn't have the temperment for it. I really respect him for that. Gotta watch the egos as well. Some egos are quite problematic. Is the instructor open to learning from anyone? I take the stance that I can learn from anyone if I take the time to learn (I learned a lot even from the children I taught).
Also, keep in mind that different martial arts have different emphasis: karate was developed in Japan and Okinawa to mee the needs of the people there. Karate has an older history/tradition than Aidido (also Japaneese)but not as long a Kunf Fu. By contrast, Krav Maga is an Israeli fighting art that places a very different emphasis on self-defense than does karate, kung fu, tae kwon do or even boxing.
What do you want to get out of a martial art? Shop around and don't be pressured into anything that your gut says to be wary of. —Preceding unsigned comment added by 216.154.17.222 (talk) 19:18, 9 August 2009 (UTC)
- At the risk of making an obvious statement - an instructor who acts like Pei Mei around his students is probably more a common-or-garden asshole than a learned martial arts master. One who talks like Master Po is probably just pretentious. You don't need to learn from guys like that to be good. --Kurt Shaped Box (talk) 23:00, 9 August 2009 (UTC)
- Who??? Are you referring to this Pei Mei? Nil Einne (talk) 17:07, 10 August 2009 (UTC)
- Na, I was talking about this *Pai* Mei. Damn my tyops. --Kurt Shaped Box (talk) 23:27, 10 August 2009 (UTC)
I'd like to emphasize 216.154.17.222's point above; you need to consider what you want to get out of training. Are you looking for a cardio workout? Muay thai can sure give you that. Want to improve flexibility? Many forms of Chinese martial arts (i.e. "kung fu") place a heavy emphasis on that. If you really get into it, it can be a huge time and money investment, so shop around. If you just want to kick someone ass, try Homer Simpson's advice: "First, you gotta shriek like a woman, till he turns away in disgust. That's when it's time to kick some back! Then, when he's lyin' on the ground, kick him in the ribs, step on his neck, run like hell." Matt Deres (talk) 14:03, 10 August 2009 (UTC)
Lead lined coffins
I recently saw a TV programme where a scientist said that he had seen a woman's body who had been buried in a lead lined coffin for 200 year and yet he could still tell that her eyes were blue. Is that level of preservation really possible? Would the body have had to been subject to some other preservation? In the same programme they exhumed Mark Sykes and were able to obtain lung tissue after 90 year (even though his lead coffin was split) so I guess it is not impossible that sure amazing preservation can take place. This article on Snopes suggest that an air tight container wouldn't preserve a body very well, without other preservation techniques being used. If somebody eye colour was preserved for 200 years, I would have thought that they would be in pretty good shape for at least the first few years. Are Snopes wrong? Would lead preserve a body better than an air tight casket made of another material? Bury me in a Y shaped coffin (talk) 23:36, 7 August 2009 (UTC)
- Lead lined coffins are expensive, and person buried in them may have also been heavily embalmed - meaning that they are preserved longer.
- However, all a lead lined coffin does is prevent worms etc getting to the body - it still will decompose - into a "grave liquor" (aka "coffin liquor" "human soup") - this is well known to archaeologists, and grave diggers who have to exhume remains from old grave yards.
- http://www.hpa.org.uk/CDR/archives/CDRreview/1995/cdrr0595.pdf
Completely preserved bodies have been found in wooden coffins buried in the ground and completely decayed bodies in apparently intact lead coffins in crypts. Most lead coffins contain dry bones but some are found to be about one third full of a viscous black liquid (coffin liquor), which contains bones and (sometimes) soft tissues. Well preserved, partially mummified bodies are sometimes found and, very rarely, intact and totally preserved bodies are found that are not even discoloured. (Copyrighted PHLS 1995)
- Any bacteria sealed inside any coffin (lead, glass, stainless steel etc) will get to work, provided that they can operate in the absence of air. So just sealing isn't the answer.83.100.250.79 (talk) 16:20, 8 August 2009 (UTC)
- A sealed vessel, plus something to stop the bacteria is needed. 83.100.250.79 (talk) 16:24, 8 August 2009 (UTC)
- When the lead coffins of candidates for sainthood are opened, it is reported that some are better preserved than others[10]. Preservation is a positive mark towards beatification. The rank ones on their way to decay are said to be "walking." Edison (talk) 19:26, 8 August 2009 (UTC)
- Thanks, an interesting example. Do you know if Saint Bernadette's body underwent some kind of preservation treatment before being buried? It sounds as if she was almost completely preserved for 30 years after death, and pretty well preserved at least 20 years after that. Bury me in a Y shaped coffin (talk) 20:14, 8 August 2009 (UTC)
- As I understand it no - it's considered to be just a miracle. It's worth noting that the parts 'on display' the head and hands are actually wax. see Bernadette Soubirous#exhumations —Preceding unsigned comment added by 83.100.250.79 (talk) 20:34, 8 August 2009 (UTC)
- Thanks, an interesting example. Do you know if Saint Bernadette's body underwent some kind of preservation treatment before being buried? It sounds as if she was almost completely preserved for 30 years after death, and pretty well preserved at least 20 years after that. Bury me in a Y shaped coffin (talk) 20:14, 8 August 2009 (UTC)
Is the mind software or hardware?
I have been wondering whether if the electrical activity in the brain stopped just for a split second and was then restored, would your "mind" still be there? (I imagine that "mind" is a concept that is a can of worms, but basically would all of your memory and mental faculties be in tact). I guess that it is a bit like a PC, in that if you turn it off and then back on, the hardware is still there and so is the data on the hard drive, but any running software is gone. So is the mind software or hardware? Has anybody ever recovered from a complete cessation of electrical activity in the brain? Bury me in a Y shaped coffin (talk) 23:46, 7 August 2009 (UTC)
- I think the important question here is how is memory physically stored in the brain? Is it some chemical or electrical state that needs to be actively maintained like RAM, is it more physical changes in the ways the neurons behave like hard disk storage, or is it physical changes in the way the neurons are linked together like chip architecture. I have no expertise in neuroscience, but I think the answer is first that it's not all that well understood, and second it's probably a combination of a lot of these things. There seems to be a fair bit of information on Wikipedia about this stuff although I'm not really sure where a good place to start is. The memory article talks about the different kinds of memory. I think it's safe to assume that sensory memory (which lasts less than half a second) is probably not facilitated by any permanent physical changes in the brain. Meanwhile neuroplasticity talks about how the brain can over time rewire itself in response to experiences. Rckrone (talk) 00:45, 8 August 2009 (UTC)
- It would take a library to address all of the issues you brought up so let's grimace and push those aside. I think the core of what you're asking is "are pathways hard-coded in physical structures, or are they volatile?" It's a tough question. The brain isn't a digital circuit, it's chemical. Neurotransmitters metabolize, unused enzymes build up or break down.. The problem is, brains don't just stop working for a split second. The chemicals that are there are ionized and produce current.. how do you plan to neutralize them? Are they just going to disappear? Well they're critical to the functioning of the brain.. you might as well have asked whether a bullet ripped through your skull for just a split second would cause permanent damage. .froth. (talk) 01:41, 8 August 2009 (UTC)
- Electroconvulsive therapy rather thoroughly disrupts normal brain functioning for a fraction of a second, which is fairly similar to what you are asking. There is a lot of electrical activity going on in that case, unlike none as in your question, but the electrical activity that’s going on during that instant is a far cry from what would normally be happening in the brain. The immediate short-term effects of electroconvulsive therapy are confusion and loss of short-term memory, but the confusion goes away, and long-term memories are largely retained. Red Act (talk) 02:39, 8 August 2009 (UTC)
- As best we understand it (which is not great), long term memory is in 'hardware' - hard connections that grow between neurons. Short term memory is 'software' - electrical signals circulating around the brain that holds the information until neural connections can fill in and make the memory "permenant". This is kinda backed up by the fact that people who have accidents and lose consciousness often lose all memory of the incident - presumably because the software short term memory disappears (just like losing RAM memory in your computer if you turn it off). SteveBaker (talk) 02:50, 8 August 2009 (UTC)
- Try mind, electroencephalogram, coma, and brain death. When awake, the brain always has electrical activity, there's no such thing as electrical activity stopping for a "split second." During anesthesia, loss of consciousness can be associated with a flatline on EEG (though this may be more reflective of the limited sensitivity of EEG as opposed to actual complete electrical inactivity). When brought back to consciousness, the "mind" is unaltered. Complete loss of electrical activity on EEG is sometimes used as a criteria for determining brain death. The brain death article references the case of a man who was declared "brain dead" but later recovered. The references cited in the article indicate that he had no cerebral metabolism on positron emission tomography scan shortly after an injury, though this is not quite the same thing as declaring that there is no electrical activity. He apparently remembers hearing the doctors say that he was dead, so presumably his short term memory (and therefore electrical activity) were working normally. As a hand-waving answer to your questions, it seems as though the brain structures (hardware) are what enable the mind (software) to run and that memories can be written to short-term RAM (as with SteveBaker's analogy) or long-term ROM to be accessed by the mind when needed. --- Medical geneticist (talk) 03:22, 8 August 2009 (UTC)
Thanks to everyone for the input, I have been reading around this subject a bit (including the articles suggested above), I have also found the following of interest: Information-theoretic death and Cryonics#Premises_of_cryonics. Bury me in a Y shaped coffin (talk) 09:53, 8 August 2009 (UTC)
- As I think of it, the 'brain' is the hardware (like RAM, hard drive) but 'mind' is software. When we first 'boot up', most mental activity is crude ... but as the hardware gets more input, chemical pathways are established that eventually causes 'mind' - or self-awareness at least - to pop into existence.
Most people aren't conscious of the first few years of their life. Perhaps that is the period before the 'ignition' of mind. At that moment we become aware of the existence of our memories.
Primitive software, like machine language, then assembly language, is still very close to the hardware. But as time goes by, the software is increasingly abstracted from the hardware. Scripting languages are very far away from the underlying hardware. Clearly the software could rise to higher and higher levels of abstraction, while still running on the same hardware. It could be the same way with mind.
What does the program consist of in a computer? A varying pattern of energy levels spread across many transistors. Is the *pattern* made of electrons? No. It is the changing relationship between the electrons!! Which weighs nothing ... it's a complete abstraction! Yet, the program 'works' ... and so does the mind.
Certainly the mind 'ceases running' when the brain is badly damaged. But, like the program, the mind never tangibly existed. So in some sense, it never goes away. Twang (talk) 09:06, 9 August 2009 (UTC)
- I don't buy "ignition", if you mean a discrete moment when a child's mind fundamentally changes. I don't remember any events at age 3, but I believe I did remember them when I was 4 (the time of my oldest accessible memories). My guess: as we learn how to learn, our internal language develops, and eventually the encoding of new memories is no longer compatible with that of the oldest memories — but there's no transition, just as (we assume) there was no sharp transition between Latin and modern Spanish. —Tamfang (talk) 04:26, 11 August 2009 (UTC)
- 'Ignition' may not have been the best choice of words (is 'launch' better?). Because there's a period 'before' without recoverable memories and a period 'after' with recoverable memories, there must be a threshold. It may not matter whether it's quick or not. Is this experience 'private' and ineffable, or could it actually be observed? I don't know of any serious work. (I'm trying to be practical and stay away from metaphysics questions like 'is there mind before there's memory'.)
You may be right: it may be that our personal primitives are slowly massaged into better and better conformity with our sense data without any transition. But I suspect there comes a day for most of us when we first understand that there's an 'I'. That *might* be the day when memory kicks in. Twang (talk) 06:18, 11 August 2009 (UTC)
- 'Ignition' may not have been the best choice of words (is 'launch' better?). Because there's a period 'before' without recoverable memories and a period 'after' with recoverable memories, there must be a threshold. It may not matter whether it's quick or not. Is this experience 'private' and ineffable, or could it actually be observed? I don't know of any serious work. (I'm trying to be practical and stay away from metaphysics questions like 'is there mind before there's memory'.)
- I don't buy "ignition", if you mean a discrete moment when a child's mind fundamentally changes. I don't remember any events at age 3, but I believe I did remember them when I was 4 (the time of my oldest accessible memories). My guess: as we learn how to learn, our internal language develops, and eventually the encoding of new memories is no longer compatible with that of the oldest memories — but there's no transition, just as (we assume) there was no sharp transition between Latin and modern Spanish. —Tamfang (talk) 04:26, 11 August 2009 (UTC)
Complete cessation of brain electrical activity is the standard medical criterion for death. ECT doesn't cause a cessation of electrical activity, only a disruption. In principle it might be possible to cause a very brief cessation, followed by a return to life, by giving a single large DC stimulus -- but it would be a very tricky thing to do. There are some species of animals that can survive freezing, which involves a complete cessation of electrical activity. There are hopes that such a thing might eventually be possible with humans, but it isn't yet.
Most neuroscientists believe that there are two kinds of memory in the brain, long term memory which is encoded by structural changes, and dynamic memory which is encoded by ongoing electrical activity. Disruption of electrical activity, for example by a seizure, would clear the dynamic memory, but leave the long term forms intact. So in the OP's terminology (which is not standard), there are both hardware and software forms of memory -- at least we think there are. Looie496 (talk) 05:05, 11 August 2009 (UTC)
August 8
Ringworm
What causes ringworm to be circular shaped? --jpgordon::==( o ) 00:06, 8 August 2009 (UTC)
- It's a fungal infection. Probably it starts as a tiny dot at the center - as it spreads outwards, the damage it does is being repaired by the victims' body - so you wind up with a circular infected area with a smaller circular healed area in the middle. But that's just a guess...I don't know for sure. SteveBaker (talk) 02:38, 8 August 2009 (UTC)
- That seems to be vaguely confirmed by the literature (it seems the fungus feeds on keratin) - though I can't find an explicit statement of the reason.83.100.250.79 (talk) 15:38, 8 August 2009 (UTC)
- The same principle applies, but not the same effect results, in Fairy Rings. 86.4.181.14 (talk) 07:17, 8 August 2009 (UTC)
- <curmudgeon> Does circular shaped mean something other than circular ? </curmudgeon> —Tamfang (talk) 04:28, 11 August 2009 (UTC)
- In some contexts, yes. A circular argument or a circular definition is quite different from a circular-shaped argument or a circular-shaped definition. In British English, a circular might also be a piece of junk-mail - which might or might not be circular-shaped. SteveBaker (talk) 13:10, 11 August 2009 (UTC)
Negative Matter Superluminal Travel???
Suppose there is Negative Matter. Assume we have a negative positron at position p. When it moves to another position, say k, subluminally external observers may interpret this as an electron moving back in time. Is this counted as superluminal travel? Also, this travel into the past does not violate casualty because the information is sent from future to the present, which affects the future.The Successor of Physics 04:18, 8 August 2009 (UTC)
- What is "Negative Matter"? --Tango (talk) 04:49, 8 August 2009 (UTC)
- Do you mean Antimatter? Mitch Ames (talk) 04:56, 8 August 2009 (UTC)
- Antimatter moves through normal space in the normal way. It does not travel back in time.
- There is no such thing as a "negative positron".
- I don't understand how moving a "negative positron" from one place to the other would be the same as moving an electron back in time.
- If information was sent from the future to the present it would absolutely violate causality as we understand it.
- You might be trying to describe Tachyons. Keep in mind that Tachyons are a hypothetical construct. There is no theoretical or experimental evidence of their existence. APL (talk) 05:29, 8 August 2009 (UTC)
- I think the OP might be talking about Feynman's idea of anti-particles being thought of as their regular counterparts traveling backward through time. It's important to stress that this is just an interpretation used to help intuitively understand what's happening. A given particle exists at various point in time-space, but it's not really meaningful to say that a particle is traveling in one direction along that path or the other. There's also no violation to causality. If we assume determinism in both directions, then the state of the universe at one time determines both the state immediately before and immediately after. The direction of causation is which ever direction we choose to look at things from, so it's not really meaningful to say that in this case events in the future are causing events in the past. It's just a matter of perspective. Rckrone (talk) 05:53, 8 August 2009 (UTC)
- Guys, I think you should take a read on the (redirected, but still) page on Negative matter. It's not the same as antimatter. (One may not exist, while the other certainly does!) -- Aeluwas (talk) 07:36, 8 August 2009 (UTC)
- Thanks, Aeluwas, thats the negative matter I was discussing!
The Successor of Physics13:22, 8 August 2009 (UTC)- "Negative matter" doesn't mean anything to a physicist, the existence of a Wikipedia redirect notwithstanding. It could just as well redirect to antimatter. The anti-positron is the electron, but exotic matter is supposed to be an entirely different kind of matter that has negative mass (and is presumably also different in other ways), so there's no such thing as an exotic positron. I don't think it's true that negative mass going backwards in time would be like positive mass. Mass isn't like charge—there's no symmetry of nature that exchanges positive and negative mass (in the presence of gravity). CPT symmetry swaps positive and negative charge as it reverses the direction of time, but it doesn't swap positive and negative mass. -- BenRG (talk) 16:19, 8 August 2009 (UTC)
- Thanks, Aeluwas, thats the negative matter I was discussing!
- Guys, I think you should take a read on the (redirected, but still) page on Negative matter. It's not the same as antimatter. (One may not exist, while the other certainly does!) -- Aeluwas (talk) 07:36, 8 August 2009 (UTC)
Negative matter has been hypothesised, the fact that it remains unobserved doesn't detract from it status as a hypothesis. Having said that, I would otherwise agree with BenRG that inversion of T is equivalent to inversion of C and P, but no such known symmetry exists for mass. On an ending note, a symmetry that involves the inversion of T does not constitute superluminal travel. The particle is still going forward in time. —Preceding unsigned comment added by 92.8.8.69 (talk) 22:28, 9 August 2009 (UTC)
- Purple dancing interstellar hippo's have also been hypothesised (I just hypothesised them - so I can say this with confidence!) - that doesn't give them any more scientific status than any other hypothesis...including negative matter. Being unobserved certainly doesn't detract from the hippo's status as a hypothesis either. But being a "hypothesis" doesn't confer any kind of meaning onto some vague concept - it's just a fancy term for "a wild-assed idea that someone thought up when they had nothing better to do". The hypothesis of negative matter doesn't appear to solve any problems that physics currently has - there is no proposed experiment that would reveal whether the stuff exists - until or unless one or other of those things becomes true, we can consign this to Occam's razor. Without anything more than a hypothesis - there is no possible way to answer the OP's question...no more than if (s)he had asked whether those hippo's do the tango or the waltz. (Actually, they can-can - but that's another matter). SteveBaker (talk) 14:08, 10 August 2009 (UTC)
- A big difference between hypothetical negative mass and Baker's Hippo Burlesque is that negative mass is actually an interesting mathematical abstraction. Negative mass is something that, even if it doesn't actually exist, can still be examined in theoretical physics, asking "what would happen if this existed?" Abstract mathematical curiosity is in itself interesting and occasionally produces side results that are also practical. Another difference is that negative mass is something that has actually been explored in reliable scientific sources, whereas (far as I know) purple dancing hippos haven't. So the moral is that just because something probably doesn't exist doesn't mean it's not worth exploring the concept hypothetically. 63.95.36.13 (talk) 14:33, 10 August 2009 (UTC)
- Purple dancing interstellar hippo's have also been hypothesised (I just hypothesised them - so I can say this with confidence!) - that doesn't give them any more scientific status than any other hypothesis...including negative matter. Being unobserved certainly doesn't detract from the hippo's status as a hypothesis either. But being a "hypothesis" doesn't confer any kind of meaning onto some vague concept - it's just a fancy term for "a wild-assed idea that someone thought up when they had nothing better to do". The hypothesis of negative matter doesn't appear to solve any problems that physics currently has - there is no proposed experiment that would reveal whether the stuff exists - until or unless one or other of those things becomes true, we can consign this to Occam's razor. Without anything more than a hypothesis - there is no possible way to answer the OP's question...no more than if (s)he had asked whether those hippo's do the tango or the waltz. (Actually, they can-can - but that's another matter). SteveBaker (talk) 14:08, 10 August 2009 (UTC)
slow cooking of pork -- where does all the fat go?
