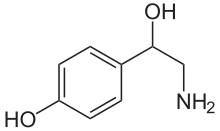
Octopamine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | OCT, Norsympathol, Norsynephrine, para-Octopamine, beta-Hydroxytyramine, para-hydroxy-phenyl-ethanolamine, α-(Aminomethyl)-4 hydroxybenzenemethanol, 1-(p-Hydroxyphenyl)-2-aminoethanol |
| Routes of administration | Oral |
| ATC code | |
| Physiological data | |
| Source tissues | invertebrate nervous systems; trace amine in vertebrates |
| Target tissues | system-wide in invertebrates |
| Receptors | TAAR1 (mammals) OctαR, OctβR, TyrR (invertebrates), Oct-TyrR |
| Agonists | Formamidines (amitraz (AMZ) and chlordimeform (CDM)) |
| Antagonists | epinastine (3-amino-9, 13b-dihydro-1H-dibenz(c,f)imidazo(1,5a)azepine hydrochloride) |
| Precursor | tyramine |
| Biosynthesis | tyramine β-hydroxylase; dopamine β-hydroxylase |
| Metabolism | p-hydroxymandelic acid;[1][2] N-acetyltransferases; phenylethanolamine N-methyltransferase |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 99.42 % |
| Metabolism | p-hydroxymandelic acid;[1][2] N-acetyltransferases; phenylethanolamine N-methyltransferase |
| Elimination half-life | 15 minutes in insects. Between 76 and 175 minutes in humans |
| Excretion | Up to 93% of ingested octopamine is eliminated via the urinary route within 24 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.002.890 |
| Chemical and physical data | |
| Formula | C8H11NO2 |
| Molar mass | 153.181 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Octopamine (molecular formula C8H11NO2; also known as OA, and also norsynephrine, para-octopamine and others) is an organic chemical closely related to norepinephrine, and synthesized biologically by a homologous pathway. Octopamine is often considered the major "fight-or-flight" neurohormone of invertebrates. Its name is derived from the fact that it was first identified in the salivary glands of the octopus.
In many types of invertebrates octopamine is an important neurotransmitter and hormone. In protostomes — arthropods, molluscs, and several types of worms — it substitutes for norephinephrine and performs functions apparently similar to those of norepinephrine in mammals, functions that have been described as mobilizing the body and nervous system for action. In mammals octopamine is found only in trace amounts, and no biological function has been solidly established for it. It is also found naturally in numerous plants, including bitter orange.[3][4] Octopamine has been sold under trade names such as Epirenor, Norden, and Norfen for use as a sympathomimetic drug, available by prescription.
Functions
Cellular effects
Octopamine exerts its effects by binding to and activating receptors located on the surface of cells. These receptors have mainly been studied in insects, where they can be divided into distinct types:
- OctαR (alpha-adrenergic-like), are structurally and functionally similar to noradrenergic alpha-1 receptors in mammals. There are multiple subtypes of the OctαR receptor. For example, the kissing bug (Rhodnius prolixus) has Octα1-R, Octα2R.[5]
- OctβR (beta-adrenergic-like), are structurally and functionally similar to noradrenergic beta receptors in mammals. There are multiple subtypes of the OctβR receptor. For example, the fruit fly (Drosophila melanogaster) has DmOctβ1R, DmOctβ2R, and DmOctβ3R.[6]
- OAMB. The diversity of this receptor is relatively unknown. The fruit fly (Drosophila melanogaster) has two distinct isoforms which are functionally distinct: OambK3 and OambAS.[7]
- TyrR (mixed octopamine/tyramine receptors), which are structurally and functionally similar to noradrenergic alpha-2 receptors in mammals.[8] Receptors in the TyrR class, however, are generally more strongly activated by tyramine than by octopamine.[8]
Phylogenetic studies claim that in ancient bilaterians such as Platynereis dumerilii there is a co-existence of norepinephrine, tyramine and octopamine receptor signaling. However, due to partial overlapping in their signalling functionality tyramine and octopamine receptors have been lost in vertebrates.[9]
