Sulazepam: Difference between revisions
Appearance
Content deleted Content added
m Bot: fix deprecated Citation Style 1 parameters (Task 9) |
Argenteus.CG (talk | contribs) No edit summary |
||
| Line 40: | Line 40: | ||
}} |
}} |
||
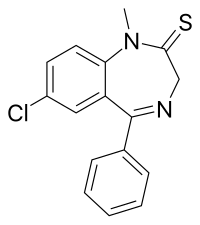
'''Sulazepam''' is a [[benzodiazepine]] derivative. It is the [[thioamide]] derivative of [[diazepam]]. It is metabolised into [[diazepam]], [[desmethyldiazepam]] and [[temazepam|oxydiazepam]]. It has [[sedative]], [[muscle relaxant]], [[hypnotic]], [[anticonvulsant]] and [[anxiolytic]] properties like those of other benzodiazepines.<ref>{{Cite web | url = http://www.psychotropics.dk/moleculeView/default.aspx?ID=1444&Catalogtype=A&ChapterID=1&Thissortorder=41 | title = sulazepam | accessdate = 29 December 2008 | year = 2003 | publisher = psychotropics.dk }}</ref><ref>{{cite journal |author=Golovenko NIa, Zin'kovskii VG |title=(title in Russian) |trans-title=Analysis of the structure of the components of the convulsive action of corazole following administration of sulazepam and its metabolites to mice |language=Russian |journal=Biull Eksp Biol Med |volume=82 |issue=9 |pages=1078–1081 |date=September 1976 |pmid=11012 |doi= |url=}}</ref> It was never marketed. |
'''Sulazepam''' is a [[benzodiazepine]] derivative. It is the [[thioamide]] derivative of [[diazepam]]. It is metabolised into [[diazepam]], [[desmethyldiazepam]] and [[temazepam|oxydiazepam]]{{Citation needed|date=June 2018}}. It has [[sedative]], [[muscle relaxant]], [[hypnotic]], [[anticonvulsant]] and [[anxiolytic]] properties like those of other benzodiazepines.<ref>{{Cite web | url = http://www.psychotropics.dk/moleculeView/default.aspx?ID=1444&Catalogtype=A&ChapterID=1&Thissortorder=41 | title = sulazepam | accessdate = 29 December 2008 | year = 2003 | publisher = psychotropics.dk }}</ref><ref>{{cite journal |author=Golovenko NIa, Zin'kovskii VG |title=(title in Russian) |trans-title=Analysis of the structure of the components of the convulsive action of corazole following administration of sulazepam and its metabolites to mice |language=Russian |journal=Biull Eksp Biol Med |volume=82 |issue=9 |pages=1078–1081 |date=September 1976 |pmid=11012 |doi= |url=}}</ref> It was never marketed. |
||
==Synthesis== |
==Synthesis== |
||
Revision as of 17:35, 12 June 2018
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H13ClN2S |
| Molar mass | 300.81 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Sulazepam is a benzodiazepine derivative. It is the thioamide derivative of diazepam. It is metabolised into diazepam, desmethyldiazepam and oxydiazepam[citation needed]. It has sedative, muscle relaxant, hypnotic, anticonvulsant and anxiolytic properties like those of other benzodiazepines.[1][2] It was never marketed.
Synthesis

Treatment of diazepam with phosphorus pentasulfide produces the corresponding thionamide, sulazepam.
See also
References
- ^ "sulazepam". psychotropics.dk. 2003. Retrieved 29 December 2008.
- ^ Golovenko NIa, Zin'kovskii VG (September 1976). "(title in Russian)" [Analysis of the structure of the components of the convulsive action of corazole following administration of sulazepam and its metabolites to mice]. Biull Eksp Biol Med (in Russian). 82 (9): 1078–1081. PMID 11012.
- ^ Archer, G. A.; Sternbach, L. H. (1964). "Quinazolines and 1,4-Benzodiazepines. XVI.1Synthesis and Transformations of 5-Phenyl-1,4-benzodiazepine-2-thiones". The Journal of Organic Chemistry. 29: 231. doi:10.1021/jo01024a511.