A lot of recipes say the fat "melts" after a long slow roast (or a braising) ... and sure enough, the huge chunk of fat on the pork slowly seems to dissolve; if I'm braising the liquid becomes quite oily. What happens to the fat? Is it broken down into smaller fatty acids (and maybe some of it gets oxidised into CO2 and water?). I also note that we generally use acidic liquids like tomato sauce, fruit juice, beer, or some liquid with a bit of vinegar tossed into it ... does some sort of acid-catalysed elimination reaction occur? John Riemann Soong (talk) 10:35, 8 August 2009 (UTC)
If you're heating triglycerides (i.e. fat) in an acidic aqueous solution, the fat is hydrolysed to glycerin and free fatty acids (the glycerin is fully water-soluble, the fatty acids only partly water-soluble); however, oxidation to CO2 and H2O does not take place. 98.234.126.251 (talk) 10:52, 8 August 2009 (UTC)
- Animal fats are just solid oils. (Or rather, edible oils are simply liquid fats). You said it yourself - the braising liquid becomes oily. No quotation marks needed, the fat literally is melting, and is being added to the drippings. If you poured off the liquid and separated out the oil, not only would it solidify when it cooled, but it would probably be equivalent in volume/weight to the fat lost from the roast. One caveat to this would be that some of the fat may be emulsified into the water portion. I seriously doubt that a significant portion of the fat has been converted to fatty acids, as free fatty acids are, well, soap. (Your bath bar possibly lists sodium tallowate as an ingredient - that's saponified (hydrolyzed) beef fat.) A small amount of free fatty acids as well as mono- and diglycerides may form, which would help to emulsify the fats, but if a significant amount of free fatty acids were formed, your roasts would taste "soapy". -- 76.201.158.47 (talk) 14:36, 8 August 2009 (UTC)
- Soap gets part of its taste from the sodium in it, hydrolysed fats would be the free acid, and have a different taste. Mostly though the fat just melts, rather than reacting as mentioned above.83.100.250.79 (talk) 15:31, 8 August 2009 (UTC)
Light as a feather, stiff as a board
There is a fairly common "party trick" where somebody sits in a chair and a small group of people lift the chair and the person, using only one finger each (alternatively the lifters put their fingers under the subjects armpits and knees). I have found some discussions about how it works (it seems that it is called "light as a feather, stiff as a board" by some) but no definitive answers as to how it works. It seems to me that it is likely the case that the combined lifting force of the lifters is simply sufficient when the weight is shared between them (even though that seems counter-intuitive). Is it that, or is something else involved, or maybe is it really a "trick" with the lifted individual or one or more of the lifters in on the trick and somehow "cheating"? There is a Wikipedia article on the game, but it seems pretty vague, I am hoping that there is, in fact, a recognised scientific explanation for it. Bury me in a Y shaped coffin (talk) 10:57, 8 August 2009 (UTC)
We have an article Light as a feather, stiff as a board on that, it doesn't seem to suggest it is anything special - just that a number of people can easily lift a person and so the 'effort' is a trick of the mind (though it has a 'dubious' statement around it). ny156uk (talk)
- Thanks, yes that is the article that I mentioned. However, it does not say conclusively how it works. Because this is so common, and yet at first sight so counter-intuitive, I imagine that there must be a definitive answer somewhere. Bury me in a Y shaped coffin (talk) 15:59, 8 August 2009 (UTC)
- I think that's more a function of journaled researchers not spending a lot of time playing a 12-year old girls' sleepover game than anything else. The article offers a number of suggestions, however, all of which probably work in concert. Essentially, if you're trying to lift a 125 pound individual with 5 people, no one needs to lift more than 25 pounds. ~ Amory (user • talk • contribs) 16:17, 8 August 2009 (UTC)
- I think that you are forgetting that many of the reseachers did spend some time as 12 year old girls at some point in their lives, and the rest spent some time as 12 year old boys. My point being that this "trick" seems to have been very well known for centuries and, if it didn't have an obvious explanation, it would have been investigated by somebody. I am surprised that there is no definitive published answer on this, although I appreciate your input and that of the other people who have responded. Bury me in a Y shaped coffin (talk) 18:40, 8 August 2009 (UTC)
- I suspect many people, even when they were 12 years old, didn't play games like this. Even if they did, many probably didn't or don't now think there's anything extraordinary going on here. (The 'don't now' is perhaps a key point. Even if they really thought there was something extraordinary then, many would probably regard it now as such one of the silly things they believed while young.) Most scientists only really study things that are of interest to someone, probably them. There are some who do try to discredit pseudoscience or and few who perhaps are more open and willing to study pseudoscientific claims in their own right, but I don't think many people actually believe or claim there's anything unusual going on with the trick. (There are a lot of other areas where there are far more people who believe in some sort of pseudoscientific explaination which many would regard as more important to study or discredit.) In other words, the vast majority of people are unlikely to have any interest in studying the trick. In any case, it's not entirely clear what there is to study since as has been explained, there are many rather obvious things that could be going on here which most would consider entirely explain why some people think there's something unusual. Edit: It seems there's so brief discussion here [11] Nil Einne (talk) 10:00, 9 August 2009 (UTC)
- I don't think that there is anything pseudoscientific here. You have a phenomenom were people seem to be able to lift a large amount of weight, yet they feel that it is very light. This has been experienced by large number of people, it is not an unsubstantiated claim. Now, someone of a scientific mindset is likely to wonder what is going on here and want to be able to explain it to themselves. Now, there is either a very obvious explanation (obvious to somebody with the required knowledge) which you would think that somebody would state as the definitive explanation or somebody would be interested enough to investigate (not to the extent of multi-billion dollar research, but some kind of investigation). Surely this situation of spotting a phenomena, yet not being able to explain it, is the basis of a lot of good science (not pseudoscience). Remember also that I didn't suggest that this would be heavily researched, I just asked if there was a recognised scientific explanation. Bury me in a Y shaped coffin (talk) 14:21, 9 August 2009 (UTC)
- I suspect many people, even when they were 12 years old, didn't play games like this. Even if they did, many probably didn't or don't now think there's anything extraordinary going on here. (The 'don't now' is perhaps a key point. Even if they really thought there was something extraordinary then, many would probably regard it now as such one of the silly things they believed while young.) Most scientists only really study things that are of interest to someone, probably them. There are some who do try to discredit pseudoscience or and few who perhaps are more open and willing to study pseudoscientific claims in their own right, but I don't think many people actually believe or claim there's anything unusual going on with the trick. (There are a lot of other areas where there are far more people who believe in some sort of pseudoscientific explaination which many would regard as more important to study or discredit.) In other words, the vast majority of people are unlikely to have any interest in studying the trick. In any case, it's not entirely clear what there is to study since as has been explained, there are many rather obvious things that could be going on here which most would consider entirely explain why some people think there's something unusual. Edit: It seems there's so brief discussion here [11] Nil Einne (talk) 10:00, 9 August 2009 (UTC)
- I think the key thing is that a finger locked out straight is pretty much as strong as the arm it is attached to. Since the finger is locked the muscles controlling it don't have to do any work, they just have to maintain the tension. People think that the finger is doing the lifting when it really isn't, the arm is doing the lifting and of course an arm can lift a fifth of a person. --Tango (talk) 17:34, 8 August 2009 (UTC)
- I think that you are forgetting that many of the reseachers did spend some time as 12 year old girls at some point in their lives, and the rest spent some time as 12 year old boys. My point being that this "trick" seems to have been very well known for centuries and, if it didn't have an obvious explanation, it would have been investigated by somebody. I am surprised that there is no definitive published answer on this, although I appreciate your input and that of the other people who have responded. Bury me in a Y shaped coffin (talk) 18:40, 8 August 2009 (UTC)
- I think that's more a function of journaled researchers not spending a lot of time playing a 12-year old girls' sleepover game than anything else. The article offers a number of suggestions, however, all of which probably work in concert. Essentially, if you're trying to lift a 125 pound individual with 5 people, no one needs to lift more than 25 pounds. ~ Amory (user • talk • contribs) 16:17, 8 August 2009 (UTC)
Pushing things out of earth orbit
Would it be possible for an astronaut on the International Space Station to easily shove some garbage in the optimal direction so that it fell to earth and got burnt up? Or in reality is this not possible? If something was pushed towards earth, then as there is virtually no friction in space it should keep on going to earth, at least it would seem that way when viewed from the Space Station. 84.13.197.233 (talk) 14:55, 8 August 2009 (UTC)
- No. Unless the astronaut pushes really, really fast (as in much faster than any astronaut could conceivably push any significant amount of garbage), the garbage will keep orbiting, just in a slightly different orbit. —Preceding unsigned comment added by 81.11.170.162 (talk) 16:59, 8 August 2009 (UTC)
- No, it's quite impossible. Pushing something like this changes its speed (called giving it a delta-v, a change of velocity). Changing the speed of an orbiting body causes it to move into a different orbit (slow it down and it goes into a tighter orbit, speed it up and it goes into a wider orbit). To get from a low-Earth-orbit (LEO) to land on the earth takes a delta-v of more than 8km/s or more (per the diagram on the delta-v article), because that's how fast the astronaut and the ISS are orbiting. So the change to that 8km/s that the astronauts throw would induce would be so trivial that it would make a trivial difference to the orbit of the garbage. In fact the garbage will reenter in time (but so will the astronaut and the ISS, unless they do something about it), as they're all orbiting in the Thermosphere, one of the upper layers of the Earth's atmosphere. Although the drag from the very thin atmosphere is very little, it all adds up, and eventually it burns off enough velocity from things orbiting at the altitudes we're talking about for them to reenter. -- Finlay McWalter • Talk 17:34, 8 August 2009 (UTC)
- Yes, they can, because there is still a considerable amount of atmospheric drag at the altitude of the ISS. The ISS is boosted every few months, if the garbage isn't boosted it will eventually burn up. You don't actually want to push it towards Earth, you want to push it backwards along the line of the orbit. This will slow it down so it goes into a lower orbit where the drag will be more significant. I'm not quite sure what would happen if you threw it straight down - it certainly wouldn't go straight down since it would still have all its sideways momentum. I think you would actually increase the height of the orbit (well, it would become elliptical with the height at the point you threw it the same as it was before and higher on the other side of the planet). --Tango (talk) 17:30, 8 August 2009 (UTC)
- Come on Tango, the question was clearly wheather a person would have enough arm power to do it, not wheather it is theoretically possible and as Finlay pointed out the delta-v is pohibitivily high. BTW the orbit you described at the end of your post would happen if the person through the object forward, not earthward as you described.Dauto (talk) 18:23, 8 August 2009 (UTC)
- If you're already in a low enough orbit that you experience some atmospheric drag, then the garbage is already on a trajectory that will send it burning up eventually. Any way you throw it with any amount of force will work. It would be impossible for a person to throw it hard enough that it fell straight to Earth (completely stopping the object) but I don't think that's what the OP was asking.
- As for the orbit resulting from throwing it in the direction of Earth, if the final velocity toward Earth is small compared to the angular velocity, the new orbit would be elliptical but with roughly the same total energy. Since the push is perpendicular to the direction of motion it doesn't significantly affect the kinetic energy. You would see it falling away from you, but as it did it would accelerate in the direction of the orbit, and as it accelerated, the speed of it's fall would slow, until eventually it would start rising again. Rckrone (talk) 19:14, 8 August 2009 (UTC)
- Come on Tango, the question was clearly wheather a person would have enough arm power to do it, not wheather it is theoretically possible and as Finlay pointed out the delta-v is pohibitivily high. BTW the orbit you described at the end of your post would happen if the person through the object forward, not earthward as you described.Dauto (talk) 18:23, 8 August 2009 (UTC)
- The retro-rocket fire slows the speed by about 1% to cause reentry. Atmospheric drag does the rest of the delta v. Any delta-v in the retro direction would hasten reentry a bit, but shoving something, throwing it or pushing it would be far less than 1% or orbital velocity. For the ISS. 1% of velocity would be 77 m/sec. A baseball player might achieve a 100 mph or 45 m/s fastball. A cricket ball might reach the same speed [12]. It would clearly deorbit sooner than if not thrown. A tossed sack of garbage might be 1/10 of that speed or less. A kicked U.S. football might hit 27 m/sec [13]. A golf ball might hit 78 m/sec [14] and a jai alai ball could hit 84 m/s [15], and either should de-orbit nicely. Edison (talk) 19:19, 8 August 2009 (UTC)
- Something on the order of 1% is needed if you want to land in the next few minutes or hours, but for disposing of garbage it doesn't matter if it takes days or weeks to burn up (much longer than that would be annoying because you would need to keep tracking it to avoid collisions). --Tango (talk) 19:34, 8 August 2009 (UTC)
- The retro-rocket fire slows the speed by about 1% to cause reentry. Atmospheric drag does the rest of the delta v. Any delta-v in the retro direction would hasten reentry a bit, but shoving something, throwing it or pushing it would be far less than 1% or orbital velocity. For the ISS. 1% of velocity would be 77 m/sec. A baseball player might achieve a 100 mph or 45 m/s fastball. A cricket ball might reach the same speed [12]. It would clearly deorbit sooner than if not thrown. A tossed sack of garbage might be 1/10 of that speed or less. A kicked U.S. football might hit 27 m/sec [13]. A golf ball might hit 78 m/sec [14] and a jai alai ball could hit 84 m/s [15], and either should de-orbit nicely. Edison (talk) 19:19, 8 August 2009 (UTC)
- It's not just a matter of it being possible - it has actually been done. I can't remember which EVA it was, but I do recall reading about a broken part that was being replaced (or something like that) being disposed of by the astronaut throwing it away and letting it burn up over the next few weeks). --Tango (talk) 19:34, 8 August 2009 (UTC)
- The astronaut "throwing" has nothing to do with it; indeed, the astronaut didn't "throw", they just let go. Things in the ISS orbit lose speed, and thus altitude, all by themselves, due solely to atmospheric friction. Left alone the ISS itself would burn up for the same reason - it loses 100m of altitude per day, and requires an occasional kick of +ve delta-v from Progress or ATV - this burn gave it an additional 7km. -- Finlay McWalter • Talk 23:24, 8 August 2009 (UTC)
- They do throw things - otherwise there is a risk of them hitting the ISS. --Tango (talk) 23:37, 8 August 2009 (UTC)
- The astronaut "throwing" has nothing to do with it; indeed, the astronaut didn't "throw", they just let go. Things in the ISS orbit lose speed, and thus altitude, all by themselves, due solely to atmospheric friction. Left alone the ISS itself would burn up for the same reason - it loses 100m of altitude per day, and requires an occasional kick of +ve delta-v from Progress or ATV - this burn gave it an additional 7km. -- Finlay McWalter • Talk 23:24, 8 August 2009 (UTC)
If an astronaut pushed something towards earth, then wouldnt she/he see it continually coasting towards earth at a constant speed? As it got closer to earth, then it would be slowed down by atmospheric friction, and accelerate downwards due to gravity? That is how the astronaut would see it - from a static viewpoint it would seem to spiral down towards earth. 89.240.34.84 (talk) 22:50, 9 August 2009 (UTC)
- It wouldn't go straight down. The orbital period would not remain the same as the astronaut's. It would only spiral down because of drag, if it was a high enough orbit that drag was negligible it would go into a periodic orbit, just a more eccentric (elliptical) than before. --Tango (talk) 23:22, 9 August 2009 (UTC)
Tyres and Dry-Rot
Hello! I have a "project car" in my garage, and I've got a lead on a good deal for new tyres. But - The car won't be road ready for maybe a year or 2 at the rate I'm working on it! So if I buy these new tyres, and they go on the car now, will they dry-rot if they just sit there not rolling for extended periods? I usually roll the car forward and back 10-20 feet or so for various access while working, but nothing more than that. They are new-new as in 2008-2009 manufacture ,and not new-old, if that makes any difference. Cheers! —Preceding unsigned comment added by 71.62.88.123 (talk) 16:50, 8 August 2009 (UTC)
- Tires bought 2 years ahead of when you will start driving the car will obviously not give as many miles of use as if you bought them when you needed them. But the "good deal" might make up for that, I suppose. Edison (talk) 19:05, 8 August 2009 (UTC)
- Store your new tyres in a cool, dry, shaded place until you need them. Stack the tyres flat on their sides. Remember to check the tyre pressures when it's time to use them. Cuddlyable3 (talk) 22:50, 8 August 2009 (UTC)
- It is completely normal to keep using the same tires for a decade or more, e.g. if you have snow tires that you put on the car for just a few months a year and don't drive much during those months. Snow tires are usually left in the garage during the summer too. 67.117.147.249 (talk) 01:58, 9 August 2009 (UTC)
- I'd keep them in a cool, dry, dark place - resting on their sides, not inflated and not mounted onto wheels. I think they'll last you a good few years that way. (So what are you restoring? My current 'baby' is a 1963 Mk I Mini...tyres are no problem...but a replacement engine block is!) If the tyres you need are still being made - and if you don't actually need them yet - then I'd hold off buying them. There's bound to be another good deal coming along in a couple of years time - and since about 80% of restoration projects are never completed, you might be wasting your money. I rarely pre-buy anything - I get just the parts I'm going to use in the next month or two. SteveBaker (talk) 13:55, 10 August 2009 (UTC)
Detecting nuclear weapons
Lets say a terrorist has a suitcase nuclear bomb. Does that bomb emit any particles that can be detected at all? Perhaps neutrinos? This is all before the bomb detonates, not after. ScienceApe (talk) 19:51, 8 August 2009 (UTC)
- I'm almost certain the short answer is "yes", but I don't believe the radioactivity of bomb materials (plutonium and/or enriched uranium) is really that great (I don't believe proneness to an actual chain reaction is too closely correlated with radioactivity levels, though if someone wants to correct ME I'd be interested). Also, the casing will presumably be lead, which will block most of that radiation. As for the neutrinos, well, they're pretty hard to detect as they only interact weakly (the weak interaction).--Leon (talk) 19:57, 8 August 2009 (UTC)
- Mostly it would be gamma rays that could be detected externally. In principle, the gamma rays could be blocked or reduced below background radiation levels but it would probably take several centimeters of lead shielding on all sides - at the very least, and the "briefcase" would easily end up weighing as much as a small car. Yes, lead is really that dense! 69.140.12.180 (talk) 20:25, 8 August 2009 (UTC)Nightvid
- Here is an excellent article on this topic. --Sean 20:43, 8 August 2009 (UTC)
- Neither neutrinos nor gamma rays are produced by U235. Neutrinos are produced during beta-decay while gamma rays are produced during a gamma-decay (hence the name). U235 decays through a alpha-decay chain producing alpha-particles which can be easily stopped by a sheet of paper for instance. Dauto (talk) 20:47, 8 August 2009 (UTC)
- Isn't there some beta decay eventually down the chain? Would that produce a detectable amount of beta radiation? I guess it's kind of moot though since beta particles are easy to block too and neutrinos are too hard to detect. Rckrone (talk) 21:14, 8 August 2009 (UTC)
- The uranium and plutonium used in nuclear weapons are very pure and therefore practically free of radioactive decay products. Also, they have a very long half-life, so an appreciable amount of decay products simply cannot build up. So the answer to your question is a definite "no". 98.234.126.251 (talk) 21:26, 8 August 2009 (UTC)
- In the case of 235U, even if it's totally pure, the daughter nuclid 231Th decays emitting beta radiation and it has a half life of only 25.5 hours. The short half life means that you approach the equilibrium at which there is one 231Th decay for each 235U decay pretty quickly (after 25.5 hours there will be 1/2 231Th decay for each 235U decay, after 51 hours 3/4 etc.). But beta radiation isn't difficult to shield, only the neutrinos are, but they are also too difficult to measure. By the way, the uranium and plutonium in nuclear weapons are maybe very pure chemically, but certainly not very pure with respect to unwanted isotopes. Icek (talk) 17:45, 9 August 2009 (UTC)
- The uranium and plutonium used in nuclear weapons are very pure and therefore practically free of radioactive decay products. Also, they have a very long half-life, so an appreciable amount of decay products simply cannot build up. So the answer to your question is a definite "no". 98.234.126.251 (talk) 21:26, 8 August 2009 (UTC)
- Isn't there some beta decay eventually down the chain? Would that produce a detectable amount of beta radiation? I guess it's kind of moot though since beta particles are easy to block too and neutrinos are too hard to detect. Rckrone (talk) 21:14, 8 August 2009 (UTC)
- Neither neutrinos nor gamma rays are produced by U235. Neutrinos are produced during beta-decay while gamma rays are produced during a gamma-decay (hence the name). U235 decays through a alpha-decay chain producing alpha-particles which can be easily stopped by a sheet of paper for instance. Dauto (talk) 20:47, 8 August 2009 (UTC)
- That is an excellent, not to mention worrying article. I am surprised that the detectors they have in place seem to be so ineffective (although it seems that it is not really possible to construct effective ones), but I am amazed by the idea that a gunlike nuclear weapon could be so easy to build (even to the point that just dropping one piece of enriched uranium onto another could potentially create a 1 kiloton explosion). I had always assumed that you would need the technological infrastructure of a state to build such a weapon. I can see now why people can be so very concerned about smuggling of enriched uranium. Bury me in a Y shaped coffin (talk) 23:01, 8 August 2009 (UTC)
- The single hardest step of building an atomic bomb is the enrichment. Dauto (talk) 01:40, 9 August 2009 (UTC)
- Yes, it would seem so. This article from a few years back seems to suggest that designing a crude weapon would certainly not be beyond the ability of a group that could recruit a couple of PhD students. Of course, the last pieces of the puzzles are the fabrication and assembly of the bomb components. I wonder what level of expertise and equipment is required for that. Bury me in a Y shaped coffin (talk) 10:43, 9 August 2009 (UTC)
- I guess that one bit of good news is that the quality of enriched uranium being produced by states that are more likely to lose it (or deliberately provide it to groups) is likely much lower than that discussed in the Scientific American article (which they state could cause a 1 kt explosion just by dropping one piece onto another), meaning that a bomb that used it would need to be more sophisticated (in terms of the accuracy of the components) and therefore less easy to construct. Bury me in a Y shaped coffin (talk) 10:48, 9 August 2009 (UTC)
- The hard parts of making a "suitcase" nuclear bomb are not the conceptual parts (which any Ph.D. student would have no trouble grasping). Probably, most college sophomore engineering students can handle all the conceptual details of the designs. But the execution of the extreme precision engineering required for all parts of the device are very hard to do. Refining the radioactive elements is the most commonly cited "difficult engineering task" - conceptually simple, but actually manufacturing and refining pure U235 or plutonium is so nontrivial that it really does take a sizeable operation. But this is only one single part of the process! While reading today about the Nagasaki bombing, I read about the fuzing device - an Exploding-bridgewire detonator (designed by a Ph.D. student at Berkeley, no less). But the precision engineering required on the Nagasaki bomb required a timing control on the chemical reaction of the detonators on the order of microseconds - else the shockwave does not properly yield the explosive lens effect necessary for achieving the the critical density for fission. Surely there are thousands of other extremely precise mechanical, chemical, nuclear, and electronics details. Even with a small team of highly trained experts, there's not a very good way to get these engineering details worked out - a large infrastructure base is required for the kinds of details necessary. In brief, there is a huge difference between understanding the process to make pure U235, and being able to make it. The same applies for every other engineering detail of these weapons - they're really fragile, balanced on a knife-edge betwen functional- and dud- so any little slip-up would result in a heavy lead-lined suitcase with some sort of hazardous stuff in it (but no nuclear weapon). Nimur (talk) 19:28, 9 August 2009 (UTC)
- See Radioactive Boy Scout. Apparently it isn't too hard for a 17-year-old. -RunningOnBrains(talk) 06:31, 10 August 2009 (UTC)
- You're confusing a tiny "reactor" of sorts with an actual bomb. Not the same, at all. If he had run his reactor for the rest of his life, he wouldn't have had an atomic bomb. More relevant article: Nth Country Experiment. --98.217.14.211 (talk) 00:30, 11 August 2009 (UTC)
- See Radioactive Boy Scout. Apparently it isn't too hard for a 17-year-old. -RunningOnBrains(talk) 06:31, 10 August 2009 (UTC)
- Make sense, although the bomb dropped on Nagasaki was of an implosion design rather than a gun-type weapon. I understand that implosion weapon are many times more difficult to create because of the timing issues that you mention. Not sure if you have had a chance to read the article linked by Sean above, but it suggests that gun-type designs are much easier to fabricate and, if the uranium is sufficiently enriched, you could potentially even get a detonation by just dropping one piece on the other. In a gun type weapon you are basically just slamming two pieces of uranium together, but of course the engineering required is still going to be slightly complex, I just wonder how complex. Bury me in a Y shaped coffin (talk) 20:00, 9 August 2009 (UTC)
- You'd need a gun that can shoot uranium at high speeds; that's easy enough to make. You'd also need neutron reflectors to cause as many fission events as possible before the bomb disintegrates, but I don't see anything challenging about surrounding the gun with reflector material. Whether all of this can fit in a suitcase, I have no idea. --Bowlhover (talk) 22:52, 9 August 2009 (UTC)
- Probably, if you have the right materials and know what you are doing. They used gun-type weapons in developing nuclear artillery shells which are about the size of a large suitcase. --98.217.14.211 (talk) 00:30, 11 August 2009 (UTC)
- You'd need a gun that can shoot uranium at high speeds; that's easy enough to make. You'd also need neutron reflectors to cause as many fission events as possible before the bomb disintegrates, but I don't see anything challenging about surrounding the gun with reflector material. Whether all of this can fit in a suitcase, I have no idea. --Bowlhover (talk) 22:52, 9 August 2009 (UTC)
- The hard parts of making a "suitcase" nuclear bomb are not the conceptual parts (which any Ph.D. student would have no trouble grasping). Probably, most college sophomore engineering students can handle all the conceptual details of the designs. But the execution of the extreme precision engineering required for all parts of the device are very hard to do. Refining the radioactive elements is the most commonly cited "difficult engineering task" - conceptually simple, but actually manufacturing and refining pure U235 or plutonium is so nontrivial that it really does take a sizeable operation. But this is only one single part of the process! While reading today about the Nagasaki bombing, I read about the fuzing device - an Exploding-bridgewire detonator (designed by a Ph.D. student at Berkeley, no less). But the precision engineering required on the Nagasaki bomb required a timing control on the chemical reaction of the detonators on the order of microseconds - else the shockwave does not properly yield the explosive lens effect necessary for achieving the the critical density for fission. Surely there are thousands of other extremely precise mechanical, chemical, nuclear, and electronics details. Even with a small team of highly trained experts, there's not a very good way to get these engineering details worked out - a large infrastructure base is required for the kinds of details necessary. In brief, there is a huge difference between understanding the process to make pure U235, and being able to make it. The same applies for every other engineering detail of these weapons - they're really fragile, balanced on a knife-edge betwen functional- and dud- so any little slip-up would result in a heavy lead-lined suitcase with some sort of hazardous stuff in it (but no nuclear weapon). Nimur (talk) 19:28, 9 August 2009 (UTC)
- The single hardest step of building an atomic bomb is the enrichment. Dauto (talk) 01:40, 9 August 2009 (UTC)
- The theory is only that you have to take two or more sub-critical-mass chunks and push them together to make a critical mass chunk and kaboom! The practical problem (which is why the guys at the Manhattan project had such a hard time making something that actually worked) is that when the two sub-critical mass pieces get close - the amount of heat and radiation goes up very rapidly, and this can be enough to rip the bomb to shreds without it actually going critical and producing the expected yield. The call this a "fizzle". So simply dropping one chunk of material onto another doesn't produce the large yield you need to take out a city...although it might be enough to spread radioactive material over a few city blocks...a "dirty bomb". You could do as well by just wrapping some radioactive material around a few sticks of dynamite. The whole business of experimenting with "gun" and "implosion" designs was to solve the question of how to get the fissile material close enough together and to hold it there for long enough for the chain reaction to really get going. Slamming the parts together RAPIDLY seems to have been the key - and for that you need either a "gun" or an "implosion" device...but small, subtle details of the design - and high engineering tolerances - seem to be a vital part of making something that actually works without fizzling. Given the ruinously expensive cost of the fissile material, this kind of thing can't really be done experimentally by anyone short of a major governement - so you are left with needing to steal someone else's design and doing some really first class precision machining - or employing a LOT of very smart people to figure out a new design with a real chance of working the very first time you try it. SteveBaker (talk) 13:48, 10 August 2009 (UTC)
- I just want to note that it's easy to deride a fizzle as not being very powerful, but if you set off a .5kt explosion in a major city, it would without a doubt be the most deadly terrorist attack ever perpetrated. When you start talking about nuclear yields, it's easy to fall into the "if it's smaller than Hiroshima, it's a dud" mindset, but if you take the time to calculate how bad that would actually be, it's pretty bad if you get an appreciable yield at all (which for U-235, you probably would, if you dropped 1.5 crits together in a crude way). The Oklahoma City bomb was only .002 kt. If you can get anything appreciable nuclear yield, then you're still talking about a relatively large explosion.