In vertebrates no octopamine-specific receptors have been identified. Octopamine binds weakly to receptors for norepinephrine and epinephrine, but it is not clear whether this has any functional significance. It binds more strongly to trace amine-associated receptors (TAARs), especially TAAR1.[8]
Invertebrates
Octopamine was first discovered by Italian scientist Vittorio Erspamer in 1948[10] in the salivary glands of the octopus and has since been found to act as a neurotransmitter, neurohormone and neuromodulator in invertebrates. Although Erspamer discovered its natural occurrence and named it, octopamine had actually existed for many years as a pharmaceutical product.[11] It is widely used in energy-demanding behaviors by all insects, crustaceans (crabs, lobsters, crayfish), and spiders. Such behaviors include modulating muscle tension,[12] flying,[13] ovulation and egg-laying,[14][15][16][17][18][19][20] and jumping.[21][22]
Octopamine in non-insect invertebrates
In lobsters, octopamine seems to direct and coordinate neurohormones to some extent in the central nervous system, and it was observed that injecting octopamine into a lobster and crayfish resulted in limb and abdomen extension.[23]
In the nematode, octopamine is found in high concentrations in adults, decreasing egg-laying and pharyngeal pumping behaviors with an antagonistic effect to serotonin.[24]
Octopaminergic nerves in the mollusc may be present in the heart, with high concentrations in the nervous system.[25]
In larvae of the oriental armyworm, octopamine is immunologically beneficial, increasing survival rates in high-density populations.[26]
Octopamine in non-Drosophila insects
In insects, octopamine is released by a select number of neurons, but acts broadly throughout the central brain, on all sense organs, and on several non-neuronal tissues.[27][28] In the thoracic ganglia, octopamine is primarily released by DUM (dorsal unpaired median) and VUM (ventral unpaired median) neurons, which release octopamine onto neural, muscular, and peripheral targets.[29][30] These neurons are important for mediating energy-demanding motor behaviors, such as escape-induced jumping and flight. For example, the locust DUMeti neuron releases octopamine onto the extensor tibia muscle to increase muscle tension and increase relaxation rate. These actions promote efficient leg muscle contraction for jumping.[27] During flight, DUM neurons are also active and release octopamine throughout the body to synchronize energy metabolism, respiration, muscle activity and flight interneuron activity.[13] Octopamine in locusts is four times more concentrated in the axon than in the soma, and decreases the locust's myogenic rhythm.[31]
In the honey bee, octopamine has a major role in learning and memory. In the firefly, octopamine release leads to light production in the lantern.[32][33]
The emerald cockroach wasp stings the host for its larvae (a cockroach) in the head ganglion (brain). The venom blocks octopamine receptors[34] and the cockroach fails to show normal escape responses, grooming itself excessively. It becomes docile and the wasp leads it to the wasp's den by pulling its antenna like a leash.[35]
Octopamine in Drosophila
Octopamine effects almost every process of the fruit fly and is widely present in both the adult and larval fly. A non-exhaustive list of some of the areas in which Octopamine modulates:
- Learning and memory[36][37][38]
- Ovulation and Egg-Laying[14][15][16][17][18][19][20]
- Locomotion[39][40][41]
- Muscle Physiology[42][43]
- Aggression [44][45][46]
- Alcohol and drug tolerance[47][48][49][50]
- Feeding[51][52]
- Microbiome and gut physiology[53][54]
- Sleep [55][56]
- Modulating effects of exercise [57][58]
- Metabolism[59][60]
Vertebrates
In vertebrates, octopamine replaces norepinephrine in sympathetic neurons with chronic use of monoamine oxidase inhibitors. It may be responsible for the common side effect of orthostatic hypotension with these agents, though there is also evidence that it is actually mediated by increased levels of N-acetylserotonin.
One study noted that octopamine might be an important amine that influences the therapeutic effects of inhibitors such as monoamine oxidase inhibitors, especially because a large increase in octopamine levels was observed when animals were treated with this inhibitor. Octopamine was positively identified in the urine samples of mammals such as humans, rats, and rabbits treated with monoamine oxidase inhibitors. Very small amounts of octopamine were also found in certain animal tissues. It was observed that within a rabbit's body, the heart and kidney held the highest concentrations of octopamine. Octopamine was found to be 93% eluted by urine within 24 hours of being produced in the body as a byproduct of Iproniazid in rabbits.[11]
Pharmacology
Octopamine has been sold under trade names such as Epirenor, Norden, and Norfen for use in medicine as a sympathomimetic drug, available by prescription. Very little information exists concerning its clinical usefulness or safety, though.[61]
In mammals, octopamine may mobilize the release of fat from adipocytes (fat cells), which has led to its promotion on the internet as a slimming aid. However, the released fat is likely to be promptly taken up into other cells, and there is no evidence that octopamine facilitates weight loss. Octopamine may also increase blood pressure significantly when combined with other stimulants, as in some weight loss supplements.[62]
The World Anti-Doping Agency lists octopamine as a banned substance for in competition use, as a "specified stimulant"[63] on the 2019 Prohibited List.
Insecticides
The octopamine receptor is a target of insecticides, as its blockage leads to decreased cAMP levels. Essential oils can have such a neuro-insecticidal effect,[64] and this octopamine-receptor mechanism is naturally utilized by plants with active insecticidal phytochemicals.[65]
Biochemical mechanisms
Mammals
Octopamine is one of four primary endogenous agonists of human trace amine-associated receptor 1 together with 3-iodothyronamine, dopamine and tyramine.[66][67]
Invertebrates
Octopamine binds to its respective G-protein coupled receptors (GPCRs) to initiate a cell signal transduction pathway. At least three groups of octopamine GPCR have been defined. OctαR (OCTOPAMINE1 receptors) are more closely related to α-adrenergic receptors, while OctβR (OCTOPAMINE2 receptors) are more closely related to β-adrenergic receptors. The Octopamine/Tyramine receptors (including Oct-TyrR) can bind both ligands, and display agonist-specific coupling. Oct-TyrR is listed in both OCTOPAMINE and TYRAMINE RECEPTORS gene groups.[68]
Biosynthesis
In insects
Octopamine acts as the insect equivalent of norepinephrine and has been implicated in regulating aggression in invertebrates, with different effects on different species. Studies have shown that reducing the neurotransmitter octopamine and preventing coding of tyramine beta hydroxylase (an enzyme that converts tyramine to octopamine) decreases aggression in Drosophila without influencing other behaviors.[69]

In humans
See also
References
- ^ a b Hengstmann, J. H.; Konen, W; Konen, C; Eichelbaum, M; Dengler, H. J. (1974). "The physiological disposition of p-octopamine in man". Naunyn-Schmiedeberg's Archives of Pharmacology. 283 (1): 93–106. doi:10.1007/bf00500148. PMID 4277715. S2CID 35523412.
- ^ d’Andrea, Giovanni; Nordera, Gianpietro; Pizzolato, Gilberto; Bolner, Andrea; Colavito, Davide; Flaibani, Raffaella; Leon, Alberta (2010). "Trace amine metabolism in Parkinson's disease: Low circulating levels of octopamine in early disease stages". Neuroscience Letters. 469 (3): 348–51. doi:10.1016/j.neulet.2009.12.025. PMID 20026245. S2CID 12797090.
- ^ Tang F, Tao L, Luo X, Ding L, Guo M, Nie L, Yao S (September 2006). "Determination of octopamine, synephrine and tyramine in Citrus herbs by ionic liquid improved 'green' chromatography". Journal of Chromatography A. 1125 (2): 182–8. doi:10.1016/j.chroma.2006.05.049. PMID 16781718.
- ^ Jagiełło-Wójtowicz E (1979). "Mechanism of central action of octopamine". Polish Journal of Pharmacology and Pharmacy. 31 (5): 509–16. PMID 121158.