- I also want to object that only governments could come up with a gun-type design that would work. I suspect a small team of people with the right backgrounds (some physics, some explosives, some engineering) could easily come up with a Hiroshima-style weapon IF they had the U-235. It's a shop-class sort of project, and you don't need to waste U-235 to experiment about it (they didn't during the Manhattan Project, and things are a lot better known today than they were then... you can get a million times more information about fission processes through a nuclear engineering course than they ever had during the war). Implosion designs are much, much harder, and require the whole team of very smart people to work on (even if it is not as revolutionary as it once was). For U-235, the only significant hurtle is obtaining the material—which is why it is a much, much more dangerous element from the point of view of terrorism than plutonium. --98.217.14.211 (talk) 00:30, 11 August 2009 (UTC)
- On detecting nuclear weapons: Robert Oppenheimer was asked by a Senator once, in Congress, what instrument he would use to detect an atomic bomb. "A screwdriver," he replied—to open every suitcase coming in. --98.217.14.211 (talk) 00:30, 11 August 2009 (UTC)
Antenna length
(apologies if I'm not on the right desk) I have one of those portable TVs with a whip antenna attached. (You can extend the antenna to a length of about 30 inches, and retract it to about 5 inches.) What is the best length of this antenna - half or quarter wavelength? Xenon54 (talk) 21:41, 8 August 2009 (UTC)
- What frequency or station are you trying to tune to?83.100.250.79 (talk) 22:21, 8 August 2009 (UTC)
- Specifically, it's WJZ-TV on channel 13 (211 MHz). Quarter-wavelength is 13.3 inches (I think), but something's telling me "No! It's half!" and I can't remember which it is. Xenon54 (talk) 22:54, 8 August 2009 (UTC)
- Dipole antenna is 1/2 , Whip antenna is 1/4 which must 'be grounded'. (I get about 13.9" for 1/4) I assume the article is right? 83.100.250.79 (talk) 23:47, 8 August 2009 (UTC)
- Specifically, it's WJZ-TV on channel 13 (211 MHz). Quarter-wavelength is 13.3 inches (I think), but something's telling me "No! It's half!" and I can't remember which it is. Xenon54 (talk) 22:54, 8 August 2009 (UTC)
- At 211MHz, the wavelength = 1.42 meters = 56 inches. 1/4 wavelength is 14 inches.
Generally speaking, a longer antenna always 'intercepts' more energy. I'll go with the half-wavelength (28") being 'better' for reception ... 'in free space' at least.
Twang (talk) 08:44, 9 August 2009 (UTC)- Responders have assumed that "best" means maximum sensitivity. That could be true when seeking a distant station. It's more usual to have adequate signal strength but receive a picture that is degraded by "ghosting" due to delayed signal(s) from reflection(s). A whip aerial has little or no directivity but you can experiment with its length and position to minimise ghosts. Cuddlyable3 (talk) 12:42, 9 August 2009 (UTC)
- A bit of OR here, I've had good results with ghost removal in a weakish reception area, by resting the tip of the antenna against an aluminium venetian blind.-KoolerStill (talk) 18:25, 9 August 2009 (UTC)
- Dipole antennas for televisions are very weakly tuned anyway, (and they must pick up a reasonably wide-band channel, with various different wavelengths). The "quarter-wavelength" or "half-wavelength" concept is usually less relevant in this situation than other factors - most importantly, the dipole is directional, so changing its orientation will affect your signal reception capability. Also, the total received power will vary (roughly linearly) with the length of the antenna - so a longer antenna, which de-tunes from the correct frequency, may still yield better total signal reception due to better received power. Your specific dipole antenna circumstances may vary... Nimur (talk) 19:17, 9 August 2009 (UTC)
August 9
Is this guy a crank?
http://www.wholeapproach.com/index.php
I tried to google criticism of this website but no results. Any help?--12.48.220.130 (talk) 03:11, 9 August 2009 (UTC)
- The evidence for these yeast syndromes is dubious at best, the condition is not widely accepted among the mainstream medical and scientific community. See [16] and [17] Rockpocket 03:55, 9 August 2009 (UTC)
- While not specifically answering the question, Googling for criticism of a website is often ineffective because unless the person/website is a notable crank, it's likely no one has discussed it. Googling for the claims made or therapy or whatever is likely to be more effective presuming the person didn't come up with some 'novel' idea. Even better of course is to look into the science and website. Does the website mention any science to support it? Is there any science to support it? If for example, the website is describing some sort of treatment, then looking into the condition the treatement is alleged to help should be illuminating. Ultimately the best thing is perhaps not to ask is the person a crank but is there any evidence the person is not a crank? Nil Einne (talk) 17:01, 10 August 2009 (UTC)
Where can I find more about that? —Preceding unsigned comment added by 12.48.220.130 (talk) 04:35, 9 August 2009 (UTC)
- See the references for Candidiasis#Alternative views. Red Act (talk) 10:12, 9 August 2009 (UTC)
Zero Velocity but accelerating!!!!!
Can an object moving with zero velocity have some acceleration?? -- 04:17, 9 August 2009 (UTC)
- Momentarily, yes. I suspect this is homework, so I won't give the detailed answer. However, watch the pendulum and you may be enlightened :) --Dr Dima (talk) 04:25, 9 August 2009 (UTC)
- This is not homework. I just happened to ponder about it! I always get such stupid questions in my mind!!! -- 04:34, 9 August 2009 (UTC)
Plot the trajectory of a ball thrown up into the air as a function of distance versus time. Take the derivative, to find velocity. Is the function defined at y=0? If so, then the object experienced zero velocity even undergoing constant gravitational acceleration. John Riemann Soong (talk) 06:00, 9 August 2009 (UTC)
Can an object be not moving and yet is accelerating at the same time? The answer is yes. But the "amount of time" the object spent at this strange state is infinitesimally small. 122.107.207.98 (talk) 11:39, 9 August 2009 (UTC)
- Until you put it into a simple harmonic motion system that is. Well okay, it's still going to be infinitesimally small (thanks to the cardinality of the continuum), but it'll repeat that moment every time the cycle repeats. John Riemann Soong (talk) 14:11, 9 August 2009 (UTC)
- The original questioner might want to contemplate the conceptual difference between velocity and instantaneous velocity. A particle can have an instantaneous velocity of 0, and a non-zero acceleration; but after an infinitely small instant of time, it will soon have a non-zero velocity as well. This thought-experiment is often used to introduce the concept of the derivative with respect to time, in an elementary mechanics or calculus class. Nimur (talk) 19:14, 9 August 2009 (UTC)
- Bringing in the ideas that went into special relativity, a person sitting in a box that travel upwards at a constant acceleration, cannot distinguish this from sitting in a box at rest, in a gravitational field. (on the ground) This really goes back to Newton since the force the ground is pushing at you with is given as F = m*a where a is the gravitational acceleration. EverGreg (talk) 12:09, 10 August 2009 (UTC)
- A train set out at midday, travelling (as they do) from A to B at 100mph. A common housefly sets out at the same time, travelling from B to A at 1mph. At point C, the fly hits the front of the train. In this instant, it is momentarily stationary. Since the fly and the train are both at point C - then either the fly's velocity changed from 1mph to -100mph in zero time (which would require an infinite acceleration and hence an infinite force) - or the fly slowed down, was briefly stationary, then gained a finite acceleration up to -100mph. In which case, was the train also stationary at some point? Did the fly stop the train? The problem with these thought-experiments is that they neglect the elasticity of objects at macro-scales and the sheer weirdness of things at the nano-scale. In theory, the fly reached zero velocity had an acceleration that was non-zero - but it can only have that for an amount of time equal to zero. In practice, time, position, momentum and force are all quantized in weird and wonderful ways - so that this issue simply doesn't come up as a practical problem. When the fly hit the train, both fly and train deformed, atom by atom - some atoms in the train did reverse direction briefly. The forces between the atoms acted to repel them from each other and cause a gradual slow-down, then acceleration of the two surfaces at the nano-scale. You really can't ask these questions at the 'macro' scale and get a sensible answer. SteveBaker (talk) 13:34, 10 August 2009 (UTC)
Untitled Query
how to split hydrogen and nitrogen from urea. please say the method in detail. —Preceding unsigned comment added by Studiousvenkat (talk • contribs) 05:06, 9 August 2009 (UTC)
- Welcome to the Wikipedia Reference Desk. Your question appears to be a homework question. I apologize if this is a misevaluation, but it is our policy here to not do people's homework for them, but to merely aid them in doing it themselves. Letting someone else do your homework does not help you learn how to solve such problems. Please attempt to solve the problem yourself first. If you need help with a specific part of your homework, feel free to tell us where you are stuck and ask for help. If you need help grasping the concept of a problem, by all means let us know. Thank you. ~ Amory (user • talk • contribs) 06:12, 9 August 2009 (UTC)
- Subscription required. If you can't access that article, then google "10.1039/b905974a" (that will bring up references to the paper). 152.16.59.102 (talk) 06:14, 9 August 2009 (UTC)
- Uhh, how do you want your hydrogen and nitrogen? As ammonia? As N2 and H2? John Riemann Soong (talk) 06:18, 9 August 2009 (UTC)
- Use a mass spectrometer - that will provide N , C, O and H atoms!83.100.250.79 (talk) 12:39, 9 August 2009 (UTC)
- A related chemical process is the Haber process - an efficient method for producing ammonia, given nitrogen and hydrogen. The process is reversible, so under some conditions, you can produce nitrogen and hydrogen from ammonia. So, one method to solve the initial question is to decay the urea into ammonia (via hydrolysis) - and then use the ammonia into a reversed Haber process. You might also want to read about the urea cycle. Nimur (talk) 19:11, 9 August 2009 (UTC)
Suppose you're standing on the surface of a balloon
Further suppose that this balloon is in empty space, far away from any other matter, but you have your own air supply. Further suppose that this (very large) balloon is very elastic. Air is being pumped into it rapidly, and it is expanding. It has negligible mass (compared to, say, a planet), but because the surface is expanding, you are being accelerated.
Consequently (because your body has inertia), you are able to walk around on the surface. Because it's expanding at just the right rate, you feel a 'weight' just like you do when you walk around on the surface of the Earth.
Here's the question: how is this 'force' -- caused by expansion of the balloon -- fundamentally different (apart from its cause) from the force of gravity? Twang (talk) 08:25, 9 August 2009 (UTC)
- Other than observing the relative motion of nearby objects (if any), I can think of no way to tell the difference, though the expansion would have to be in space rather than of space. Dbfirs 08:32, 9 August 2009 (UTC)
- You wouldn’t be able to tell the difference, with local experiments. See Einstein equivalence principle. Red Act (talk) 10:26, 9 August 2009 (UTC)
- A local experiment: Stop walking, stand and look at your feet gradually moving apart. Cuddlyable3 (talk) 12:31, 9 August 2009 (UTC)
- "Local" is a technical term, it means (roughly speaking) that the experiment only concerns a single point (or an arbitrarily small region around that point). Your experiment isn't local since your feet are in two different places. --Tango (talk) 15:21, 9 August 2009 (UTC)
- A local experiment: Stop walking, stand and look at your feet gradually moving apart. Cuddlyable3 (talk) 12:31, 9 August 2009 (UTC)
Wouldn't your acceleration be different depending on what sort of mass you had? What if you were as twice as heavy? John Riemann Soong (talk) 15:38, 9 August 2009 (UTC)
- Yes, but if the pressure in the balloon was high enough you compressing the balloon would be a negligible factor. --Tango (talk) 15:52, 9 August 2009 (UTC)
I suppose it depends on how you define fundamentally, but the acceleration you'd feel on the balloon is essentially pressure, caused by the air being pumped in (work is being done). Gravity is gravity. ~ Amory (user • talk • contribs) 15:58, 9 August 2009 (UTC)
- In science, things are only considered to be different if there is an experiment that tells them apart. According to General Relativity, there is no experiment that can tell acceleration (whatever the cause) and gravity apart, so they are the same thing. --Tango (talk) 16:03, 9 August 2009 (UTC)
- This was meant as a response to Bus stop's removed comment, but it's applicable here. I was trying to imply that the fact that the two "forces" are from different causes matters here. I failed miserably. At any rate, the "weight" on the balloon is caused due to its expansion due to the pumping-in of air, as I said. What that means, however, is that more and more air must be pumped in in order to sustain that weight, as implied in the question. As the balloon expands further and further, you move farther and farther away from a given point, something you do not notice under the influence of gravity unless no other forces are in play. If you did the same thing with gravity (move away from the center of gravity), you would begin to weigh less and less as the distance between you increased. The question was about forces and weight, not accelerations, and our weight on Earth rarely involves a significant amount of acceleration. ~ Amory (user • talk • contribs) 16:36, 9 August 2009 (UTC)
- I'm believe Amory has a point, but I disagree with the significance of the issue, or more properly, I feel that the response needs more qualification. With a very large balloon, on which the surface is approximately flat in a decent-sized neighbourhood of the observer, he needn't notice the change in its shape (which I'll add Amory very rightly did not mention), and let's suppose he build a house and other landmarks on this near-flat surface. How would he know he was moving? With reference to applicable landmarks, he wouldn't be. So he needn't notice.--Leon (talk) 16:46, 9 August 2009 (UTC)
- Actually I think he would notice. While the shape doesn't noticeably change the size does. The radius of the balloon needs to increase with an acceleration of 9.8m/s2. The distance between two points is the angle between them (in radians) multiplied by the radius, so that will be increasing at a speed equal to the speed the radius is enlarging multiplied by that angle. Assuming the balloon started at rest the size of the Earth and the most distant parts of the house were 20m apart, that corresponds to an angle of 20/6400000=1/320000 radians. The size of the house is then R/320000, where R=6400000+1/2*9.8*t2. If we assume the house can survive an expansion of 1% before it collapses then it will collapse when (6400000+1/2*9.8*t2)/320000=20.2, ie. when 1/2*9.8*t2/320000=0.2, which is 3 hours and 38 minutes after the start. So this guy's house would collapse in less than 4 hours - he would notice that! It is only locally that acceleration and gravity are equivalent, a house is an extended object so it can tell the difference (at least, it can tell that the acceleration/gravity isn't uniform, you probably could describe the house collapsing in terms of tidal forces in some gravitational field). --Tango (talk) 17:22, 9 August 2009 (UTC)
- That's true; though the larger the balloon's radius the longer the house would stand! However, more germanely, the environment would change globally, which makes creating some sort of "static" local environment difficult. I suppose that if the surface has zero friction, one could place some extremely large platform upon it to build such an environment. In which case I don't think he'd notice.--Leon (talk) 17:31, 9 August 2009 (UTC)
- The larger the balloon's starting radius, the longer the house would stand. If I'd assumed the balloon had started at zero size and then the house was built once it reached the Earth's size, it wouldn't have lasted anywhere near as long (probably seconds, I haven't done the sums). I think if you had an enclosed platform that met the balloon tangentially (ie. at a single point - not possible in real life, but good enough for a thought experiment like this one), you wouldn't be able to tell the difference between that and a similar platform on Earth. --Tango (talk) 17:36, 9 August 2009 (UTC)
- It’s possible to perform experiments that would distinguish between the two situations, even within an enclosed platform, if the distances and times involved in the experiment are too large for the experiment to qualify as being local, as is the case with the house example. Suppose that 20m wide house isn’t attached to the balloon, but instead is engineered to allow the balloon to expand under it. Suppose the house has a precisely flat, perfectly frictionless, 20m-long table in it, that’s been balanced with the use of a super-precise level placed at the center of the table. Take two perfectly frictionless objects, and place them so they’re precisely motionless at the ends of the table. Wait the 3 hours and 38 minutes. If the objects have moved together by about 1%, you’re on earth. If the objects are where you left them, your house is being pushed along by the expanding balloon. Or if you’re impatient, you can just weigh yourself with super-precise scales on the house’s ground floor, and in the house’s attic. If you weigh less in the attic, you’re on Earth. If you weigh the same in both places, you’re on the balloon. Red Act (talk) 20:00, 9 August 2009 (UTC)
- You make a good point, I was so wrapped up in the details of the balloon I forgot the Earth's gravitational field wasn't uniform! --Tango (talk) 20:26, 9 August 2009 (UTC)
- It’s possible to perform experiments that would distinguish between the two situations, even within an enclosed platform, if the distances and times involved in the experiment are too large for the experiment to qualify as being local, as is the case with the house example. Suppose that 20m wide house isn’t attached to the balloon, but instead is engineered to allow the balloon to expand under it. Suppose the house has a precisely flat, perfectly frictionless, 20m-long table in it, that’s been balanced with the use of a super-precise level placed at the center of the table. Take two perfectly frictionless objects, and place them so they’re precisely motionless at the ends of the table. Wait the 3 hours and 38 minutes. If the objects have moved together by about 1%, you’re on earth. If the objects are where you left them, your house is being pushed along by the expanding balloon. Or if you’re impatient, you can just weigh yourself with super-precise scales on the house’s ground floor, and in the house’s attic. If you weigh less in the attic, you’re on Earth. If you weigh the same in both places, you’re on the balloon. Red Act (talk) 20:00, 9 August 2009 (UTC)
- The larger the balloon's starting radius, the longer the house would stand. If I'd assumed the balloon had started at zero size and then the house was built once it reached the Earth's size, it wouldn't have lasted anywhere near as long (probably seconds, I haven't done the sums). I think if you had an enclosed platform that met the balloon tangentially (ie. at a single point - not possible in real life, but good enough for a thought experiment like this one), you wouldn't be able to tell the difference between that and a similar platform on Earth. --Tango (talk) 17:36, 9 August 2009 (UTC)
- That's true; though the larger the balloon's radius the longer the house would stand! However, more germanely, the environment would change globally, which makes creating some sort of "static" local environment difficult. I suppose that if the surface has zero friction, one could place some extremely large platform upon it to build such an environment. In which case I don't think he'd notice.--Leon (talk) 17:31, 9 August 2009 (UTC)
- Actually I think he would notice. While the shape doesn't noticeably change the size does. The radius of the balloon needs to increase with an acceleration of 9.8m/s2. The distance between two points is the angle between them (in radians) multiplied by the radius, so that will be increasing at a speed equal to the speed the radius is enlarging multiplied by that angle. Assuming the balloon started at rest the size of the Earth and the most distant parts of the house were 20m apart, that corresponds to an angle of 20/6400000=1/320000 radians. The size of the house is then R/320000, where R=6400000+1/2*9.8*t2. If we assume the house can survive an expansion of 1% before it collapses then it will collapse when (6400000+1/2*9.8*t2)/320000=20.2, ie. when 1/2*9.8*t2/320000=0.2, which is 3 hours and 38 minutes after the start. So this guy's house would collapse in less than 4 hours - he would notice that! It is only locally that acceleration and gravity are equivalent, a house is an extended object so it can tell the difference (at least, it can tell that the acceleration/gravity isn't uniform, you probably could describe the house collapsing in terms of tidal forces in some gravitational field). --Tango (talk) 17:22, 9 August 2009 (UTC)
- I'm believe Amory has a point, but I disagree with the significance of the issue, or more properly, I feel that the response needs more qualification. With a very large balloon, on which the surface is approximately flat in a decent-sized neighbourhood of the observer, he needn't notice the change in its shape (which I'll add Amory very rightly did not mention), and let's suppose he build a house and other landmarks on this near-flat surface. How would he know he was moving? With reference to applicable landmarks, he wouldn't be. So he needn't notice.--Leon (talk) 16:46, 9 August 2009 (UTC)
- It's really worth noting that large spheres of gas have extremely appreciable gravity. In fact, most of the gravitating objects in our universe are large spheres of gas. How can the force of gravity be considered "negligible" if there is enough gas to make a balloon like the one that's being described? Nimur (talk) 22:01, 9 August 2009 (UTC)
- Those large spheres of gas aren't made of gas as we know it. Most of Jupiter is metallic hydrogen. Stars are made of plasma and behave very differently to gasses like those in Earth's atmosphere. An Earth-sized balloon filled with Earth-like air would have a pretty low mass, even lower if you filled it with hydrogen instead. I'm not sure exactly what because I'm not sure how much it would compress under its own gravity. The density will also depend on the nature of the balloon itself. --Tango (talk) 22:14, 9 August 2009 (UTC)
- Given the radius of Earth as 6371km, and a density of Hydrogen of 0.08988 g/L (massive underestimate), an Earth-sized ball of hydrogen would be approximately 1020kg (if my quick math is right), a full 10,000 times less than the current Earth, but hardly negligible (compare to the mass of the Moon). ~ Amory (user • talk • contribs) 22:34, 9 August 2009 (UTC)
- Where did that density come from? If that's under STP, it's pretty meaningless - why would this balloon be under STP? Don't forget you are further away from the centre of gravity than you would be on the moon, so surface gravity would be lower than you might expect. 10,000 times less density means 10,000 less surface gravity, so that's 0.00098m/s2, only 100 times more than microgravity on the ISS [18] and that is often described as zero-g. It's pretty negligible, if you ask me. --Tango (talk) 22:52, 9 August 2009 (UTC)
- If you're interested Tango (or others) I responded on my talk page here, not wanting to derail this (now lovingly answered) question further. ~ Amory (user • talk • contribs) 23:44, 9 August 2009 (UTC)
- Where did that density come from? If that's under STP, it's pretty meaningless - why would this balloon be under STP? Don't forget you are further away from the centre of gravity than you would be on the moon, so surface gravity would be lower than you might expect. 10,000 times less density means 10,000 less surface gravity, so that's 0.00098m/s2, only 100 times more than microgravity on the ISS [18] and that is often described as zero-g. It's pretty negligible, if you ask me. --Tango (talk) 22:52, 9 August 2009 (UTC)
- Given the radius of Earth as 6371km, and a density of Hydrogen of 0.08988 g/L (massive underestimate), an Earth-sized ball of hydrogen would be approximately 1020kg (if my quick math is right), a full 10,000 times less than the current Earth, but hardly negligible (compare to the mass of the Moon). ~ Amory (user • talk • contribs) 22:34, 9 August 2009 (UTC)
- It's a thought exercise - the question is about the specific acceleration and forces on the balloon, trying to ignore all other influences. Clearly it's not realistic, but it's useful for our understanding to deconstruct a problem. ~ Amory (user • talk • contribs) 22:19, 9 August 2009 (UTC)
- Those large spheres of gas aren't made of gas as we know it. Most of Jupiter is metallic hydrogen. Stars are made of plasma and behave very differently to gasses like those in Earth's atmosphere. An Earth-sized balloon filled with Earth-like air would have a pretty low mass, even lower if you filled it with hydrogen instead. I'm not sure exactly what because I'm not sure how much it would compress under its own gravity. The density will also depend on the nature of the balloon itself. --Tango (talk) 22:14, 9 August 2009 (UTC)
- It's really worth noting that large spheres of gas have extremely appreciable gravity. In fact, most of the gravitating objects in our universe are large spheres of gas. How can the force of gravity be considered "negligible" if there is enough gas to make a balloon like the one that's being described? Nimur (talk) 22:01, 9 August 2009 (UTC)
- In response to an earlier objection that the expanding balloon would tear the house apart, there are casters and sliding pads which would allow the house to be supported at four or more points which would slip along the surface as the balloon expanded. The base of the house could also be a Teflon covered flat or curved plate or diaphragm which would slip nicely. Edison (talk) 02:48, 10 August 2009 (UTC)
- We weren't trying to build a house, though, we were trying to hide the fact that the balloon was expanding. You could tell the balloon was expanding by watching the wheels turn around or whatever. --Tango (talk) 11:36, 10 August 2009 (UTC)
- Couldn't you tell the difference with the Cavendish experiment? AlmostReadytoFly (talk) 11:48, 10 August 2009 (UTC)
- So if you jump off of the surface of the balloon, there is nothing to reduce your outward velocity (no gravity) - but it seems like there is because the balloon is accelerating upwards towards you at exactly the rate that gravity would be lowing you down. However, there is an experiment you can do - which is to toss a ball vertically upwards at "escape velocity" for the planet you think you're standing on. If there is real gravity - then the ball will never return to you - if there is only this 'fake' gravity, then the ball will always come back eventually, no matter how fast you throw it. As others have pointed out, it's only the same as gravity "locally" - and as soon as you can do any kind of non-local experiment, you can tell the difference. You specified an otherwise empty universe - and that's just as well because otherwise we'd be able to measure the red-shift of nearby stars and discover that they too are accelerating towards us in a manner that could not happen if this were a gravitational field because they are too far away. The illusion of gravity is perfect for strictly 'local' observations...but is easily detectable with almost any kind of non-local experiment. The Cavendish experiment uses non-locality. SteveBaker (talk) 13:18, 10 August 2009 (UTC)
- Actually, I don’t see how doing the Cavendish experiment would enable you to tell the difference. The Cavendish experiment doesn’t rely on non-locality at all. What the Cavendish experiment measured was the gravitational attraction between lead balls separated by a few centimeters, by measuring miniscule changes in the motion of a rod that only oscillated back and forth by about 4 mm. The distances involved are too small for variations in the Earth’s gravitational field to affect the measurements, and the masses of the balls are vastly too small for gravity’s nonlinearity to have a significant effect. So within experimental error, the Cavendish experiment would measure the gravitational attraction between the balls to be the same, whether you were on Earth or on the balloon. Red Act (talk) 15:19, 10 August 2009 (UTC)
- Indeed. The Cavendish experiment is done horizontally so local gravity or vertical acceleration won't make any difference - that's really the whole point, you want to remove any contributions from anything other than the gravity of the balls. --Tango (talk) 15:30, 10 August 2009 (UTC)
- Actually, I don’t see how doing the Cavendish experiment would enable you to tell the difference. The Cavendish experiment doesn’t rely on non-locality at all. What the Cavendish experiment measured was the gravitational attraction between lead balls separated by a few centimeters, by measuring miniscule changes in the motion of a rod that only oscillated back and forth by about 4 mm. The distances involved are too small for variations in the Earth’s gravitational field to affect the measurements, and the masses of the balls are vastly too small for gravity’s nonlinearity to have a significant effect. So within experimental error, the Cavendish experiment would measure the gravitational attraction between the balls to be the same, whether you were on Earth or on the balloon. Red Act (talk) 15:19, 10 August 2009 (UTC)
Thanks, y'all. I hope you had as much fun with it as I did reading the replies. As a result, in time I may return with a more refined round 2 question. (Not that there's any comparison, but ... No damn wonder Niven took so long after Ringworld!!) Twang (talk) 04:22, 11 August 2009 (UTC)
I don't understand the 3-body problem
I tried looking at the article and I don't "get" it. Where can I have it explained in simpler terms?--12.48.220.130 (talk) 13:38, 9 August 2009 (UTC)
- What don't you get? The idea is that we have 3 bodies (eg planets or something), and we also have their intial positions and speeds, as well as formula showing how they interact with each other (eg gravity in this case).