- ^ Hana, Sam; Lange, Angela B. (26 September 2017). "Cloning and Functional Characterization of Octβ2-Receptor and Tyr1-Receptor in the Chagas Disease Vector, Rhodnius prolixus". Frontiers in Physiology. 8: 744. doi:10.3389/fphys.2017.00744. ISSN 1664-042X. PMC 5623054. PMID 29018364.
- ^ Maqueira, Braudel; Chatwin, Heather; Evans, Peter D. (15 June 2005). "Identification and characterization of a novel family of Drosophilaβ-adrenergic-like octopamine G-protein coupled receptors: Insect β-adrenergic-like octopamine receptors". Journal of Neurochemistry. 94 (2): 547–560. doi:10.1111/j.1471-4159.2005.03251.x. PMID 15998303. S2CID 83666118.
- ^ Lee, Hyun-Gwan; Rohila, Suman; Han, Kyung-An (5 March 2009). "The Octopamine Receptor OAMB Mediates Ovulation via Ca2+/Calmodulin-Dependent Protein Kinase II in the Drosophila Oviduct Epithelium". PLOS ONE. 4 (3): e4716. Bibcode:2009PLoSO...4.4716L. doi:10.1371/journal.pone.0004716. ISSN 1932-6203. PMC 2650798. PMID 19262750.
- ^ a b c Pflüger HJ, Stevensonb PA (2005). "Evolutionary aspects of octopaminergic systems with emphasis on arthropods". Arthropod Structure & Development. 34 (3): 379–396. doi:10.1016/j.asd.2005.04.004.
- ^ Bauknecht, Philipp; Jékely, Gáspár (2017). "Ancient coexistence of norepinephrine, tyramine, and octopamine signaling in bilaterians". BMC Biology. 15 (1): 6. doi:10.1186/s12915-016-0341-7. PMC 5282848. PMID 28137258.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Erspamer, V. (2009). "Active Substances in the Posterior Salivary Glands of Octopoda. II. Tyramine and Octopamine (Oxyoctopamine)". Acta Pharmacologica et Toxicologica. 4 (3–4): 224–47. doi:10.1111/j.1600-0773.1948.tb03345.x.
- ^ a b Kakimoto Y, Armstrong MD (February 1962). "On the identification of octopamine in mammals". The Journal of Biological Chemistry. 237 (2): 422–7. doi:10.1016/S0021-9258(18)93937-2. PMID 14453200.
- ^ Ormerod, Kiel G.; Hadden, Julia K.; Deady, Lylah D.; Mercier, A. Joffre; Krans, Jacob L. (15 October 2013). "Action of octopamine and tyramine on muscles of Drosophila melanogaster larvae". Journal of Neurophysiology. 110 (8): 1984–1996. doi:10.1152/jn.00431.2013. hdl:10464/6361. ISSN 0022-3077. PMID 23904495.
- ^ a b Orchard, I; Ramirez, J M; Lange, A B (January 1993). "A Multifunctional Role for Octopamine in Locust Flight". Annual Review of Entomology. 38 (1): 227–249. doi:10.1146/annurev.en.38.010193.001303. ISSN 0066-4170.
- ^ a b Lee, Hyun-Gwan; Seong, Chang-Soo; Kim, Young-Cho; Davis, Ronald L; Han, Kyung-An (1 December 2003). "Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster". Developmental Biology. 264 (1): 179–190. doi:10.1016/j.ydbio.2003.07.018. ISSN 0012-1606. PMID 14623240.
- ^ a b Li, Yong; Fink, Christine; El-Kholy, Samar; Roeder, Thomas (March 2015). "THE OCTOPAMINE RECEPTOR octß2R IS ESSENTIAL FOR OVULATION AND FERTILIZATION IN THE FRUIT FLY Drosophila melanogaster: octß2R is essential for ovulation". Archives of Insect Biochemistry and Physiology. 88 (3): 168–178. doi:10.1002/arch.21211. PMID 25353988.