- The problem is to find equations describing the positions of the bodies after time t.
- The thing about it is that it's very difficult to solve...
- You're probably familiar with simple 2 body problems such as the motion of a small ball under gravity (which is solved eaasily by assuming that the earth (that causes gravity) is so big that it doesn't move at all (an approximation) - the three body problem is to find the same sort of things for 3 things, with no approximations. (Probably you don't get a lot of the article because it's complicated maths - to be honest - neither do I)83.100.250.79 (talk) 15:15, 9 August 2009 (UTC)
I mean is it of the same nature as Fermat's last theorem or something?--12.48.220.130 (talk) 16:01, 9 August 2009 (UTC)
- No. it's just a difficult set of equations to solve.83.100.250.79 (talk) 16:05, 9 August 2009 (UTC)
- You should also read N-body_problem#King_Oscar_II_Prize_about_the_solution_for_the_n-body_problem which roughly says "stop asking stuff about things which you have no way of knowing if you've got the answer or not or fuck off" 83.100.250.79 (talk) 16:08, 9 August 2009 (UTC)
- Try starting with something simpler first - do you get the 2-body problem?83.100.250.79 (talk) 16:43, 9 August 2009 (UTC)
Well yeah. That makes sense. So why can't three objects all revolve around the same barycenter as well?--12.48.220.130 (talk) 18:42, 9 August 2009 (UTC)
- They can - but that's just one special case of a three body problem - one which happens to be much easier to solve. It's the general solution that is hard to get...83.100.250.79 (talk) 19:03, 9 August 2009 (UTC)
Well what's an example of a generic case that is hard to find a solution for?--12.48.220.130 (talk) 20:06, 9 August 2009 (UTC)
- A mass 4 at (3,0,0) initial velocity (1,2,2)
- B mass 10 at (3,4,3) initial velocity (0,-2,1)
- C mass 3 at (-1,3,3) initial velocity (7,0,5)
- All subject to gravitational type force (inverse square) eg kM1M2/r2 (units in SI units or whatever)
- What are the positions of A, B and C as a function of time?
- (edited twice)83.100.250.79 (talk) 20:32, 9 August 2009 (UTC)
- What makes generic examples as the one described above is the fact that the bodies will swarm around each other following open orbits (that do not repeat themselves) and will ocasionally suffer arbitrarilly close encounters. The closer the encounter the harder it becomes to predict what happens next due to Oberth effect which implies that an arbitrarily small imprecision in the orbits before the close encounter can turn into an arbitrarily large imprecision after it making the general problem impossible to handle. Dauto (talk) 21:04, 9 August 2009 (UTC)
- The Oberth effect is only one of the many issues that plague a numerical solution to this problem. A naive iterative solution of the gravitational force equation, using Euler method iteration and incrementing the velocity and position of each body for each time step, is numerically unstable, and diverges due to accumulating error. For the non-technical reader: This is the simplest way you might think you can program a computer to solve the gravity equation - just solve for the force, update the acceleration, and update the velocity and position - but it doesn't work! This is the case even for the 2-body problem, which has an analytic solution. See "Inaccuracy and Instability of Euler's method" from the University of Buffalo's Math Department for a graphic visualization of numerical error on a very simple equation. Even some more advanced ordinary differential equation solvers tend towards numerical instability on the force law iteration. This doesn't even consider whether the problem suffers from an analytic instability - this is strictly about propagation of error in the numerical differential equation solver. With an n-body problem, you have a large matrix of coupled differential equations, and you will find that the errors are not only propagating forward in time, but coupling in unusual ways throughout the problem. You certainly have to worry about this issue if you are solving the 3-body problem on a computer. Nimur (talk) 22:10, 9 August 2009 (UTC)
- I remember for the intro programming class I took at school, one of the first things we did was program a solar system simulator that used Euler's method approximation. It generally worked alright if you didn't look too closely, but when objects got very close to each other, they would suddenly get flung off the screen at high speed never to be seen again. Rckrone (talk) 23:08, 9 August 2009 (UTC)
- That's really at the heart of the problem. You can use numerical approaches to get an approximation to the correct answer - and you can make the time steps arbitarily small to get an arbitarily good answer - but the 3 body problem is chaotic in nature - small changes in the short term tend to blow up into arbitarily massive changes in the long term for all but the most stable cases. So you can't ever get a good answer over long time periods using a numerical method with finite precision. With the 2 body problem, the equations exist and can be solved for any arbitary time 't' and you can plug any 't' into the equation and get a good answer. I'm a computer game programmer - and for exactly these reasons, doing N body dynamics problems is one of the hardests parts of what we do. Even simulating something as seemingly simple as a pile of boxes sitting perfectly still on the ground becomes quite tricky in general-purpose dynamics software. If you naively plug in the math and naively simulate them numerically then they start jiggling, then bouncing - then they fly apart at crazy speeds...just like your solar system simulator! All sorts of cheats and kludges are required to make the 'real world' math function believably in a numerically simulated world. SteveBaker (talk) 13:05, 10 August 2009 (UTC)
- I remember for the intro programming class I took at school, one of the first things we did was program a solar system simulator that used Euler's method approximation. It generally worked alright if you didn't look too closely, but when objects got very close to each other, they would suddenly get flung off the screen at high speed never to be seen again. Rckrone (talk) 23:08, 9 August 2009 (UTC)
- The Oberth effect is only one of the many issues that plague a numerical solution to this problem. A naive iterative solution of the gravitational force equation, using Euler method iteration and incrementing the velocity and position of each body for each time step, is numerically unstable, and diverges due to accumulating error. For the non-technical reader: This is the simplest way you might think you can program a computer to solve the gravity equation - just solve for the force, update the acceleration, and update the velocity and position - but it doesn't work! This is the case even for the 2-body problem, which has an analytic solution. See "Inaccuracy and Instability of Euler's method" from the University of Buffalo's Math Department for a graphic visualization of numerical error on a very simple equation. Even some more advanced ordinary differential equation solvers tend towards numerical instability on the force law iteration. This doesn't even consider whether the problem suffers from an analytic instability - this is strictly about propagation of error in the numerical differential equation solver. With an n-body problem, you have a large matrix of coupled differential equations, and you will find that the errors are not only propagating forward in time, but coupling in unusual ways throughout the problem. You certainly have to worry about this issue if you are solving the 3-body problem on a computer. Nimur (talk) 22:10, 9 August 2009 (UTC)
- A couple decades back I wrote a program to simulate a ship launched from Earth orbit towards the Moon's future position. It was designed so that the user had to find the right set of initial values to get the ship to orbit the Moon at least once. To make it as realistic as possible (but 'fly' in reasonable CPU time for that day), I consulted with a math prof and we came up with a Runga-Kutta 'solution' that could be quite accurate.
After toying with that for several months, I developed a MUCH deeper appreciation for any team that managed to get a real satellite into lunar orbit ... Twang (talk) 04:43, 11 August 2009 (UTC)- Yep - and that's not even a proper 3-body problem since the gravitational force exerted by the satellite onto the earth and moon is negligable. SteveBaker (talk) 13:03, 11 August 2009 (UTC)
- A couple decades back I wrote a program to simulate a ship launched from Earth orbit towards the Moon's future position. It was designed so that the user had to find the right set of initial values to get the ship to orbit the Moon at least once. To make it as realistic as possible (but 'fly' in reasonable CPU time for that day), I consulted with a math prof and we came up with a Runga-Kutta 'solution' that could be quite accurate.
What is the name of this insect?
Does anyone know the name of this insect, photographed in South Germany? It must be approximately 2 cm long. Thanks a lot... --Edcolins (talk) 18:00, 9 August 2009 (UTC)
-
Unknown insect, first picture.
-
Unknown insect, second picture.
- Nice photo, this site should be able to help you. [19]. 86.4.181.14 (talk) 21:32, 9 August 2009 (UTC)
- Could it be a firefly/lightning bug? Firefly is found here. Bus stop (talk) 21:55, 9 August 2009 (UTC)
- His name is Friedrich, and he would be happy to autograph your pictures. B00P (talk) 00:41, 10 August 2009 (UTC)
- Actually it's his brother Willem, he parts his antennae the other way. 86.4.181.14 (talk) 06:16, 10 August 2009 (UTC)
- Thanks for your answers... Could it be a female stictoleptura rubra (stictoleptura rubra) maybe? --Edcolins (talk) 18:42, 10 August 2009 (UTC)
What the heck is going on in this video?
http://strangepaths.com/canon-1-a-2/2009/01/18/en/
--12.48.220.130 (talk) 18:35, 9 August 2009 (UTC)
- Nothing much. The score sounds musical played from left to right as well as from right to left, and still sounds musical when both are played together. The mobius strip in the video is just a visualisation and (doesn't seem) to have anything particularily to do with it - the music could have been written on one side of a normal ring of paper and it still would have worked.
- Was there something else mysterious.?83.100.250.79 (talk) 19:01, 9 August 2009 (UTC)
- After edit conflict - The musical score is unfolded into a Moebius strip, which has unique mathematical properties. I'm pretty sure this is only a loose analogy to the musical score, though - notice that the note-bobs lose synchronization with the audio while traversing the score in Moebius strip format. Nimur (talk) 19:03, 9 August 2009 (UTC)
- @83.100.250.79: It would NOT have worked, as the staff is "turned upside down" for the part of the table canon. --Cookatoo.ergo.ZooM (talk) 21:50, 9 August 2009 (UTC)
- I didn't notice that.83.100.250.79 (talk) 22:57, 9 August 2009 (UTC)
- @83.100.250.79: It would NOT have worked, as the staff is "turned upside down" for the part of the table canon. --Cookatoo.ergo.ZooM (talk) 21:50, 9 August 2009 (UTC)
- Yes, it does work. You need to have the staff be upside down on the “back” side before doing the twist, in order to have it be right side up after the twist. To check that it works, try it by writing a line of text on a thin strip of paper, and turning it into a Moebius strip by following the video (I did). The sync is a little off when the Moebius strip is playing, in that the music starts about one whole note later than it should, but other than that, it’s accurate. If the sync looks way off for part of it, it’s because the music being played at that point is on the “back” of the strip at that point, not the “front” part that you can see. Notice that the “cursors” are solid on the side of the paper that’s being played at the moment, and have a gap on the “back” side of the paper, that isn’t being played. When following the cursors, it also helps to remember that the balls on the cursors are on the “up” side of the staff, when the cursor is viewed from the side of the paper that’s being played. Red Act (talk) 01:11, 10 August 2009 (UTC)
- There's no good reason to put it on a Moebius strip or any sort of loop at all, since the piece doesn't loop. They play it through once in both directions at once and when they get to ends they stop. What makes the topology of a Moebuis strip or a regular loop interesting is what happens at the ends or when we go around in a full circle, but nothing like that is relevant here. Rckrone (talk) 01:36, 10 August 2009 (UTC)
Changes in DNA
Is an acquired mutation in the DNA still being instructed to create the mutated cells still controled at the mRNA level, telling the body that even though you were not born with certain gene mutation but it may have developed because of environment or lifestyle are those mutated cells controlled by the RNA still? —Preceding unsigned comment added by 71.155.212.203 (talk) 20:58, 9 August 2009 (UTC)
- I can't make head or tail of your question. Why don't you try elaborating it a little bit more? Please do not refrain from using proper punctuation. Dauto (talk) 21:14, 9 August 2009 (UTC)
If there is a mutation in the DNA that someone is not born with, for example cancer cells that is not inherited rather is a result from environmental factors, are those cells still directed by the RNA telling or continuing to give the bad/mutated genes or cells and continue to make the mutated genes/cells (it doesn't come from few bad cell that breaks away to duplicate other cells)?
- You still aren't making such sense. For example, what does "give the bad/mutated genes or cells" mean? However, the DNA replication process isn't likely to be affected by small mutations - the RNA, DNA polymerase, etc. will all do what they usually do. --Tango (talk) 22:19, 9 August 2009 (UTC)
Your question appears to violate universal grammar. I'll try to make some sense of it though. Cells with acquired mutations can purge them with DNA repair enzymes; I imagine DNA repair enzymes are located in protected regions of the genome in such a way that for those loci to acquire mutations would require some pretty massive damage (such massive damage would trigger apoptosis and kill the cell). However cancer cells probably arise where mutations knock out DNA repair enzymes, cell cycle control genes, etc. through the generations and it's done gradually enough that the cell doesn't go into apoptotic shock.
Are you asking whether damage to RNA post-transcription factors could lead to mutations? John Riemann Soong (talk) 13:36, 10 August 2009 (UTC)
- This is my best guess at the parsing of the OPs two paragraphs:
- "Is mutated DNA (which is still being instructed to create mutated cells) still controled at the mRNA level? Is mutated DNA still controlled by RNA?"
- "If there is a new mutation in the DNA, for example in cancer cells, are those cells still directed by the RNA to make mutated genes or cells? Or do these new genes or cells come from a few bad cells that break away to duplicate other cells?" 89.240.199.45 (talk) 13:57, 11 August 2009 (UTC)
Attachment for cooling cars pre-ac?
I was at a classic car show and saw an attachment on the window of a Nash Rambler. The owner said it was for cooling the passenger compartment when traversing the desert in the Southwest US. Drivers would rent the device at one end, which would be filled with ice and attached to the window. At the other end of the journey, the device was then refilled with ice and rented to another driver going the opposite direction. What is the actual name of this attachment, and where can I find more details? DarkAudit (talk) 22:59, 9 August 2009 (UTC)
- Car cooler. -96.255.161.148 (talk) 01:48, 10 August 2009 (UTC)
- That's it. Thanks!. DarkAudit (talk) 01:57, 10 August 2009 (UTC)
- I love this place!!!!! Especially the comedy double acts!!!!! 06:14, 10 August 2009 (UTC) —Preceding unsigned comment added by 86.4.181.14 (talk)
August 10
Alice
Arg, this is driving me crazy. In Australia, isn't there some animal, or something to do with animals, that the term "alice" is applied to? Any help is appreciated. Thx. --207.206.137.204 (talk) 01:47, 10 August 2009 (UTC)
- I'm not from Australia, but googling turned up a lot of Australian slang sites that all related "Alice" to Alice Springs, Northern Territory. There was also Alice the Pig. I wish I could have found more for you. I'm sure some of our Australian editors will be by shortly to assist. 152.16.59.102 (talk) 02:13, 10 August 2009 (UTC)
- I've got that too , I'm fairly certain it's a nickname for the spiny euchidna (they call them alices), curiously there is absolutely nothing to prove this. Did you know that the platypus is austrlias only poisonous mammal?83.100.250.79 (talk) 02:23, 10 August 2009 (UTC)
- @83.100: Considering how rare venomous mammals are, that's not much of a suprise, is it? --Scray (talk) 03:46, 10 August 2009 (UTC)
- Subtle recommendation to use the term venomous by Scray noted -- "poisonous" refers to organisms that affect their predator upon consumption (or heterotroph, more specifically), whereas "venomous" more precisely refers to those organisms that envenomate other organisms without the need for consumption. I don't think the spur is a "last-licks" on the part of the male platypus to "get" the guy that eats him. DRosenbach (Talk | Contribs) 14:14, 10 August 2009 (UTC)
- @83.100: Considering how rare venomous mammals are, that's not much of a suprise, is it? --Scray (talk) 03:46, 10 August 2009 (UTC)
- I'm Australian and I've never heard of Alice as a nickname for an animal. It is definitely used to refer to Alice Springs and perhaps sometimes as a nickname for a place like it, a dry remote town, of which there are a lot of in the outback. Vespine (talk) 03:24, 10 August 2009 (UTC)
You also have lice in Australia but you don't say "a lice" you would say a louse. Graeme Bartlett (talk) 21:48, 10 August 2009 (UTC)
- You could however say all lice which would sound the same. Googlemeister (talk) 13:17, 11 August 2009 (UTC)
- There is a koala called Alice in something called Animal Crossing.She is named for Alice Springs, about the only thing abbreviated to "Alice" here. - KoolerStill (talk) 20:17, 11 August 2009 (UTC)
Total Perspective Vortex IRL
Has an effect comparable to that of the Total Perspective Vortex from The Restaurant at the End of the Universe been observed e.g. in astronomy students? If so, how is it treated? NeonMerlin 02:52, 10 August 2009 (UTC)
- Well there certainly haven't been any proven cases of "brain annihilation!" All facetiousness and humor aside, the controversial concept of Depressive realism is probably the closest concept. As for Astronomy students, they are probably some of the most grounded people, if only because they tend to form tigh-knit groups since they are often so few. Besides, our understanding of astronomy isn't nearly as wide-reaching as everything. ;) ~ Amory (user • talk • contribs) 03:09, 10 August 2009 (UTC)
Evolution and Paganism
I've heard that the theory of evolution has pagan origins in Babylon, 2000 years before Christ. Is that true?
Bowei Huang (talk) 04:04, 10 August 2009 (UTC)
- No. No, it is not. Algebraist 04:15, 10 August 2009 (UTC)
- And even if it were, scientific theories are to be judged on the veracity or falsehood of their predictions, not on their origins. —Preceding unsigned comment added by 81.11.170.162 (talk) 06:03, 10 August 2009 (UTC)
It wouldn't surprise me if that was true, to some limited extent - coming up with the concept of animals adapting over time to fit their surroundings is not an immense leap of intellect. Some pagans quite easily could have seen, say, a green insect living in grasslands and a similar-looking but brown insect in a nearby forest. From there it's not too hard to suggest that perhaps those two animals "evolved" to fit their surroundings. However, having said that it's far from being the "origin" of the theory of evolution - for one, there are very limited records from that far in the past, so the chances of any reasonable scientific thought surviving to be verified in the present day are very slim indeed. So yes - ideas that were perhaps related to evolution quite possibly were thought of that long ago, but it's unlikely that any evidence remains, and even if so it's unlikely if any of it was used in the formulation of the modern theory. ~ mazca talk 07:58, 10 August 2009 (UTC)
- It is possibe that there were early theories of evolution, just as there were early theories of cosmology and early atomic theories. However, today's theories in these fields do not "have their origins" in the earlier theories. Today's theories in all three fields originated independently of the earlier theories. This is not true for all scientific theories: for instance, medicine, agronomy, and chemistry (and their theories) can be traced more or less continuously back to antiquity, even though the modern theories in these fields bear little relationship to those in antiquity. -Arch dude (talk) 12:11, 10 August 2009 (UTC)
- I would have thought the the notion of breeding to develop characteristics (e.g. domesticating dogs etc) are likely to be old enough for this to be plausibly dating 2000BC. However on "pagan origins in Babylon, 2000 years before Christ" I think you may have misheard someone talking about the Biblical creation narratives which certainly derive in parts (order from chaos etc) from their Babylonian predecessors (or so we are taught in school). --BozMo talk 12:25, 10 August 2009 (UTC)
- I think the OP is really asking whether the hypothesis of evolution was around back then - and I think it's extremely likely that it was. However, turning a hypothesis into a theory (in the scientific meaning of the term) is a more formal process - and that was certainly not done back in those early times. Merely having an idea that fortuitously turns out to be right doesn't qualify you as the inventor of a theory. It's only when observations, experiments and careful testing are performed and can exclude other possible causes that a hypothesis can be labelled as a theory in the scientific meaning of that word. There is no evidence that I could find that the Babylonians did that (and it's very unlikely that they would - the 'scientific method' is really only a couple of hundred years old). SteveBaker (talk) 12:51, 10 August 2009 (UTC)
- People have understood the practical implications of evolution for thousands of years, even if they didn't have an understanding of molecular genetics. Basic concepts of breeding have existed for 10,000 years. People have long understood that one can control which traits are passed on through offspring to create slow changes in a population. There is no fundemental difference between traits that are encouraged by deliberate human intervention versus traits that are encouraged by adaptation to a changing environment. The only significant barrier to the modern theory of evolution is only the time scales involved. Once you detatch yourself for the need for the earth to only be 6000 years old, and are willing to accept an earth that is billions of years old, then the rest of it falls into place pretty easily. --Jayron32 18:20, 10 August 2009 (UTC)
Yet I don't think anyone before the 19th ct. made that connection. The principles of selection-by-man were not extended to create the concept of selection-by-nature. Answering the original question - no, the scientific theory of natural selection is not known to have been inspired, directly or indirectly, by any non-Christian beliefs. On the other hand, there are many cases of stories about species developing from each other through some kind of mystical force or magic, or through the will of a deity (thus, not through evolution in the modern scientific sense of the word). One commonly occurring belief is that some or all animals are descended from humans who were transformed into animals for one reason or another (sometimes as punishment for some transgression, but in other cases they are regarded as ancestors worthy of veneration). The opposite direction of development is less common, but not unheard of. I think I recall that in the creation myth of a certain Australian aboriginal tribe, fish miraculously turned into lizards, lizards turned into some kind of mammals and the mammals turned into a humans. I can't find the reference though, and I can't find any mention of this elsewhere, so I might be wrong. I do, however, find sources saying that in a couple of tribes in Tibet and in South Africa, humans were said to originate from apes or monkeys. It's a nice illustration of the difference between religion and science - the claim can happen to be the same, the difference is in the explanations and the methods to reach it.--91.148.159.4 (talk) 21:38, 10 August 2009 (UTC)
Bowie, if you're interested for a careful, interesting historical account about the development of evolutionary theory—which has its origins long before Darwin, and indeed has interesting connections to ideas about society, morality, and religion!—check out the two-volume biography of Darwin by Janet Browne. It does not strive to convince you of anything you don't want to believe (you can keep the wool in your ears if you'd like), but it will give you a much deeper understanding of where these ideas came from. The truth of it is far more interesting (and potentially more scandalous!) than anything your conspiracy theories will have up their sleeves. --98.217.14.211 (talk) 00:10, 11 August 2009 (UTC)
- It's fairly well established that the "ancient" Greek astronomers learned much from the (far more ancient) Babylonians about astronomy. So the ancient Babylonians were certainly trying out their powers of observation to help them make predictions, not just consulting sheep intestines.
In a long lifetime of science reading, I've never heard anyone make the remarkable claim that the Babylonians invented a theory of evolution -- but I can imagine such a speculative hypothesis arising in modern times, because I heard people like H.W. Armstrong baying at the moon on AM radio. Twang (talk) 06:39, 11 August 2009 (UTC)
Relativistic Doppler effect-not theta_s?
I am trying to derive the formula
in Relativistic Doppler effect#Motion in an arbitrary direction. I used an equivalent of Lorentz transformation to transform the light cone for an observer, A, to the shape in a coordinate system for another observer, B. A point at the edge of the light cone for A in the coordinate system A, with an angle around t-axis in the x-y plane, starting from x-axis, , is
times a distance from origin after transformation. (In Lorentz transformation and Relativistic Doppler effect, directions of v seems opposite, and this is in Relativistic Doppler effect way)
I think this is the rate of change in wavelength. Changing it to frequency,
- .
This is like the one at the beginning, but with instead of . How can I derive the correct one? Like sushi (talk) 05:03, 10 August 2009 (UTC)
- That formula can be obtained by combining the effect due to the movement of the source away from the observer (that's the same effect that gives you the non-relativistic doppler effect) with an extra gamma factor due to time dilation. The first effect gives you a factor
- and the second effect gives the factor
- It is as simple as that. Dauto (talk) 05:43, 10 August 2009 (UTC)
- Alternatively, you can use the fact that and form a 4-vector in the observer's reference frame and apply a Lorentz transformation to get in the sources reference frame. Choosing the x-axis along the direction of the source motion, we have
- But we also have , where the minus sign is due to the fact that the source is assumed to be moving away from the observed while the light ray must be moving towards the observer (otherwise it would never be detected). Putting all that together with the simple formula we get
which yields the desired formula. Dauto (talk) 06:16, 10 August 2009 (UTC)
- Thank you for quick answer. But why is it not but ? I do not understand the alternative explanation. And I hope you will answer in a way that guides me from what I supposed in the first post.
- Like sushi (talk) 06:31, 10 August 2009 (UTC)
- The angle comes about when you take the component of the source velocity along the line connecting source and observer. That velocity is measured from the point of view of the observer (from the source's point of view it is not moving at all, obviously) which means that is the correct angle. Dauto (talk) 06:52, 10 August 2009 (UTC)
- Here's a nice simple way to derive the redshift without coordinates. At the end I'll introduce coordinates and get back the usual formula. I'll use "1+z" for the ratio of received wavelength to transmitted wavelength because it's just about the only standardized notation for redshift out there. Ignore the added 1 and just think of "1+z" as a single symbol.
- Let xs and vs be the sender's spacetime location and four-velocity at the time of sending and let xr and vr be the receiver's location and velocity at the time of receiving. Let X = xr − xs be the separation between them. Note X is a lightlike vector because xs and xr are connected by a light signal.
- The sender waits an interval dτ, measured on her wristwatch, and then sends a second signal (or a second wavefront of the same light beam, if you prefer). The receiver receives this after an interval (1+z)dτ, measured on his wristwatch. (This is the definition of the redshift 1+z.) These second sending and receiving events also have to be separated by a lightlike vector—that is,
- It's now just a matter of solving this for 1+z. Multiply out the square to get
- The first term disappears because X is lightlike and the third term is negligible because we're assuming dτ is small. Dividing what's left by 2 dτ and rearranging we get
- which is the coordinate-free redshift formula. I don't know why anyone would want to uglify it by introducing coordinates, but here goes. Let X = (Δt, Δx) and vs = (γs, γsvs) and vr = (γr, γrvr). Then we have
- Now Δx/Δt is a vector with magnitude 1 (= c), so (Δx/Δt)·v gives the component of v along the path of the light, which I'll call v||. Thus
- which I'll grant you is also pretty easy to remember, though you have to be careful about the signs. As I've defined it, v|| is positive for motion in the same direction as the light. Usually people pick coordinates where the receiver is at rest, in which case . Then they introduce an angle θ instead of writing v|| and you have to remember how the angle was defined and whether it's a sine or a cosine and whether it's added or subtracted along with everything else and it just becomes more and more confusing, so I'm going to stop here. Incidentally, the coordinate-free formula works in general relativity also if you suitably interpret X (it can't be a vector anymore because the light signal crosses a large region of spacetime where curvature can't be neglected). You can derive the gravitational redshift and cosmological redshift formulas from it by choosing different coordinates and background geometries. -- BenRG (talk) 10:44, 11 August 2009 (UTC)
Ring Singularities
In a rotating black hole there is supposed to be a ring singularity, and if you fall through it you go to a negative universe(universe with gravity repulsive). Is there space in the middle of a ring singularity? I imagine it to be no, and you fall through the ring singularity to the other side of the spacetime surface, and because the spacetime(or only time) is curved "downwards" from the original universe, the spacetime is curved "upwards" on the other side, so gravity is repulsive. I also imagine that the fields may travel through the spacetime surface so that the only thing different about the negative and the ordinary universes are the gravitational fields.
The Successor of Physics 05:59, 10 August 2009 (UTC)
- I've never heard of this "negative universe". I think you just end up in a new universe with the same laws of physics (in fact, if it is possible to travel between the two it would be reasonable to consider then parts of the same universe). I think you would come out of a white hole, but they aren't repulsive, they actually look identical to black holes to an outside observer (this is very counter-intuitive, but it is what the maths says). --Tango (talk) 11:43, 10 August 2009 (UTC)
- There is a region of repulsive gravity through the ring singularity in the Kerr-Newman metric. That's not a "universe with gravity repulsive", though, it's just the equivalent of a negative-mass object in our universe. If you brought two ordinary objects through the ring singularity they would still attract each other. Probably that region of the solution doesn't have any physical significance. Don't make the mistake of thinking that attractive gravity is "downwards" curvature and repulsive gravity is "upwards". The curvature is intrinsic—there's no up and down. The difference between attractive and repulsive gravity is more like the difference between elliptical and hyperbolic space. You might be thinking of the "gravity well" or "rubber sheet" picture of Newtonian gravity, where a valley is attractive and a hill is repulsive. A lot of books confuse that with spacetime curvature. -- BenRG (talk) 20:54, 10 August 2009 (UTC)
- On a tangent, it's kind of funny when people talk about or movies and tv shows show things going "through" a black hole into a new universe or back in time, etc. Fraser Cain on the Astronomy Cast podcast had a good line about this. He said it's like a frog looking up at a blender and wondering "If I jump inside the blender will I go to a new universe? Or looking at a trash compactor and wondering if you can go to a new dimension. Unfortunately, if you go into a black hole, you just end up being blended and compacted into the stuff that's in the singularity itself (whatever form of stuff that is). 63.95.36.13 (talk) 22:00, 10 August 2009 (UTC)
- An idealised black hole is a connection to "another universe", although it is impossible to actually travel between them, even if you could avoid being ripped to pieces, the connection doesn't exist long enough for even light to traverse it. However, even that doesn't matter because real black holes aren't ideal, when a black hole forms from the collapse of a star (rather than existing forever) the connection never exists in the first place. It is best depicted using a Penrose diagram like this one. Roughly speaking space is horizontal and time is vertical with up being forward in time - only one dimension of space is shown, the other two are the same, though. Travelling at the speed of light corresponds to travelling along a 45 degree line, travelling slower than light means being closer to vertical than that, that is why you can't leave the black hole once you are in it, you would have to move along a line more than 45 degrees off vertical, which means travelling faster than light. The connection between the two universes is a single point, you can't get through it without ending up in the black hole. (Ignore the electrically charged/rotating version - that's just weird! That is where the ring shaped singularities come into it, though.) --Tango (talk) 00:20, 11 August 2009 (UTC)
- Thanks!
The Successor of Physics 04:36, 11 August 2009 (UTC)
First major sport to get the Deep Blue treatment?
Interesting line of discussion, I think...
Which major sport will be the first to get the Deep Blue treatment -- i.e., someone creates a robot that can match (if not exceed) the best human athlete?
Now certain sports like target shooting could almost certainly already be "solved" if anyone bothered, although sporting clays would present some difficulty!
But restricting it to major sports, which do you think would be easiest to beat? Which would be the last to go down?
Personally, I think golf will be the first to fall because it lacks the collisions and speed of other sports. The range-finding and condition assessment would seem perfect for a computer.
As for the last sport to fall? I think it would probably be basketball - satisfying the dribbling requirements and keeping track of the ball in such a confined, cramped space would present all sorts of difficulties... and that'd be after you solved all the basic mobility challenges...
What says the mighty Science Desk?
218.25.32.210 (talk) 07:54, 10 August 2009 (UTC)
- I think that this is beyond our wisdom (no offence, anyone), but first, do you mean with robots performing the physical side of things? If not, what do you propose instead? Human bodies controlled by computer? And do other board games like Go count as "sports"? If so, I'd guess we'll crack them all first. Of course, by "board game" I mean "discrete" game, in which the set of possible states is finite. Even relatively simple games like pool are principally continuous, with an infinite number of states, though one could probably discretize the table into squares at minimal loss of performance. --Leon (talk) 08:56, 10 August 2009 (UTC)
- Beyond whose wisdom? Here's a video of my classmates' robot playing Shuffleboard. Clearly, computers no longer need to stick to nerdy games like "chess" and "Go" - soon robots will be lining up on cruise ship decks and hogging all the shuffleboards with their precise, calibrated technique! Nimur (talk) 16:12, 10 August 2009 (UTC)
- I thought I explained it well enough, but I guess not. Fully autonomous robots competing shoulder to shoulder against humans. Go, however much its proponents made enjoy trumpeting its complexity, is quite clearly not a "major sport" - let's keep it on topic! 218.25.32.210 (talk) 09:23, 10 August 2009 (UTC)
- In that case, I haven't a clue. And I'm not convinced that the question is very clear. For instance, are the robots allowed some sort of "telepathy", meaning invisible radio contact between them? The rules of sports don't specify that humans can't be telepathic, to my knowledge! Are we placing limits on size (mass and volume) of these machines? If not, couldn't quite simple robots pummel humans with ease, in sports like boxing? Are robots allowed wheels, or must they have legs, like humans? With wheels, I'd imagine we could engineer some very fast robots. --Leon (talk) 09:32, 10 August 2009 (UTC)
- What are you defining as a major sport? You've already ruled out target shooting, which does seem an obvious choice.
- The men's world record for discus is only 74.08 m, whereas clay pigeon traps can shoot up to 100m, according to our article. Hammer throw would also be a relatively easy problem to solve.
- I understand getting humanoid robots to balance is a difficult problem, which suggests sports involving lots of balancing and moving around would be difficult (e.g. basketball as suggested, gymnastics). Swimming might be easier, with the possibility of very hydrodynamic designs. AlmostReadytoFly (talk) 09:55, 10 August 2009 (UTC)
- In that case, I haven't a clue. And I'm not convinced that the question is very clear. For instance, are the robots allowed some sort of "telepathy", meaning invisible radio contact between them? The rules of sports don't specify that humans can't be telepathic, to my knowledge! Are we placing limits on size (mass and volume) of these machines? If not, couldn't quite simple robots pummel humans with ease, in sports like boxing? Are robots allowed wheels, or must they have legs, like humans? With wheels, I'd imagine we could engineer some very fast robots. --Leon (talk) 09:32, 10 August 2009 (UTC)
- Against topic drift, the gods themselves contend in vain. (Redlink?!) —Tamfang (talk) 04:51, 11 August 2009 (UTC)
OP back, now at home hence the different IP. Here, I'll throw out some guidelines for the sake of discussion.
Rule 1 Robot must have a body similar to a human - 2 legs, 2 arms, walk upright, similar articulation (elbows, knees, ankles), sensors in the "head", dimensions equivalent with professional athletes of the same sport -- weight is unrestricted in non-contact sports (golf, tennis, swimming), but must also be equivalent in contact sports (football, rugby, sumo)
Rule 2 Robots may not communicate with teammates in any fashion that a human could not - so no infrared, no wi-fi - only auditory / visual / gesture based communication
Rule 3 Robots are autonomous during competition, not remotely controlled.
Off the top of my head, let's confine it to the following sports: swimming, track & non-throwing field (pole vault, high jump, etc), wrestling, sumo, American football, world football, golf, tennis, badminton, basketball, baseball, ice hockey, volleyball, and cycling.
I think cycling is particularly interesting - the energy efficiency advantages would be offset as it'd have to balance itself on a bicycle and rely on cameras for depth perception and road surface interpretation... 213.146.164.143 (talk) 12:05, 10 August 2009 (UTC)
- My kid used to have a radio-controlled toy motorcycle. It balances very easily without any clever software, cameras or anything else. Once a bike is moving fast enough, the balance is rather automatic. SteveBaker (talk) 14:22, 10 August 2009 (UTC)
- I don't know what qualifies you to set up the rules. You asked a question - the answer doesn't necessarily conform to your guidelines! Actually, your 'rules' are incredibly stupid...why should (say) a robot soccer player have to look and speak like a human? We didn't require Deep Blue to use neurons to think with - why should a robot have to have things like arms and legs and communicate with it's teammates using crappy audio links? Do you require 'eyes' to be made with a gelatinous lens projecting the image on to a curved interior surface - instead of just using off-the-shelf cameras? Do we have to build our audio detectors using fluid-filled chambers with little hairs protruding into them instead of microphones? Drawing the line where you drew it is an entirely arbitary decision - and one that completely precludes us from researching a proper answer. What you are asking is really "When will the first fully humanoid robot be built?" and that's not at all the same question - and because the definition of 'fully humanoid' is an arbitary one too - we're not going to be able to answer that one if you nail down the preconditions so tightly. It's like asking "When will the first robot appear that's painted pink with little blue stripes?"...the answer can only be "Nobody knows until it happens". SteveBaker (talk) 12:39, 10 August 2009 (UTC)
- Ping pong was the first physical sport to be attacked robotically...without a doubt. A guy called Russell Anderson built a fairly successful ping pong playing robot back in 1986 - he even wrote a book about it: [20]. There were robot ping-pong contests held all over the place back in the 1980's - research into the subject more or less fizzled out once it was realised that the problem was pretty much solved. There is currently quite a bit of interest in robot soccer - and (to 'level the playing field') "segway soccer" between humans and robots. Someone mentioned shooting - there are several robotic paintball guns out there that can out-shoot a human with ease...especially on moving targets...switching out the painball gun for a 'real' gun would be a little dangerous - and doesn't really prove anything. Robotic gliders have beaten the human glider endurance record. It would be completely trivial to build a robot that could beat a human on any track running event...in fact anything like the shot putt, discus, javalin, etc should be entirely trivial to win robotically. In case you insist that the robots use legs for running...let me point out that I can build a robot to beat people in the wheelchair track events at the para-olympics when legs are specifically not allowed in the rules! SteveBaker (talk) 12:39, 10 August 2009 (UTC)
- Which glider record were you thinking of, Steve. This article
claims a team is still trying to reach a 142-mile robotic flight while the human record seem to be 2200 km by Steve Fossett. 75.41.110.200 (talk) 14:52, 10 August 2009 (UTC)
- You might like Robot competition. Personally my favourite is the dance competition. How long till the judges say robots are more artistic, have better costumes, express the music than humans? Next they'll be competing in another athletic sport as in A.I. Artificial Intelligence... Dmcq (talk) 14:02, 10 August 2009 (UTC)
- I would imagine that you could design a robotic pitcher for baseball where one of it's arms is essentially a cannon capable of shooting the ball at a speed too high for a human player to hit, say 300 mph. That would far surpass any human achievements in that part of the sport. Even if he strikes out every time he bats, it would still be a major net gain for the team, so you wouldn't even have to program that part. 65.121.141.34 (talk) 16:12, 10 August 2009 (UTC)
- I suppose Pitch-o-mat 5000 was just a modified howitzer? Nimur (talk) 21:13, 10 August 2009 (UTC)
- I would imagine that you could design a robotic pitcher for baseball where one of it's arms is essentially a cannon capable of shooting the ball at a speed too high for a human player to hit, say 300 mph. That would far surpass any human achievements in that part of the sport. Even if he strikes out every time he bats, it would still be a major net gain for the team, so you wouldn't even have to program that part. 65.121.141.34 (talk) 16:12, 10 August 2009 (UTC)
'Bacteriocidality' of penicillin
"β-lactam antibiotics work by inhibiting the formation of peptidoglycan cross-links in the bacterial cell wall. The β-lactam moiety (functional group) of penicillin binds to the enzyme (DD-transpeptidase) that links the peptidoglycan molecules in bacteria, which weakens the cell wall of the bacterium (in other words, the antibiotic causes cytolysis or death due to osmotic pressure). In addition, the build-up of peptidoglycan precursors triggers the activation of bacterial cell wall hydrolases and autolysins, which further digest the bacteria's existing peptidoglycan.
Are these cross-links continually constructed during the lifecycle of the bacteria -- otherwise, penicillin should be merely bacteriostatic? Or is the secondary effect the major (I assume it's not, as it's secondary). DRosenbach (Talk | Contribs) 14:04, 10 August 2009 (UTC)
- The cross-linking doesn't have to be continuous - it just has to be necessary for both daughter cells on division. If both daughters die on division then any active colony will collapse, and therefore penicillin would be bacteriocidal (This bit is just a thought experiment - read on for a real answer). If I remember my microbiology lectures correctly, during growth the cell wall is actually continually synthesized "on the inside" (closest to the inner membrane) and broken down by autolysins on the outside. This is to allow for expansion (the newly synthesized cell wall can stretch as it is not yet cross-linked). As, at least under ideal lab conditions, bacteria are continually growing then yes, the cross links are pretty much continually constructed during the lifecycle. 84.12.138.49 (talk) 22:50, 10 August 2009 (UTC)
Tornandos and Hurricanes in US
I didn't hear of a lot of press about a lot of tornados out in the Midwest. Was this a milder year than average? Does that have anything to do with the milder temperatures we had out here on the East Coast? Will that affect how many hurricanes we can expect out here this season? --Reticuli88 (talk) 15:45, 10 August 2009 (UTC)
- For the latter two questions, our understanding of weather is still primitive enough that we can't draw any firm conclusions about wildly disparate weather trends being linked. For example, our tornado article notes a correlation with the Southern Oscillation (El Nino), which is also cited as a rationale for this year's lower-activity hurricane forecast. However, we note that the tornado-SO correlation is weak and moreover ties to winter tornadoes -- which have not yet occurred. Finally, even if borne out, the research doesn't create a causative tie between tornadoes and hurricanes; they would be merely correlated at best. — Lomn 16:12, 10 August 2009 (UTC)
- Going back to the former, our article on tornadoes of 2009 cites 1053 US tornadoes as of 31 July; our tornado climatology article notes that the US reports about 1200 tornadoes annually. I don't find this year to be an outlier in terms of low tornadic activity. — Lomn 16:15, 10 August 2009 (UTC)
- (ec) Generally, the cause of hurricanes in the United States is elevated water temperatures in the eastern Atlantic ocean, spurning west-bound low pressure systems; while the cause of tornados are large pressure variations in the air masses that originated over the western part of North America and are moving eastbound. If these two effects are correlated, or causally linked, I am not aware of any research indicating so. As Lomn pointed out, we don't really have a good model of how global weather patterns interplay - so it's hard to say for sure. Nimur (talk) 16:17, 10 August 2009 (UTC)
- Pedantic nit-pick: I assume you really meant spawning, not spurning? 87.81.230.195 (talk) 00:01, 11 August 2009 (UTC)
- That we haven't heard much about tornadoes this year is mainly a matter of luck; the worst tornadoes have been staying away from population centers, so the death toll has been much lower. While this May was unusually quiet as far as tornadoes go, this summer has been unusually active in the Northeastern United States.
- The formative ingredients for tornadoes and hurricanes are so different, it is really impossible to discuss any correlation between the two with our current understanding of meteorology/climatology. Landfalling hurricanes do tend to produce tornadoes in their outer rainbands, but whether or not a tropical cyclone hits land is also just a matter of luck.-RunningOnBrains(talk) 17:00, 10 August 2009 (UTC)
- (ec) Generally, the cause of hurricanes in the United States is elevated water temperatures in the eastern Atlantic ocean, spurning west-bound low pressure systems; while the cause of tornados are large pressure variations in the air masses that originated over the western part of North America and are moving eastbound. If these two effects are correlated, or causally linked, I am not aware of any research indicating so. As Lomn pointed out, we don't really have a good model of how global weather patterns interplay - so it's hard to say for sure. Nimur (talk) 16:17, 10 August 2009 (UTC)
- Going back to the former, our article on tornadoes of 2009 cites 1053 US tornadoes as of 31 July; our tornado climatology article notes that the US reports about 1200 tornadoes annually. I don't find this year to be an outlier in terms of low tornadic activity. — Lomn 16:15, 10 August 2009 (UTC)
Neodymium Chloride
Hello. Does neodymium chloride (NdCl) exist? Thanks in advance. --Mayfare (talk) 17:48, 10 August 2009 (UTC)
I don't see why not -- its oxidation states probably means it would form analogues to iron chloride. John Riemann Soong (talk) 17:55, 10 August 2009 (UTC)
- Like this maybe Neodymium(III) chloride .83.100.250.79 (talk) 18:09, 10 August 2009 (UTC)
- @ [21] ~£1/g 83.100.250.79 (talk) 18:13, 10 August 2009 (UTC)
- Indeed, similarly to iron, neodymium tends to have a +2 or +3 oxidation state. That means one would expect to have chloride salts NdCl2 and NdCl3 but probably not NdCl. I don't see an article for any ones for any other truly ionic salts (except for the previously-noted Neodymium(III) chloride) or any +2 valence compounds in Category:Neodymium compounds. DMacks (talk) 18:15, 10 August 2009 (UTC)
- (ec)Yes, I think it does. Those, by the way, are the first four hits on Google. ~ Amory (user • talk • contribs) 18:21, 10 August 2009 (UTC)
- There's probably a 1:1 ratio NdCl, but that's a different story, and not the common chloride that you can get in a tin, the orginal question ask-er would have to ask about it if they specifically wanted to know. (feel free)83.100.250.79 (talk) 19:39, 10 August 2009 (UTC)
- There is probably not a stable NdCl monochloride. It does not get a mention in my copy of Advanced Inorganic Chemistry. To get this you may have to look in the gas phase. Graeme Bartlett (talk) 08:49, 11 August 2009 (UTC)
- There's probably a 1:1 ratio NdCl, but that's a different story, and not the common chloride that you can get in a tin, the orginal question ask-er would have to ask about it if they specifically wanted to know. (feel free)83.100.250.79 (talk) 19:39, 10 August 2009 (UTC)
- (ec)Yes, I think it does. Those, by the way, are the first four hits on Google. ~ Amory (user • talk • contribs) 18:21, 10 August 2009 (UTC)
The CRC handbook's table Physical Constants of Inorganic Compounds lists the following:
- NdCl2, CAS # 25469-93-6, green hygroscopic solid, melting point 841 °C
- NdCl3, CAS # 10024-93-8, violet hexagonal crystals, melting point 759 °C, boiling point 1600 °C, density 4.13 g cm−3
- NdCl3·6H2O, CAS # 13477-89-9, purple crystals, decomposes at 124 °C, density 2.3 g cm−3
A search of crystallography databases also lists NdCl2.31 and Nd14Cl33.
Ben (talk) 09:29, 11 August 2009 (UTC)
spirit fire
What proof would an alcoholic beverage need to be before you can light it on fire with a match? would 80 proof be sufficient? I want something exciting for a party. Googlemeister (talk) 18:31, 10 August 2009 (UTC)
- Definitely. Most spirits are around 80 proof and they all light no problem. Be careful, though - flames from burning alcohol can be almost invisible unless the room is quite dark and they can leave the container dangerously hot after the flames are out. --Tango (talk) 18:38, 10 August 2009 (UTC)
- You might find Alcoholic proof interesting. Edison (talk) 18:55, 10 August 2009 (UTC)
- I'd suggest using Bacardi 151 - afaik, it's sort of the standard for flaming shots and the like. It's
shittycheap enough and burns better than the 80%proof stuff. ~ Amory (user • talk • contribs) 19:01, 10 August 2009 (UTC)- I'd avoid that; it burns too well. And for god's sake don't confuse percentage with proof like that. Please see alcoholic proof. Matt Deres (talk) 19:13, 10 August 2009 (UTC)
- Oops, thanks! ~ Amory (user • talk • contribs) 19:55, 10 August 2009 (UTC)
- Bacardi-151 is good, but 190-proof Everclear is better. Alas, it's illegal in too many places now. Twang (talk) 06:48, 11 August 2009 (UTC)
- Oops, thanks! ~ Amory (user • talk • contribs) 19:55, 10 August 2009 (UTC)
- I'd avoid that; it burns too well. And for god's sake don't confuse percentage with proof like that. Please see alcoholic proof. Matt Deres (talk) 19:13, 10 August 2009 (UTC)
- (e/c) Be extremely careful when lighting alcohol. If you're planning on doing a flambé kind of thing, a few quick tips: keep the lid to the vessel in your hand at all times. If the fire gets out of hand, the lid will cut off the oxygen supply. Keep a fire extinguisher within arms reach. And this sounds obvious, but turn off your stove, especially if you're using a gas stove - in fact, take your pan off the heat altogether. Gas plus fire = well, you get the idea. Also, the vapours coming off the alcohol normally ignite first, so expect the flame to start before you'd think you should expect the flame to start. Our article suggests adding cinnamon to the dish; I can't vouch for that myself. Why not try an electric pickle instead? What, no article??? Matt Deres (talk) 19:11, 10 August 2009 (UTC)
- Here's an exciting party trick (I saw it done with some high tequila content mix drink). Get some small shot glasses. Fill it up and light it up. Quickly (before it gets too hot) cover it with your hand's palm extinguishing the fire. As the flame is extinguished it consumes O2 reducing the pressure in the cup enough to make it stick to your palm. Visibly shake your hand up and down mixing your drink Hold back the urge to let people know wheather/that you have slighly burned your hand. Dare other people to do the same. Tease the ones that refuse. Rinse and repeat untill bored or drunk. Make sure you have an bucket with ice "for drinks" near by just in case. Have fun. Dauto (talk) 19:15, 10 August 2009 (UTC)
- Partial-vacuum is also substantially due to cooling of the headspace vapor after the flame is out. Burning does chemically consume the O2, but two thirds of the oxygen gas consumed by burning ethanol winds up as CO2 gas, so not nearly the "gas loss" one might expect. On the other hand, dropping the temperature of a water-vapor/CO2/atmosphere from flame temp to hand temp is a noticeable pressure difference. See also Fire cupping. DMacks (talk) 19:39, 10 August 2009 (UTC)
- I need to reinforce one point for our OP. The light you get from burning alcohol is NOT spectacular. It's almost invisible except in a fairly dark room when there is a faint blue light. To get something impressive there needs to be something besides the alcohol that's being burned. SteveBaker (talk) 12:56, 11 August 2009 (UTC)
Orthodontic braces
I know someone who has braces who is about the mid 20's agewise. Does an adult need braces longer then a teen would to correct the same problem? Googlemeister (talk) 18:37, 10 August 2009 (UTC)
- According to this and this, yes it does, although there are some discrepancies. I am not a dentist (IANAD, that's a new one) but I presume it has to do with the fact that as a teen, one's teeth are still moving into their "final" position and can thus be tampered with more easily. As an adult, the teeth are relatively set, and it's harder to move them without altering all the others. Many still consider it worthwhile because of the long-lasting changes it will likely have. At any rate, if you're considering getting them, you should speak with a dentist to get the full picture. ~ Amory (user • talk • contribs) 18:55, 10 August 2009 (UTC)
- If I'm not mistaken, getting braces also has long-lasting consequences: you'll be wearing a retainer to sleep for the rest of your life if you want your teeth to be straight. I would like to be wrong on this. Vranak (talk) 20:41, 10 August 2009 (UTC)
- Like most answers, it depends. It depends on what needs to be done. There are many types of malocclusions that can warrant the need for braces, and there are issues of minor tooth movement. Even with a full mouth case, there are various levels of severity, that would range from closing some spaces to correcting crossbites and exposing impacted canines. If it's just about closing some spaces, it shouldn't take any longer, but if it's something much more complex, it might take longer than 18 months...but then so might a child who presents with some very complex case. DRosenbach (Talk | Contribs) 21:15, 10 August 2009 (UTC)
- If I'm not mistaken, getting braces also has long-lasting consequences: you'll be wearing a retainer to sleep for the rest of your life if you want your teeth to be straight. I would like to be wrong on this. Vranak (talk) 20:41, 10 August 2009 (UTC)
Questions/suggestions for Fetch (game)
On a whim I was looking at Fetch (game) and noticed the article didn't address some questions I was curious about. (I also posted these question in the article's discussion page, but figured you folks would be more reliable for answers.) First, do all dog breeds like to play fetch instinctively, or are there certain breeds who don't have an inclination for the game without training? It's unlikely that newborn pups play fetch, so at what stage of development do dogs show an interest in the game? And is there a researched evolutionary reason for dogs learning to play fetch (eg maybe dogs have been trained for thousands of years to fetch prey for hunters so the trait is now ingrained)?
And on a related note, what species other than dogs like to play fetch? Will some species of cats or birds play fetch, and if so how much training does it require if any? Is falconry a related activity, for example?
Finally, if anybody has good sources for answers to these questions, I'd recommend appending them to the Fetch (game) article. It would be a nice addition. 63.95.36.13 (talk) 21:52, 10 August 2009 (UTC)
- I know that retrievers are specificially bred to retrieve which is like fetch. I don't know much beyond that. Rckrone (talk) 22:28, 10 August 2009 (UTC)
- I've had an Uncle and Aunt who had a cat that would play fetch, and I don't believe they had tried to teach that particular cat to play fetch. But I would expect this to be a rare behavior in cats. 131.191.87.100 (talk) 23:37, 10 August 2009 (UTC)
- Of the two cats I've had, both have "fetched" toy mice without any training, although as is usually the case with cats they're only letting you play with them, and on their terms. Both these cats eventually figured out that if they chase after the mouse but don't bring it back, the loving human will eventually walk over to where they are and then re-throw the toy. That's not exactly fetch anymore, but they did figure out how to game the system in their favor! ~ Amory (user • talk • contribs) 00:57, 11 August 2009 (UTC)
- I have two Giant Schnauzers who have never in their entire lives even looked after a ball when it was thrown, let alone gone to fetch it. They might fit under your idea of a dog who doesn't have an inclination for the game without training. However, they were both raised in a kennel where there weren't many balls to be fetched, so that may have something to do with it. They are also super couch potatoes...208.65.223.146 (talk) 03:04, 11 August 2009 (UTC)
- Cats'll play with a laser-pen spot for a while too, then lose interest in that. (I don't have any dog data on the subject.)
- Of the two cats I've had, both have "fetched" toy mice without any training, although as is usually the case with cats they're only letting you play with them, and on their terms. Both these cats eventually figured out that if they chase after the mouse but don't bring it back, the loving human will eventually walk over to where they are and then re-throw the toy. That's not exactly fetch anymore, but they did figure out how to game the system in their favor! ~ Amory (user • talk • contribs) 00:57, 11 August 2009 (UTC)
I about 250 million years we will have second Pangaea. But which oneis most likely? Pangaea Ulitma, Amasia or NovoPangaea. What is a novopangaea?--69.229.108.245 (talk) 22:03, 10 August 2009 (UTC)
- According to our Pangaea Ultima article, Novopangaea is just another name for that predicted configuration. As to whether this or Amasia is most likely, I doubt there is any way to tell. 250 million years is a very long way ahead to predict. Mikenorton (talk) 22:14, 10 August 2009 (UTC)
Do South Africa tip (Cape Town) and tip of South America snow every year at basin (not the mountain) but surface level like metro-los Angeles-orange-county type land? Idoubt Cape Town often gets land-level snow altugh it's winter can be bone-chilly. Cape horn is just bad-weather, since it's coastal island, it is unlikely too to have a fixate snow seansons.--69.229.108.245 (talk) 22:21, 10 August 2009 (UTC)
- Cape Town, South Africa, is at 33 degrees south latitude. this the the same distance from the equator as the southern shore of the Mediteranian (straits of Gibraltar, Tangier, etc.) Snow is not likely. By contrast, Cape Horn, at 55.5 degrees south, is much further away from the equator and is famous for nasty winter weather. -Arch dude (talk) 23:02, 10 August 2009 (UTC)
- (After looking as some more pages) Cape town's record low temperatures is -1 degree C, or 30 degrees F. and it's average lows are higher than those of Los Angeles, which is farther from the equator. Cape Horn is considerably farther from the equator than Kiska, in Alaska.
- I'm 28 and in my lifetime we've never had snow at ground level in Cape Town. Cape Town is a coastal city with a Mediterranean climate and quite temperate weather. We regularly get hail a few times during winter yes, but never the slow-falling snow we see on TV! On the other hand we always have snow in the higher lying areas such as the upper slopes of Table Mountain and towns further inland. Ceres, Western Cape is always inundated with Capetonians driving out every year whenever the first snowfalls of the season are reported there. We go crazy for the stuff, we're just not used to it! Also, since you do mention South Africa in your heading, I must mention that we almost certainly get snow in the Highveld areas of the country (cities such as Johannesburg and Pretoria for example), which are 100's of km above sea level and very far inland. On an aside, the coldest place in South Africa (and thus also getting regular snow) is Sutherland, a very remote inland town in the Karoo desert most famous for hosting the Southern African Large Telescope, the largest optical telescope in the southern hemisphere. Zunaid 18:51, 11 August 2009 (UTC)
Does the mass of the earth change with population growth?
Does a batch of newborns add mass to the earth? My feeling would be no, due to the law of conservation of matter. —Preceding unsigned comment added by 12.168.244.134 (talk) 00:07, 11 August 2009 (UTC)
- No, everything the new organisms are made of was already on the Earth, it is just existing matter being rearranged, nothing is created from scratch. There is some change of mass due to the atmosphere escaping, meteors hitting the Earth, etc. and also some due to energy entering and leaving the Earth as radiation (E=mc2), but that's pretty negligible. --Tango (talk) 00:10, 11 August 2009 (UTC)
- You are correct. The mass of a newborn had been counted as part of the mass of the baby’s mother before the baby was born, so childbirth results in no increase of the total mass on the earth. Red Act (talk) 00:25, 11 August 2009 (UTC)
- Even if all newborns actually came from outer space, the mass addition wouldn't matter as far as the Earth is concerned. The Earth has a mass of about 6x1024 kilograms, and if there were seven billion people alive (there aren't) and they all weighed 100 kilograms (220 lbs), that would only be 7x1011 kilos, which is a paltry contribution. ~ Amory (user • talk • contribs) 00:45, 11 August 2009 (UTC)
- A fact which I think is even more counterintuitive which I only really "got" recently is that burning a tree does not decrease any mass either.. The mass of the ashes that are left and the gasses released by the burning actually equals the mass of the tree before it was burned.. I mean I always knew all the "matter" was still there just in a different configuration, but I never really realised that the gas actually had the same mass as the tree. Vespine (talk) 01:06, 11 August 2009 (UTC)
- Strictly speaking, it is marginally less because some of that mass has been converted into energy (E=mc2 again), but since the speed of light is so enormous it is a negligible amount of mass that is lost (and it is only actually lost when that energy gets radiated into outer space). --Tango (talk) 01:18, 11 August 2009 (UTC)
- I think E=mc2 only applies to nuclear reactions. Burning a tree is a chemical reaction. (As is growing it in the first place.) Mitch Ames (talk) 11:25, 11 August 2009 (UTC)
- If you read the article it's quite clear that applies whenever there's a change in mass although as Tango mentioned and I think it's been pointed out before with actual figures the change in mass is negible. See also Wikipedia:Reference desk/Archives/Science/2009 February 24#Conservation of mass or search more in the archives Nil Einne (talk) 11:37, 11 August 2009 (UTC)
- It applies to everything. It just says that mass and energy are equivalent. You think of it as energy having mass, if energy is released its mass goes with it. --Tango (talk) 16:30, 11 August 2009 (UTC)
- I think E=mc2 only applies to nuclear reactions. Burning a tree is a chemical reaction. (As is growing it in the first place.) Mitch Ames (talk) 11:25, 11 August 2009 (UTC)
- Strictly speaking, it is marginally less because some of that mass has been converted into energy (E=mc2 again), but since the speed of light is so enormous it is a negligible amount of mass that is lost (and it is only actually lost when that energy gets radiated into outer space). --Tango (talk) 01:18, 11 August 2009 (UTC)
- A fact which I think is even more counterintuitive which I only really "got" recently is that burning a tree does not decrease any mass either.. The mass of the ashes that are left and the gasses released by the burning actually equals the mass of the tree before it was burned.. I mean I always knew all the "matter" was still there just in a different configuration, but I never really realised that the gas actually had the same mass as the tree. Vespine (talk) 01:06, 11 August 2009 (UTC)
- Would the mass converted into energy while dismantling the tree roughly equal the energy that was turned into mass by capturing the sun when the plant was growing in the 1st place? Would it be completely incorrect to say that the fire is just years of accumulated sunshine released in a (relative) instant? Maybe not strictly correct but rather poetic way to look at it I think. Vespine (talk) 04:54, 11 August 2009 (UTC)
- Once, in answering a child's question "Why is the fire hot?", Buckminster Fuller said that the fire was the sunlight the tree had absorbed "unwinding from the log". Deor (talk) 06:15, 11 August 2009 (UTC)
- Yes, that is a good way to describe it. --Tango (talk) 16:23, 11 August 2009 (UTC)
- Once, in answering a child's question "Why is the fire hot?", Buckminster Fuller said that the fire was the sunlight the tree had absorbed "unwinding from the log". Deor (talk) 06:15, 11 August 2009 (UTC)
- Even if all newborns actually came from outer space, the mass addition wouldn't matter as far as the Earth is concerned. The Earth has a mass of about 6x1024 kilograms, and if there were seven billion people alive (there aren't) and they all weighed 100 kilograms (220 lbs), that would only be 7x1011 kilos, which is a paltry contribution. ~ Amory (user • talk • contribs) 00:45, 11 August 2009 (UTC)
Howard Hughes Medical Institute
Is the Howard Hughea Medical Institute(tax-exempt) classified as a 501c(3) organization under the IRS tax code? —Preceding unsigned comment added by Bmmillar (talk • contribs) 01:04, 11 August 2009 (UTC)
- From the Wikipedia article:
Hughes was the sole trustee of HHMI and transferred all his stock of Hughes Aircraft to the institute, in effect turning the large defense contractor into a tax-exempt charity. For many years the Institute grappled with maintaining its non-profit status; the Internal Revenue Service challenged its "charitable" status which made it tax exempt
- That would seem to imply that yes, it is, despite my paltry knowledge of the tax code. ~ Amory (user • talk • contribs) 01:11, 11 August 2009 (UTC)
- No, it counts as a Medical Research Organization, which is IRS code section 170(b).[22] Red Act (talk) 01:20, 11 August 2009 (UTC)
Northern Canada, Anchorage and southern Greenland
Do Northern Canada and Anchorage, Alaska get up to 70s F in summer highs ? Well it is up in the 60 degrees away from equators. Is is possible southern Greenland can get up to 50s F in summer highs?--69.229.108.245 (talk) 02:07, 11 August 2009 (UTC)
- Anchorage can get to the 80s: [23] 75.41.110.200 (talk) 03:00, 11 August 2009 (UTC)
- Wow, that's amazing Anchorage have hit 80 F. Thanks 75.41.110.200--69.229.108.245 (talk) 03:02, 11 August 2009 (UTC)
- As you can see at our article on Anchorage, Alaska, the average summer high is above 60 degrees Fahrenheit, and the record high is 93F, so it seems likely that the temperature often reaches 70F or higher. As for Greenland, Narsarsuaq routinely gets above 60F, see here.-RunningOnBrains(talk) 03:41, 11 August 2009 (UTC)
Remember that while the summer daylight in these places is less intense than nearer the equator (because the sun is lower in the sky), there are also a lot more hours of sunlight each day in the summer (up to 24 hours a day when you get beyond the Arctic Circle), which partially offsets the weakness of it. "Summer" in climatic terms tends to be short in the arctic, limiting the kinds of plants than can grow there (for instance), but it certainly exists. --Anonymous, 04:16 UTC, August 11, 2009.
- Even today, in August, Accuweather is forecasting 69°F for Anchorage. A bit chillier in Nuuk, though! Tonywalton Talk 11:45, 11 August 2009 (UTC)
Conversions
I saw this information on YouTube. Before the explosion in the engine room aboard the SS Norway, 60,000 liters of water heated up to 500 degrees Celcius in a matter of seconds. How many gallons of water is 60,000 liters? How many degrees Fahrenheit is 500 degrees Celcuis69.203.157.50 (talk) 03:05, 11 August 2009 (UTC)
- Google is great for these kind of questions, here's the first http://www.google.com.au/search?hl=en&q=60000+l+in+gallons+&meta=&aq=f&oq= i'll let you work out how to do the temperature your self :). Vespine (talk) 03:17, 11 August 2009 (UTC)
- Well, the question is what kind of gallons you mean. Note that even though Vespine's link is to the Australian version of Google, it gave a result in US gallons. (At least, it did for me, trying it from Canada.) If you want Imperial gallons, you have to ask for them explicitly. --Anonymous, 04:21 UTC, August 11, 2009.
- 500C = 932F. (Double the C, decrease by 10%, add 32. You can do it in your head, right?) What supposedly caused this temperature increase? What was the starting temp? Where did the energy come from? How many Joules does it represent? Sounds dubious. Was it this incident? [24] A boiler rupturing and flashing to steam should not cause a temperature rise, and energy does not come to a boiler from nowhere. It normally took over 3 hours to go from cold to full pressure. Edison (talk) 04:39, 11 August 2009 (UTC)
- Just for the numbers, i have 125Gj to get that amount of water to 500 degrees, from 0. 30 tons of TNT. I'm bad at numbers tho so that could be way off.. However, i think they've just misreported it, i don't think the water would heated up to that high in a few seconds, i think the explosion and consequent vaporisation of the water took a few seconds. The tempereature was probably right up there just before the explosion. I'd be looking for a failure in the cooling system as the cause. But no doubt on a ship like this, multiple systems must have failed for this kind of accident to occur. warning sytstems, shut down systems etc... Vespine (talk) 05:09, 11 August 2009 (UTC)
- The very informative NTSB accident report says "About 0637, a boiler ruptured in the aft boiler room ... The boiler contained about 20 tons of water operating at a temperature of about 528º F under a pressure of about 60 bar (870 pounds per square inch [psi])). In the normal atmospheric pressure of the aft boiler room (14.7 psi), the pressurized hot water rapidly expanded in volume about 1,260 times into steam. The expanding steam, mixed with smoke, soot, and debris, swept through the engineering spaces, fatally injuring four engineering crewmembers who were on watch or on duty in or near the boiler room, as well as four other crewmembers who were in the crew living spaces on the starboard side of the Caribbean deck, next to the boiler room". Chronology of the incident says:
- "0637 - Rupture occurs in boiler No. 23 in aft boiler room. Rupture activates sprinkler system in areas around boiler room as well as smoke alarms locally in affected areas and remotely in wheelhouse. Pier-side Miami-Dade police officer radios report of accident to his command.
- 0638 - Boiler automation system shuts down operating main boilers, which stops steam turbine generators and causes loss of main electrical power. Ship’s battery-operated emergency systems activate and function as designed."
- Further on, the report says "Tests of the four safety valves on boiler No. 23 revealed no significant defects, indicating that they probably functioned properly at the time of the accident". The report concludes that "the probable cause of the boiler rupture on the Norway was the deficient boiler operation, maintenance, and inspection practices of Norwegian Cruise Line, which allowed material deterioration and fatigue cracking to weaken the boiler". So sounds like the boiler failed during normal operations and the steam came from the superheated water that was already in the boiler, not from external water heated by an explosion. Gandalf61 (talk) 10:08, 11 August 2009 (UTC)
- Our article says "Her engines consisted of eight high-pressure, super-heating boilers delivering 65 kg per cc and 500 degrees Celsius, all weighing 8,000 tons". This isn't referring to the accident but if I understand this correctly that is the normal operating temperature. There's a possible source but it's offline. It also says "This move cut down fuel consumption to 250 tonnes per 24 hours. The remaining four boilers and engine room were made fully automated, and operated from either a central control station below decks, or from the bridge", it's not clear if this cut down the operating temperature as well. Nil Einne (talk) 11:31, 11 August 2009 (UTC)
- The very informative NTSB accident report says "About 0637, a boiler ruptured in the aft boiler room ... The boiler contained about 20 tons of water operating at a temperature of about 528º F under a pressure of about 60 bar (870 pounds per square inch [psi])). In the normal atmospheric pressure of the aft boiler room (14.7 psi), the pressurized hot water rapidly expanded in volume about 1,260 times into steam. The expanding steam, mixed with smoke, soot, and debris, swept through the engineering spaces, fatally injuring four engineering crewmembers who were on watch or on duty in or near the boiler room, as well as four other crewmembers who were in the crew living spaces on the starboard side of the Caribbean deck, next to the boiler room". Chronology of the incident says:
- By the late 19th century, safety practices in operating steam boilers included periodic internal inspections, to detect the beginning of cracks or failure of internal bracing, and periodic testing of indicator valves and safety pressure relief valves. Boilers and other pressure vessels had to undergo hydrostatic testing, in which it was pressurized way above operating pressure by being filled with water. That way if it burst during the test, there was little or no stored energy to cause the damage and casualties that happened here. If I were investigating, I would wnt to check those tests and inspections. If a boiler had passed a pressure test, and had functioning relief valves, why would it pop like a kernal of popcorn? What was the root cause per the investigation? Malfeasance or incompetence? Murphy's Law? Bad Luck? Shit Happens? If it was a design problem, were there other similar boilers which had to be modified or taken out of service? Also, reports above list both 500 C and 528F, which are far apart as I noted. Edison (talk) 15:49, 11 August 2009 (UTC)
- The safety board investigation cited above concluded "The National Transportation Safety Board determines that the probable cause of the boiler rupture on the Norway was the deficient boiler operation, maintenance, and inspection practices of Norwegian Cruise Line, which allowed material deterioration and fatigue cracking to weaken the boiler. Inadequate boiler surveys by Bureau Veritas contributed to the cause of the accident." Pressures and temps were normal when it failed. Cracks from fatigue and corrosion extended to .55 inches in a wall .935 inches thick. Internal inspection or hydraulic testing had not been done recently on the header which failed. Edison (talk) 16:35, 11 August 2009 (UTC)
name of soviet car
What is the name of the type of car in this picture? --67.173.155.191 (talk) 04:07, 11 August 2009 (UTC)
- Dunno. It sort of resembles a ZIL-117 but some differences are visible. Maybe another model in the same series. 70.90.174.101 (talk) 04:20, 11 August 2009 (UTC)
That would be a Gaz-14 Chaika. Here's a Youtube video that nicely shows the distinctive rear.--Rallette (talk) 10:50, 11 August 2009 (UTC)
Cheap DNA restriction enzymes
To help in finding cheap sites for restriction endonuclease analysis (REA), I'm trying to compose a list of cheap endonucleases for use with my molecular cloning program. I know that, for example, EcoRI and BamHI are examples of relatively cheap enzymes, and therefore preferable candidates for REA. Can someone help me to augment this list? --94.212.39.7 (talk) 07:26, 11 August 2009 (UTC)
- New England Biolabs's website has a pricelist Rockpocket 07:45, 11 August 2009 (UTC)
- It would be a lot of work to deduce the enzymes from that price list. I was hoping someone already had a list compiled or otherwise was familiar enough with the prices to be able to reel them from the top of their head. --94.212.39.7 (talk) 10:47, 11 August 2009 (UTC)
- Page down to page 7 of the price list. On the left column, starting with product number R0101 are the restriction enzymes. They continue until page 10, with product number R3642 - basically all product numbers starting with "R" are restriction enzyme, restriction methylases and related. A quick glance shows that NEB's pricing for restriction enzymes is relatively simple, with "small" sizes in the $50-70 range, and "large" sizes in the $225-275 range. Different enzymes have different amounts of enzyme in each size, though. Uncommon or hard to purify enzymes may only have 500 units in the small size, whereas commonly used enzymes which NEB expresses recombinantly have 10,000 units in the small size. The cheapest ones are the ones you've probably heard of: EcoRI, HindIII, BamHI, and PstI all come in 10,000 units in the small size. - If you're at all interested in restriction enzymes, it pays to spend time browsing NEB's website. They have a lot more information there than the standard "this is what we sell and this is how much it costs" info. - 128.104.112.100 (talk) 16:15, 11 August 2009 (UTC)
- It would be a lot of work to deduce the enzymes from that price list. I was hoping someone already had a list compiled or otherwise was familiar enough with the prices to be able to reel them from the top of their head. --94.212.39.7 (talk) 10:47, 11 August 2009 (UTC)
Sorry, I didn't fully understand what you wanted. NEB and other companies generally price their enzymes at roughly the same price, what they vary is how many enzyme units you get for that standard price. Therefore the amount you plan use use is a factor in value for money. 128.104.112.100 is correct, though, in that most of the restriction enzymes are grouped together on that list, so it should be easy to scan down and find the best value for your application. In general, I seem to remember BamHI, EcoRI and XhoI are very inexpensive. Rockpocket 16:57, 11 August 2009 (UTC)
Battle excavations
Is it posssible to detect and excavate for example the shields, bones and other artifacts of Greek warriors at the site of Thermopylae? Also, is there a good source on battlefield excavations? 217.25.31.169 (talk) 09:13, 11 August 2009 (UTC)
- Use "find" for "archaelogy" in Battle of Thermopylae for more details, arrowheads etc have been found there. Don't know about shields - I would suspect any are long gone.
- Note that you personally cannot do this (ie start digging)- you'll get arrested by angry greeks ! - amateur archaelogy is illegal in all countries.83.100.250.79 (talk) 13:03, 11 August 2009 (UTC)
- (it might be an idea to move your question to the humanities desk)83.100.250.79 (talk) 13:38, 11 August 2009 (UTC)
- Amateur archaeology is not "illegal in all countries". Tempshill (talk) 16:10, 11 August 2009 (UTC)
- Where did that idea even come from? There are probably regulations on where digging is allowed, but anyone can do archaeology, amateur- or professional. In most places, even digging is okay. In places where archaeological artifacts are known to exist, a lot of regulations probably exist, but "illegal all countries"? Really? [citation needed]. Nimur (talk) 19:20, 11 August 2009 (UTC)
- If I remember The Mildenhall Treasure correctly, in the UK, any treasure found that is gold or silver must be reported to the authorities, and the Crown has the authority to confiscate it. Failure to report it is a crime. But conducting amateur archaeology to look for such treasure (or any other artifact) is not illegal in the UK, as far as I know. Tempshill (talk) 22:47, 11 August 2009 (UTC)
- Where did that idea even come from? There are probably regulations on where digging is allowed, but anyone can do archaeology, amateur- or professional. In most places, even digging is okay. In places where archaeological artifacts are known to exist, a lot of regulations probably exist, but "illegal all countries"? Really? [citation needed]. Nimur (talk) 19:20, 11 August 2009 (UTC)
- As long as you have the permission of the landowner, I can't see there being any problems. If you find human remains there are usually procedures to go through and there may be complicated rules about who owns whatever you find, but you amateur archaeology isn't illegal in any country as far as I know. There are archaeology societies made up of amateurs set up all over the place. --Tango (talk) 21:35, 11 August 2009 (UTC)
- Amateur archaeology is not "illegal in all countries". Tempshill (talk) 16:10, 11 August 2009 (UTC)
European Maple Syrup
Are any of the species of maple tree native to Europe usable for making syrup? --Carnildo's non-admin account (talk) 09:46, 11 August 2009 (UTC)
- I think the issue is not the species of tree but the climate. I think Maple syrup is incredibly bitter without the right weather (long cold plus correct timing). I suspect you would have to be somewhere well East in Europe to have a chance --BozMo talk 13:14, 11 August 2009 (UTC)
- Sugar maples originated in North America. In Europe I have seen some in gardens, parks and even alleys, but I am not aware of any agricultural usage of the tree. Googling indicates that the European climate is unsuitable to produce any significant yield of syrup, as this requires freezing nights and warmish days for prolonged periods. Molasses from other sources (sugar beet) were / are used in Europe. --Cookatoo.ergo.ZooM (talk) 14:52, 11 August 2009 (UTC)
- So, it appears that extreme cold is bad for sugar maples. How about warm weather? I'm currently in the Ozarks (Missouri) and there is a lot of sugar maples. A road nearby is called "Sugar Tree Hill" because the sugar maples there are supposedly extra sweet. It rarely freezes at night here - just a few weeks in February. So, I wonder if there are warmer weather sugar maples and colder weather sugar maples (or if the sugar maples here aren't sugar maples at all). -- kainaw™ 15:07, 11 August 2009 (UTC)
- Sugar maples can grow in a lot of different places. The suitability of the sap for making syrup is based both on the tree species, and on the climate. I see no reason why maple species native to Europe could not produce good syrup if grown in the right conditions, or that a sugar maple grown in improper conditions would not give good syrup. In fact, you can make syrup from birch trees. Googlemeister (talk) 15:15, 11 August 2009 (UTC)
- This site describes the European field maple, and indicates that the sap contains sugar, and can be drunk or turned into syrup, but that the sugar content is far below that of the North American sugar maple. As far as climate/weather goes, take a look at this site, but basically, it needs to get warm enough for the sap to flow well during the day, and cold enough (below freezing) to allow the tree to recover at night. Sugaring season ends when the leaves start budding because of chemical changes in the sap related to bud/leaf production which give the syrup an off taste. Some jerk on the Internet (talk) 18:48, 11 August 2009 (UTC)
- So, it appears that extreme cold is bad for sugar maples. How about warm weather? I'm currently in the Ozarks (Missouri) and there is a lot of sugar maples. A road nearby is called "Sugar Tree Hill" because the sugar maples there are supposedly extra sweet. It rarely freezes at night here - just a few weeks in February. So, I wonder if there are warmer weather sugar maples and colder weather sugar maples (or if the sugar maples here aren't sugar maples at all). -- kainaw™ 15:07, 11 August 2009 (UTC)
Relativistic Doppler effect-another problem
Relativistic Doppler effect#Motion in an arbitrary direction has relativistic abberatition formula
I just calculated putting . Then
- .
Because c>|v|, the numerator is always positive, so the denominator decides the sign of .
And because |x|>1, and c>|v|, |cx|>|v|, and the sign of x decides the sign of .
If x is positive, is positive, and if x is negative, is negative, and vice versa.
Taking |x| as large as possible, comes near 0, that is, comes near , and still the sign of is the same as .
Looking at Relativistic Doppler effect#Visualization, the direction at comes forward.
Hmmm...Like sushi (talk) 11:04, 11 August 2009 (UTC)
Sorry, I found a mistake in calculation.
Like sushi (talk) 11:39, 11 August 2009 (UTC)
Why is AIDS so Common in Africa?
I was taught that AIDS was spread through gay sex. But the infection rate--last time I checked--was ⅓ in some countries in Africa. It's hard to understand how a disease that isn't airborne could infect that many people.--IndexOutOfBounds (talk) 15:01, 11 August 2009 (UTC)
- AIDS may be spread by any unprotected sex (gay, straight, bi, tri...). It is rampant in underdeveloped countries due to a lack of education and prevention. -- kainaw™ 15:03, 11 August 2009 (UTC)
- A guy can give a girl AIDS, but not the other way around, right? At least, it'd have to involve untraditional sex for that to happen, right?--IndexOutOfBounds (talk) 15:04, 11 August 2009 (UTC)
- Wow, have you been poorly served by your public health service. Girls can give guys HIV with regular, traditional sex. Both penetrative and receptive partners can transmit and receive HIV. Here's an overview of HIV transmission. Statistically, it is less likely to occur, but it can, and does occur. Keep in mind that there are non-sexual ways to transmit it as well; mother-to-child is a very common mode of transmission in Africa in particular. --98.217.14.211 (talk) 15:08, 11 August 2009 (UTC)
- I graduated from high school in 2000 and last learned about HIV/AIDS in 1998. So, no, I don't remember everything they told us about AIDS back then, and even if they went into details such as these.--IndexOutOfBounds (talk) 15:15, 11 August 2009 (UTC)
- It's kind of a big deal, you know, how to avoid getting various diseases. I'm just saying. And it's pretty easy to look up this information. You might as well be posting on here about whether the Earth is flat or not, and then complaining that you went to school a long time ago and can't be bothering to remember such a thing. To think that AIDs is "only spread by gay sex" is an extremely, extremely ignorant thing to believe still. If that's really what you were taught in 2000 (which is not what I was taught in the 1990s), then you were, as I said, seriously poorly served by whomever taught you about public health, STDs, etc. --98.217.14.211 (talk) 16:13, 11 August 2009 (UTC)
- Just so you know, this is a reference desk where you can ask questions. I'm not a doctor. I have few college degrees, but none of them are in biology or medicine. So, to call me ignorant is (ironically) ignorant.--IndexOutOfBounds (talk) 23:45, 11 August 2009 (UTC)
- It's kind of a big deal, you know, how to avoid getting various diseases. I'm just saying. And it's pretty easy to look up this information. You might as well be posting on here about whether the Earth is flat or not, and then complaining that you went to school a long time ago and can't be bothering to remember such a thing. To think that AIDs is "only spread by gay sex" is an extremely, extremely ignorant thing to believe still. If that's really what you were taught in 2000 (which is not what I was taught in the 1990s), then you were, as I said, seriously poorly served by whomever taught you about public health, STDs, etc. --98.217.14.211 (talk) 16:13, 11 August 2009 (UTC)
- I graduated from high school in 2000 and last learned about HIV/AIDS in 1998. So, no, I don't remember everything they told us about AIDS back then, and even if they went into details such as these.--IndexOutOfBounds (talk) 15:15, 11 August 2009 (UTC)
- Are you currently in Africa? I thought we spent enough time and money in America and Europe to stamp out such ignorance. -- kainaw™ 15:10, 11 August 2009 (UTC)
- Wow. You're a really big troll. I don't think the reference desk is for you. After having to read your comments, I don't think it's for me, either.--IndexOutOfBounds (talk) 15:15, 11 August 2009 (UTC)
- Also note there is more information in the transmission section of the article on HIV. --130.216.1.16 (talk) 15:10, 11 August 2009 (UTC)
- You have kind of answered your own initial education. Poor education and ignorance (sometimes willfull) of the facts are a major factor in transmission. See Jacob Zuma and his infamous "shower cure". Fribbler (talk) 15:12, 11 August 2009 (UTC)[
- As well as Fribbler's comment, HIV conspiracy theories and AIDS denialism have also taken hold in parts of Africa. When political leaders such as Thabo Mbeki and Manto Tshabalala-Msimang are spreading misinformation about how HIV is spread, it's difficult to get the message about safer sex across. AlmostReadytoFly (talk) 15:21, 11 August 2009 (UTC)
- You have kind of answered your own initial education. Poor education and ignorance (sometimes willfull) of the facts are a major factor in transmission. See Jacob Zuma and his infamous "shower cure". Fribbler (talk) 15:12, 11 August 2009 (UTC)[
- According to the AIDS article, the primary mode of HIV infection worldwide is through sexual contact between a man and a woman. One possibility being researched as to why heterosexual transmission is more common in Africa than elsewhere is that schistosomiasis, which affects up to 50 per cent of women in parts of Africa, damages the lining of the vagina. Red Act (talk) 15:35, 11 August 2009 (UTC)
- I hope IndexOutOfBounds is not seriously offended by comments here. I can remember when doctors genuinely thought that AIDS was mainly transmitted through gay sex, but that was in the 1980s, and much has been discovered since then, and I would have thought that any educator would have updated their knowledge by 1998. Dbfirs 18:20, 11 August 2009 (UTC)
- Misinformation is a big part of it. One persistant myth amongst African men is that having sex with a virgin will cure you of AIDS. I leave it to your imagination as to (a) how such a crazy notion might spread faster than the "don't have sex" message - and (b) the devastating consequences of such dangerous claims. SteveBaker (talk) 18:33, 11 August 2009 (UTC)
- The Catholic prohibition of condoms is also a significant factor. --Tango (talk) 21:30, 11 August 2009 (UTC)
- And also the George Bush government funding AIDS awareness campaigns throughout Africa under the specific condition that abstainance be taught rather than condom use. That's such an incredibly irresponsible thing to mandate, it makes me want to scream! SteveBaker (talk) 23:33, 11 August 2009 (UTC)
- I am not aware of any sexually transmitted disease that is only spread by gay people, only spread by males, only spread by females, or in general only spread by any particular kind of person. Any person can transmit a sexually transmitted disease to any other person, and it may not even involve sex (mishandled medical equipment can also be a vector). Pathogens don't pay attention to what kind of person you are. What is correct is that there was a point at time in which AIDS was statistically more prevalent in the gay community than in the general population in the United States. I don't know whether this is still the case. Dcoetzee 23:26, 11 August 2009 (UTC)
Electric field strength
Hi, this is a homework problem, but I'm not asking for the solution, I'd just like someone to point out where I've gone wrong please. The problem is:
Given two point charges of arbitrary same-signed charge q1 and q2, at locations x1 and x2, find the point x between them at which the electric field strength is equal to zero.
What I did was make x1 equal to zero, then made this equality:
Then I rearranged that to get:
Which is just a quadratic, so then you solve that. The trouble is, the way the problem is given, values for the charges and distances are given (these change every time, it's an online thing), and the answer you check it against is the numerical one for that particular set of values, and mine keeps coming up incorrect. For example, if I have
Then I get the values of the coefficients in the quadratic as
And when I solve that, I get 0.153 m, which I add to 0.25 to get a final answer of 40.3 cm. The given solution for this set of values, however, is x=43.2 cm. I've checked and double-checked my working; could someone help me? --130.216.1.16 (talk) 15:08, 11 August 2009 (UTC)
The x2 you use in your equation is the distance between the charges, not the position x2 from the assignment. —Preceding unsigned comment added by 81.11.170.162 (talk) 15:57, 11 August 2009 (UTC)
- No, although the symbol is used to mean two different values, the distinction is maintained mentally such that that isn’t a problem. The real problem is that there’s a sign error – the expression above should be
- I don't think so. Dauto (talk) 21:44, 11 August 2009 (UTC)
- The problem is that
helicopter ejection seats
The Kamov Ka-50 was the first helicopter to be designed with a ejector seat, have there been any others with this feature? Googlemeister (talk) 15:10, 11 August 2009 (UTC)
- The Ka-52 two-seat variant also has the ejector system; I've found no reference to ejector seats in other helicopters. Rotary wing aircraft can already survive many unpowered landings via autorotation. — Lomn 15:53, 11 August 2009 (UTC)
- If it also rather difficult to eject from a helicopter since there are rotor blades above you! You have to get rid of them before you can eject, which makes for a much more complex system. They will only be fitted if it is really worth it, which it rarely is. --Tango (talk) 16:39, 11 August 2009 (UTC)
- What's wrong with ejecting downwards? Vimescarrot (talk) 17:58, 11 August 2009 (UTC)
- It prevents low-altitude ejections, and helicopters are typically in low-altitude environments. — Lomn 18:05, 11 August 2009 (UTC)
- What's wrong with ejecting downwards? Vimescarrot (talk) 17:58, 11 August 2009 (UTC)
- If it also rather difficult to eject from a helicopter since there are rotor blades above you! You have to get rid of them before you can eject, which makes for a much more complex system. They will only be fitted if it is really worth it, which it rarely is. --Tango (talk) 16:39, 11 August 2009 (UTC)
- They usually have explosive bolts to blow the rotors off...with the amount of centrifugal force, it doesn't take much for them to fly outwards at a truly spectacular speed! There have been experiments with ejecting through the floor of the helicopter also - but having large doors in places like that really messes up the structural rigidity of the thing. The US experimented with them on the Cobra - but I don't think that ever went into production. Part of the problem is that helicopters generally fly too close to the ground for a safe ejection. While there are ejection systems that can fling the seat high enough for the parachute to open - those seats have big rockets under them and are too heavy for helicopter operations...they are mostly only used for aircraft that do carrier deck landings. SteveBaker (talk) 18:30, 11 August 2009 (UTC)
- Thanks for the info! :) Vimescarrot (talk) 21:50, 11 August 2009 (UTC)
The Moon

I just read about the "Giant impact hypothesis" for the first time. Very interesting. But for that much material to have been blasted off the face of this planet, surely when the NASA guys took photos of earth, they'd have seen something that looked more like Pacman than a sphere? --Dweller (talk) 16:18, 11 August 2009 (UTC)
- The Earth’s solid crust is relatively very thin, below which there’s a liquid mantle. So an enormous hole like that would get filled in rather immediately. Red Act (talk) 16:31, 11 August 2009 (UTC)
- The mantle is a solid! See rheid. It's like cheese; it moves when you push on it, but it is not a liquid. Nor is the mantle a sea of magma. Dragons flight (talk) 23:30, 11 August 2009 (UTC)
- My boss told me he had heard there was one side of the earth with less dense material (visible on a "gravitation map" or whatever) which could be due to such an impact. I don't know if he was correct or not... TastyCakes (talk) 16:33, 11 August 2009 (UTC)
- See: Physical geodesy. --Tango (talk) 16:36, 11 August 2009 (UTC)
- I'd think that since Earth is geologically active, it would have erased any gravity anomalies long ago. Anything below the crust is fluid and in a state of constant motion (although said "motion" is slower than the speed that glass flows), so density variations would even out pretty quickly. The crust is constantly being created, subducted, and moved around by plate tectonics, so very few rocks remain from the time of the impact. --Bowlhover (talk) 22:01, 11 August 2009 (UTC)
- See also: History of the Earth for a brief note about how the Earth reacted to the collision. The theory is that it became completely molten - which would cause it to quickly become nearly spherical. -- kainaw™ 17:24, 11 August 2009 (UTC)
- Even without becoming molten, gravity will reshape a body like the Earth back into the near-spherical condition of hydrostatic equilibrium (the IAU's definition of a planet notes that a defining characteristic is sufficient gravity to overcome rigidity and reach hydrostatic equilibrium). — Lomn 18:07, 11 August 2009 (UTC)
- Indeed, but a molten planet will reshape quicker. --Tango (talk) 18:30, 11 August 2009 (UTC)
- Even without becoming molten, gravity will reshape a body like the Earth back into the near-spherical condition of hydrostatic equilibrium (the IAU's definition of a planet notes that a defining characteristic is sufficient gravity to overcome rigidity and reach hydrostatic equilibrium). — Lomn 18:07, 11 August 2009 (UTC)
- My boss told me he had heard there was one side of the earth with less dense material (visible on a "gravitation map" or whatever) which could be due to such an impact. I don't know if he was correct or not... TastyCakes (talk) 16:33, 11 August 2009 (UTC)
Large population increases rate of evolution?
In a book review, game designer and former physics teacher Chris Crawford cite two writers as showing mathematically that a larger population increases the rate of evolution, even if it doesn't make previously abundant resources scarce. Can anyone here confirm that the numbers work out, whether because they've read the book or because of another source that says the same thing? NeonMerlin 17:54, 11 August 2009 (UTC)
- It seems pretty obvious - in order to have evolution, you have to have some kind of pressure on the species such that not every individual can have offspring survive into the next generation - and you have to have some source of genetic variation. A larger population means that there are more mutations - more new genes appearing and more combinations of genes showing up. Assuming there is at least some level of breeding pressure, the increased number of mutations will obviously result in faster evolution. 18:21, 11 August 2009 (UTC)
- I would think it was more the opposite. Population bottlenecks can cause rapid evolution since any mutations in the few members of the population that survive will inevitably end up in a large proportion of the population. --Tango (talk) 18:29, 11 August 2009 (UTC)
- It's both. A larger population means there is likely to be a greater number (not necessarily percent) of genetic mutations occurring, so the potential is definitely there. However, in a small population, all it takes is one particularly persistent mutation to quickly affect the entire population. ~ Amory (user • talk • contribs) 19:18, 11 August 2009 (UTC)
- I guess we have to be more careful about what we're saying when we talk about increasing the "rate" of evolution. Certainly having a smaller population would result in a new, beneficial gene spreading more rapidly - but an awful long time could go by without any new, useful mutation showing up. With a large population, beneficial genes would show up much more frequently - although it would take longer for them to spread everywhere. But the degree to which that is the case has to depend on exactly HOW beneficial the gene was. A gene that made you immune to heart disease would spread through the population incredibly quickly - but a gene that helped you survive being struck by lightning could take forever to take hold in a larger population. When you consider the evolutionary advances due to two or more rare genes that are only beneficial in combination happening to meet as a result of sexual reproduction - the advantages of a large population should be even more significant. SteveBaker (talk) 23:28, 11 August 2009 (UTC)
- It's both. A larger population means there is likely to be a greater number (not necessarily percent) of genetic mutations occurring, so the potential is definitely there. However, in a small population, all it takes is one particularly persistent mutation to quickly affect the entire population. ~ Amory (user • talk • contribs) 19:18, 11 August 2009 (UTC)
core not molten
When will Earth's core become solid and what would be the consequence for Earth? -- Taxa (talk) 18:39, 11 August 2009 (UTC)
- The inner core is solid, it's just the outer core that is molten. (The inner core is hotter, but under more pressure, so is solid despite the heat.) The Earth is slowly cooling, so I suppose it is possible that the outer core will freeze eventually. That would have a significant impact on the geomagnetic field, although I don't think it would eliminate the field entirely. I can't find a timescale for that, though - it might be longer than the life of the Sun, so would be moot. --Tango (talk) 18:56, 11 August 2009 (UTC)
- From what I understand, our knowledge of the thermal conduction properties of the inner earth are very limited. In fact, our best information on the deep earth comes from seismic analysis - which can give a good sound-speed and rock shear properties. This lets us estimate the rock composition in the mantle - but the error bars are non-trivial. We also have electromagnetic information, but it's not very conclusive as far as telling us material properties in the earth. So, from this amalgamation of data, we "guess" what sort of rock is down there, and under what conditions (pressure, temperature, etc). The next step is to estimate, or measure in a laboratory, the thermal conductivity properties of such a material - again, we don't have a great way of synthesizing molten silica or iron at the temperatures and pressures we expect to find in the deep mantle or outer core, so lab measurements are helpful only as far as data-extrapolation is reliable. Finally, the thermal conductivity problem can be solved analytically or numerically to estimate the time-constant of cooling. To my understanding, the Earth is almost in steady-state temperature, with solar energy incidence equally balanced by reradiation into space. But, with all these "Global Climate Change" scientists redefining what is meant by "Temperature of the Earth" (they usually mean the sea-level average temperature, or the average ocean temperature, or whatever - but they rarely mean the blackbody temperature or the radiation temperature as observed from space) - it's hard to be very sure whether Earth as a whole is "warming" or "cooling". On geological time scales, we can probably say that Earth is actually cooling, but very slowly; and by comparison to other Earth-like planets that we have measured (Mars, Mercury, and Moon -not really a planet, but who knows what terminology IAU will come out with next-- come to mind). All of these planets have cooled and appear to have solid interiors as evidenced by weak or absent dipole planetary magnetic fields; but our seismic experiments on the Moon were noisy and weak (so we aren't really sure). No seismic experiments have been conducted on Mars or Mercury (to my knowledge) so we have very limited understanding of the interiors of those planets. Each of those three seem to have cooled quicker than Earth - probably due to their smaller size (which means lower thermal mass, and also a greater surface-area to volume ratio). We can pretty much assume that Earth's interior will also solidify; I would estimate the timescales are on billions-of-years (which means that the steady-state approximation of solar heating will be invalid). Regarding the consequences - the biggest question is, "what will happen to our dipole magnetic field?" Mars seems to have greatly weakened and frozen some magnetism into the crustal rock; but Mercury and Moon have a neglible magnetic field. The dramatic consequences might include the disappearance of the Van Allen belts, exposing the atmosphere to the highest-energy solar wind particles; but this is speculation. It's worth noting that the most intelligent geomagnetism experts I have talked to about this always preface things by reminding me that we really have no idea why Earth has a magnetic field anyway - something about "hot iron" and maybe some kind of circular, convective motion ... but try to get a straight answer as to why this makes the planet look like a bar magnet - and you just get more questions. So - maybe a frozen interior will have no effect on our magnetic field... Nimur (talk) 19:32, 11 August 2009 (UTC)
- Given the long timescales involved, are we going to be rendered uninhabitable by the Sun before this happens? Vimescarrot (talk) 21:47, 11 August 2009 (UTC)
- The Sun will kill us in about a billion years, so if Nimur is correct and we're talking billions of years then yes, the Sun will kill us before the core cools down enough to be a serious problem (if it will ever be a serious problem). --Tango (talk) 22:59, 11 August 2009 (UTC)
- Given the long timescales involved, are we going to be rendered uninhabitable by the Sun before this happens? Vimescarrot (talk) 21:47, 11 August 2009 (UTC)
- From what I understand, our knowledge of the thermal conduction properties of the inner earth are very limited. In fact, our best information on the deep earth comes from seismic analysis - which can give a good sound-speed and rock shear properties. This lets us estimate the rock composition in the mantle - but the error bars are non-trivial. We also have electromagnetic information, but it's not very conclusive as far as telling us material properties in the earth. So, from this amalgamation of data, we "guess" what sort of rock is down there, and under what conditions (pressure, temperature, etc). The next step is to estimate, or measure in a laboratory, the thermal conductivity properties of such a material - again, we don't have a great way of synthesizing molten silica or iron at the temperatures and pressures we expect to find in the deep mantle or outer core, so lab measurements are helpful only as far as data-extrapolation is reliable. Finally, the thermal conductivity problem can be solved analytically or numerically to estimate the time-constant of cooling. To my understanding, the Earth is almost in steady-state temperature, with solar energy incidence equally balanced by reradiation into space. But, with all these "Global Climate Change" scientists redefining what is meant by "Temperature of the Earth" (they usually mean the sea-level average temperature, or the average ocean temperature, or whatever - but they rarely mean the blackbody temperature or the radiation temperature as observed from space) - it's hard to be very sure whether Earth as a whole is "warming" or "cooling". On geological time scales, we can probably say that Earth is actually cooling, but very slowly; and by comparison to other Earth-like planets that we have measured (Mars, Mercury, and Moon -not really a planet, but who knows what terminology IAU will come out with next-- come to mind). All of these planets have cooled and appear to have solid interiors as evidenced by weak or absent dipole planetary magnetic fields; but our seismic experiments on the Moon were noisy and weak (so we aren't really sure). No seismic experiments have been conducted on Mars or Mercury (to my knowledge) so we have very limited understanding of the interiors of those planets. Each of those three seem to have cooled quicker than Earth - probably due to their smaller size (which means lower thermal mass, and also a greater surface-area to volume ratio). We can pretty much assume that Earth's interior will also solidify; I would estimate the timescales are on billions-of-years (which means that the steady-state approximation of solar heating will be invalid). Regarding the consequences - the biggest question is, "what will happen to our dipole magnetic field?" Mars seems to have greatly weakened and frozen some magnetism into the crustal rock; but Mercury and Moon have a neglible magnetic field. The dramatic consequences might include the disappearance of the Van Allen belts, exposing the atmosphere to the highest-energy solar wind particles; but this is speculation. It's worth noting that the most intelligent geomagnetism experts I have talked to about this always preface things by reminding me that we really have no idea why Earth has a magnetic field anyway - something about "hot iron" and maybe some kind of circular, convective motion ... but try to get a straight answer as to why this makes the planet look like a bar magnet - and you just get more questions. So - maybe a frozen interior will have no effect on our magnetic field... Nimur (talk) 19:32, 11 August 2009 (UTC)
- The inner core started to solidify 3-4 billion years ago, and in that time ~15% of the total core has frozen. Given that time scale, the sun will almost certainly die before the core could solidify completely. Dragons flight (talk) 23:24, 11 August 2009 (UTC)
- Is it a linear thing? --Tango (talk) 23:41, 11 August 2009 (UTC)
- The inner core started to solidify 3-4 billion years ago, and in that time ~15% of the total core has frozen. Given that time scale, the sun will almost certainly die before the core could solidify completely. Dragons flight (talk) 23:24, 11 August 2009 (UTC)
esterification of edible alcohols, besides ethanol
So I got some favourable correspondence from a chef, so I'm about to refine my series of experiments. The thing is, being 19, I don't really have good access to ethanol, and I'm tired of borrowing my roommates' beer. Which is a pity, since ethanol is probably an alcohol I could use in excess, and I wanted to try frying pan syntheses of ethyl cinnamate. What are some common food items that contain favourable OH groups for esterification? (And favourable COOH groups too?) Preferably these are compounds I could react in excess (for a noticeable ester smell?).
I was thinking of using tannins, since they are abundant. must I do to get tannin OH groups to react? (If I say, added tea leaves?) Why are aryl OH groups hard to esterify in general? Are there any pleasant tannin esters, anyway? Are there any cooking pan methods to turn compounds into reactive alcohols, e.g. turning spice oils?
Are sugar's OH groups conducive to esterification? What about sweeteners? What about caramelised sugars?
Could I also use enzymes or microbes to produce alcohols besides ethanol, e.g. convert an aldehyde into an alcohol? John Riemann Soong (talk) 19:11, 11 August 2009 (UTC)
- Depending on your area, possession of ethanol for chemistry is not regulated; you might be able to order it from a chemical supplier like Fisher Scientific: Molecular Biology-grade 100.0% ethanol is available for about 25 US dollars per 100 mL. Nimur (talk) 19:54, 11 August 2009 (UTC)
- (It should be noted - DO NOT DRINK 200 proof ethanol). Nimur (talk) 19:55, 11 August 2009 (UTC)
- Wow, 200 proof ethanol is really expensive ... couldn't I get it at azeotrope concentration? I imagine that would cheapen things considerably. Or is azeotrope-distilled alcohol (without a bittering agent?) regulated? John Riemann Soong (talk) 20:08, 11 August 2009 (UTC)
- There are numerous grades available; less pure ethanol may be cheaper. You can search for them or find other vendors. Of course, if you want to eat any of this stuff, be very careful - impurities in lab-chemistry-grade chemical supply can be extremely toxic - deadly. Even the 100.0% ethanol, which claims to be very pure, still might have as high as 100 ppm acetone, and other impurities ("probably below the LD50" isn't safe enough for human consumption). Nimur (talk) 20:12, 11 August 2009 (UTC)
- In many areas, you can buy 190 proof (95%, the azeotropic ratio) in liquor stores (some places regulate it as any other drinking alcohol, other places are stricter specifically about this one). I'll second Nimur's comment though, that lab-grade chemicals, while they may have a higher % purity than food-grade, may have than smaller amount of contaminant be a more dangerous chemical; food-grade is often lower purity but you know the impurities are safe to eat. Methyl alcohol (probably 70% or 90%, maybe also 100%) is often available from pharmacies. Isopropyl is also, but I don't know of any popular esters of it. DMacks (talk) 21:02, 11 August 2009 (UTC)
- There are numerous grades available; less pure ethanol may be cheaper. You can search for them or find other vendors. Of course, if you want to eat any of this stuff, be very careful - impurities in lab-chemistry-grade chemical supply can be extremely toxic - deadly. Even the 100.0% ethanol, which claims to be very pure, still might have as high as 100 ppm acetone, and other impurities ("probably below the LD50" isn't safe enough for human consumption). Nimur (talk) 20:12, 11 August 2009 (UTC)
- Even if you do, it isn't simple to get 200% proof - this 14-page document is a summary of the rules: [25]. You only need status, power of attorney, federal inspection, tracking systems to account for it down to no more than 1% loss, etc. 75.41.110.200 (talk) 21:29, 11 August 2009 (UTC)
- Wow, 200 proof ethanol is really expensive ... couldn't I get it at azeotrope concentration? I imagine that would cheapen things considerably. Or is azeotrope-distilled alcohol (without a bittering agent?) regulated? John Riemann Soong (talk) 20:08, 11 August 2009 (UTC)
- Aryl OH is harder to esterificy than alkyl OH because of the lower nucleophilicity of Ar-OH - caused by delocalisation of the O lone pairs onto the benzene ring - the same effect causes the higher acidity of phenols compared to alcoholes (in general , for the same type of atom, higher acidity = lower nucleophilicity)
- You can get enzymes to do almost anything - both oxidations and reductions - however for a reduction you'll probably need a reducing agent to 'feed' the enzyme.
- I think tannin esters would be too involatile to have a smell. For taste see Tannin#Foods with tannins
- 83.100.250.79 (talk) 21:04, 11 August 2009 (UTC)
How do I get Ajax out of a can?
I called the company's number and they claim it can't be done. They just sent me a coupon for a new can.
However, I have three or four cans that have something left in them, but at some point even banging the can against a hard surface didn't work.
Yes, the company advised me don't keep it in a moist environment. I don't know where else to keep it. I use it in the bathroom; therefore, it goes in the bathroom. My father used to keep and use it in the kitchen (which the company doesn't advise either). I use Lysol there.
I have a can of Comet--a big one--that may soon be in the same situation. I tried Lysol toilet bowl cleaner but all those warnings scare me. Besides, Comet and Ajax are cheaper--if I can use the whole can.Vchimpanzee · talk · contributions · 19:24, 11 August 2009 (UTC)
- Can you cut the can? Tin snips ought to go through them (I think it's really just a stiff cardboard can, right?) Nimur (talk) 20:17, 11 August 2009 (UTC)
- That might work. Yes, it is cardboard. The oldest one is bent completely out of shape.
- I was thinking about Acupuncture needles or something. Thanks.Vchimpanzee · talk · contributions · 20:43, 11 August 2009 (UTC)
- As an aside, it's probably not good that your bathrooms & kitchen are that humid - you're just asking for mold to grow there. If you have an extractor fan, you should probably turn it on more often than you currently do. SteveBaker (talk) 23:18, 11 August 2009 (UTC)
plant intelligence
What is the most intelligent plant in the world? -- Taxa (talk) 19:38, 11 August 2009 (UTC)
- Define "intelligent". Plants don't have a central nervous system, which is really a requirement for any common definition of intelligence that I know of. --Tango (talk) 19:43, 11 August 2009 (UTC)
- (ec) Define "intelligent". (Wow, Tango had exactly the same knee-jerk reaction as I did!) We really can't answer this question unless you have a specific idea about what intelligence means for an organism with no central nervous system. Maybe you would find tropism helpful - a lot of response to stimuli can occur without "thinking" - so there's sort of a biological Chinese room problem. Really sophisticated behavior might or might not be due to intelligence, depending how you want to define it. Nimur (talk) 19:47, 11 August 2009 (UTC)
- Response to stimuli. -- Taxa (talk) 20:44, 11 August 2009 (UTC)
- (ec) Define "intelligent". (Wow, Tango had exactly the same knee-jerk reaction as I did!) We really can't answer this question unless you have a specific idea about what intelligence means for an organism with no central nervous system. Maybe you would find tropism helpful - a lot of response to stimuli can occur without "thinking" - so there's sort of a biological Chinese room problem. Really sophisticated behavior might or might not be due to intelligence, depending how you want to define it. Nimur (talk) 19:47, 11 August 2009 (UTC)
- That's only one response to one stimuli in a millisecond time frame limit. What about sequences that occur over broader periods of time? -- Taxa (talk) 21:00, 11 August 2009 (UTC)
- Plants bend and twist to orient themselves to an available light source. Surely that qualifies as intelligence. See auxins. Bus stop (talk) 21:11, 11 August 2009 (UTC)
- (ec) Not what you're going to want to hear, but isn't, say, the growth of an oak tree over 50 years the result of a bunch of different stimuli? Roots grow around rocks in the ground, branches grow around any obstacles that exist, the acorns are produced at a certain time of the year, and it's all powered by photosynthesis which is 'stimulated' by sunlight. Tempshill (talk) 21:14, 11 August 2009 (UTC)
- You guys are overthinking it; the question is flawed. Vranak (talk) 21:45, 11 August 2009 (UTC)
- Overthinking! Refdeskers. Preposterous! ;-) Fribbler (talk) 23:30, 11 August 2009 (UTC)
Plants sleeping
Hello, I was wondering if someone could clear something up for me. It seems that most indoor plant growers have their lights on a timer. Is this to save electricity, or do plants do better if they have regular periods of darkness? Will plants that grow in perpetual light grow faster than ones with light and dark periods? Does this depend on the type of plant? TastyCakes (talk) 19:44, 11 August 2009 (UTC)
- It depends on the type of plant. Some plants undergo CAM photosynthesis, which means that certain chemical reactions prefer to take place at night (although a day cycle is needed to absorb the light for photosynthesis) - and the entire process is more efficient, even though only half of the reaction (day- or night- reaction) is happening at any given instant. Other plants can perform better if exposed to light for 24 hours/round-the-clock. Nimur (talk) 19:51, 11 August 2009 (UTC)
- Oh ok thanks a lot, do you know if there's anywhere I could get a list of plants that fall into each category? I'm mostly interested in things you can eat... TastyCakes (talk) 19:53, 11 August 2009 (UTC)
- Our article has a section and a list of common CAM plants; these include yucca, agave, many types of cacti (which may be edible), some Sagittaria tubers, and others. Nimur (talk) 20:00, 11 August 2009 (UTC)
- Hmm ok, so typical vegetables (lettuce, carrots, tomatoes etc) are more successful in constant light? TastyCakes (talk) 20:48, 11 August 2009 (UTC)
- Our article has a section and a list of common CAM plants; these include yucca, agave, many types of cacti (which may be edible), some Sagittaria tubers, and others. Nimur (talk) 20:00, 11 August 2009 (UTC)
- Oh ok thanks a lot, do you know if there's anywhere I could get a list of plants that fall into each category? I'm mostly interested in things you can eat... TastyCakes (talk) 19:53, 11 August 2009 (UTC)
Does this sound right to you?
"According to the USDADietary Guidelines, one serving of fruit is equal to one half cup. This measurement is based on fresh fruit cut in typical slices or chunks. Of course, by cutting the fruit into small bits like we do in Fruit2day, we can fit the equivalent of a full serving into a smaller space. That’s why even though a bottle of Fruit2day is only 6.75 fluid ounces, it still contains two complete servings of natural fruit goodness."[26] Imagine Reason (talk) 20:26, 11 August 2009 (UTC)
- It is certainly true that cutting something into smaller pieces usually allows you to fit it into a smaller space since you end up with less air around the pieces. Whether a bottle of Fruit2day really counts as two servings, I have no idea, but it is entirely possible. --Tango (talk) 20:40, 11 August 2009 (UTC)
- Could also be somewhat concentrated, having a higher nutrient to volume ratio then 1 cup of fresh fruit would. Googlemeister (talk) 20:46, 11 August 2009 (UTC)
- Or it may be older and some of the more volatile things might have decayed into less nutritious stuff. But the USDA guidelines are pretty fuzzy. It's never clear what they mean when they specify stuff like that by volume rather than weight. Also - which fruit? I'm pretty sure there are more nutrients in something dense like (say) a peach than in (say) a watermelon. The watermelon is almost all water - the peach has much less. Is a half-cup of watermelon enough? Is a half cup of peach more than you need? Given how fuzzy those terms are, I'd be very wary of company claims for being significantly more nutritionally dense than "real" fruit. SteveBaker (talk) 23:15, 11 August 2009 (UTC)
- Very true, the guidelines are kept intentionally simple, but that means they are of limited use. When you start trying to use them for something they weren't designed for, your results will be highly questionable. The guidelines are designed for fresh fruit, so using them for something like Fruit2day is problematic. --Tango (talk) 23:20, 11 August 2009 (UTC)
- Or it may be older and some of the more volatile things might have decayed into less nutritious stuff. But the USDA guidelines are pretty fuzzy. It's never clear what they mean when they specify stuff like that by volume rather than weight. Also - which fruit? I'm pretty sure there are more nutrients in something dense like (say) a peach than in (say) a watermelon. The watermelon is almost all water - the peach has much less. Is a half-cup of watermelon enough? Is a half cup of peach more than you need? Given how fuzzy those terms are, I'd be very wary of company claims for being significantly more nutritionally dense than "real" fruit. SteveBaker (talk) 23:15, 11 August 2009 (UTC)
- Could also be somewhat concentrated, having a higher nutrient to volume ratio then 1 cup of fresh fruit would. Googlemeister (talk) 20:46, 11 August 2009 (UTC)
- What's the question? "does this sound right?" No, your post sounds like spam to me. "Natural fruit goodness"? No one asking a question talks like this. Vespine (talk) 23:23, 11 August 2009 (UTC)
- That's a quote from the linked website, it's not the OP's own words. --Tango (talk) 23:42, 11 August 2009 (UTC)
elemental isotopes
Is our current scientific understanding of isotopes of heavier atoms such that the % of each stable isotope of an element is even throughout the local part of the galaxy? What I mean is if we were to theoretically travel to a planet 100 ly away which had a surface of 100% water, could we expect to find 99.762% O16, the same as in the water on earth, or are those % dictated by the local star? Googlemeister (talk) 20:55, 11 August 2009 (UTC)
- The heavier elements are made inside stars - it seems likely that the isotope makeup could differ depending on the nature of the star that made those heavier atoms. In many cases, an odd-ball isotope of a stable atom will have come about through the decay of heavier, more unstable elements. Since the predominance of those must certainly depend on the history of the recent stellar neighbourhood - I'd guess it quite likely that there would be wide variation from place to place in the galaxy. SteveBaker (talk) 23:10, 11 August 2009 (UTC)
- 100ly away, maybe, it might be orbiting a star that formed from the same nebula as the Sun, so would have similar proportions. At greater distances, Steve's points mean I would expect significant variation. The stars that formed in the same area as the Sun will have spread out quite a bit by now, but their distance from the centre of the galaxy should be fairly similar. --Tango (talk) 23:18, 11 August 2009 (UTC)
The isotopic makeup of some gases is different between the atmospheres of Earth and Mars -- I assume the mechanism is that isotopes diffuse into space unequally and in a way that depends on the air pressure and gravity. This difference is one of the ways that it was confirmed that Mars meteorites on the Earth had in fact traveled from Mars. --Anonymous, 23:32 UTC, August 11, 2009.
Broken stereo?
I have a stereo system hooked up to my TV, digital converter box, and DVD player. It is at least 15 years old. Yesterday, after we lost power during a thunderstorm (lightning struck pretty close and took out our cable too), the stereo stopped working. Whenever we turn it on, it looks like it works, but then after a second or two, it turns itself off suddenly. Is the thing history, or is this a pretty simple problem to solve? Thanks —Akrabbimtalk 21:49, 11 August 2009 (UTC)
- Probably history. Modern consumer electronics are pretty much disposable once the warranties have run out - the cost of repairing them is normally such that it's more cost effective to replace them with a brand new warrantied model. Also after fifteen years parts and service manuals may be difficult to come by. Before you look at repairing, check the price of replacing with an equivalent new unit - if a new one costs $200 and it will cost $100 to repair the fifteen year old one it's really not worth repairing. Exxolon (talk) 22:47, 11 August 2009 (UTC)
- It doesn't sound easy. Easy things are broken wires and blown fuses - but if it were either of those, it would probably be just completely dead. As a matter of course, I'd pull the lid off and see if there is anything obvious such as a blackened smudge around a particular component or a wire that looks singed. The odds are fairly remote that it'll be anything that obvious though. You might be able to trick it into working by playing with the controls during the second or two it does stay on...maybe if it has some kind of a setup menu, you could get to a "reset" entry or something and reset it before it craps out. All of these are very long shots though. In all likelyhood it has a fried memory chip or something. The computer inside powers up - checks the memory, finds that nothing makes sense and just shuts down again. SteveBaker (talk) 22:48, 11 August 2009 (UTC)
- I agree, it sounds dead. Turning on for a second or do and then turning itself off sounds like the firmware is fried. It is possible you can reset it somehow, but you will probably have to get a licensed repair firm to do it and they will charge more than a 15 year old stereo is worth. --Tango (talk) 23:04, 11 August 2009 (UTC)
Herbicide
Is anyone aware of a weed killer that would prevent plant growth for a month or more? Someone vandalised my garden on the 4th July weekend, using some sort of spray, and even the weeds haven't started growing yet. The spray was applied quite locally: weeds have been growing between the lines of dead plants for a couple of weeks now. The only other thing that I know about it is that it kills what it contacts: some plants were only partially killed, such as a tomato that was mostly blackened but produced a ripe tomato just a week ago. 24.93.116.128 (talk) 21:50, 11 August 2009 (UTC)
- I don't know about herbicides, but when you mentioned the date, and then the blackened plant, I think of burning. Could there have been fireworks nearby, with someone's rocket coming down in your garden?? You don't say what evidence you used to know it was vandalized then, after all. Of course, I'm not sure if that would have an impact or not.Somebody or his brother (talk) 22:28, 11 August 2009 (UTC)
- You wouldn't get lines of dead plants from a rocket, would you? --Tango (talk) 23:06, 11 August 2009 (UTC)
Thermal cleaning of high carbon steel
In our research we need to reduce organic contaminants to a very low level. With aluminum implements, we routinely do this by baking them in air at a few hundred degrees C to force any organic compounds to boil away as CO2 and similar gases. (Note that this is not just about killing microbes, but actually removing their organic residues.)
Now I am trying to do something similar with a new tool that is high carbon steel. However, if I use the same settings as I do for aluminum, the iron in the steel will oxide (essentially causing the tool to rust). Obviously that's no good. Does anyone know how hot one can make steel before it begins to oxide significantly? Dragons flight (talk) 23:41, 11 August 2009 (UTC)















