- ^ a b Lim, Junghwa; Sabandal, Paul R.; Fernandez, Ana; Sabandal, John Martin; Lee, Hyun-Gwan; Evans, Peter; Han, Kyung-An (6 August 2014). Broughton, Susan (ed.). "The Octopamine Receptor Octβ2R Regulates Ovulation in Drosophila melanogaster". PLOS ONE. 9 (8): e104441. Bibcode:2014PLoSO...9j4441L. doi:10.1371/journal.pone.0104441. ISSN 1932-6203. PMC 4123956. PMID 25099506.
- ^ a b Lee, Hyun-Gwan; Rohila, Suman; Han, Kyung-An (5 March 2009). Louis, Matthieu (ed.). "The Octopamine Receptor OAMB Mediates Ovulation via Ca2+/Calmodulin-Dependent Protein Kinase II in the Drosophila Oviduct Epithelium". PLOS ONE. 4 (3): e4716. Bibcode:2009PLoSO...4.4716L. doi:10.1371/journal.pone.0004716. ISSN 1932-6203. PMC 2650798. PMID 19262750.
- ^ a b Lee, Hyun-Gwan; Seong, Chang-Soo; Kim, Young-Cho; Davis, Ronald L; Han, Kyung-An (December 2003). "Octopamine receptor OAMB is required for ovulation in Drosophila melanogaster". Developmental Biology. 264 (1): 179–190. doi:10.1016/j.ydbio.2003.07.018. PMID 14623240.
- ^ a b Monastirioti, Maria (December 2003). "Distinct octopamine cell population residing in the CNS abdominal ganglion controls ovulation in Drosophila melanogaster". Developmental Biology. 264 (1): 38–49. doi:10.1016/j.ydbio.2003.07.019. PMID 14623230.
- ^ a b Deady, Lylah D.; Sun, Jianjun (16 October 2015). Wolfner, Mariana Federica (ed.). "A Follicle Rupture Assay Reveals an Essential Role for Follicular Adrenergic Signaling in Drosophila Ovulation". PLOS Genetics. 11 (10): e1005604. doi:10.1371/journal.pgen.1005604. ISSN 1553-7404. PMC 4608792. PMID 26473732.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Pollack, Alan J.; Ritzmann, Roy E.; Westin, Joanne (1988). "Activation of DUM cell interneurons by ventral giant interneurons in the cockroach, periplaneta americana". Journal of Neurobiology. 19 (6): 489–497. doi:10.1002/neu.480190602. ISSN 1097-4695. PMID 3171574.
- ^ Orchard, Ian (1 April 1982). "Octopamine in insects: neurotransmitter, neurohormone, and neuromodulator". Canadian Journal of Zoology. 60 (4): 659–669. doi:10.1139/z82-095. ISSN 0008-4301.
- ^ Livingstone MS, Harris-Warrick RM, Kravitz EA (April 1980). "Serotonin and octopamine produce opposite postures in lobsters". Science. 208 (4439): 76–9. Bibcode:1980Sci...208...76L. doi:10.1126/science.208.4439.76. PMID 17731572. S2CID 32141532.
- ^ Horvitz, H. R.; Chalfie, M.; Trent, C.; Sulston, J. E.; Evans, P. D. (28 May 1982). "Serotonin and octopamine in the nematode Caenorhabditis elegans". Science. 216 (4549): 1012–1014. Bibcode:1982Sci...216.1012H. doi:10.1126/science.6805073. ISSN 0036-8075. PMID 6805073.
- ^ Dougan, D. F. H.; Duffield, P. H.; Wade, D. N.; Duffield, A. M. (1 January 1981). "Occurrence and synthesis of octopamine in the heart and ganglia of the mollusc Tapes watlingi". Comparative Biochemistry and Physiology Part C: Comparative Pharmacology. 70 (2): 277–280. doi:10.1016/0306-4492(81)90064-2. ISSN 0306-4492.
- ^ Kong, Hailong; Yuan, Lin; Dong, Chuanlei; Zheng, Minyuan; Jing, Wanghui; Tian, Zhen; et al. (December 2020). "Immunological regulation by a β-adrenergic-like octopamine receptor gene in crowded larvae of the oriental Armyworm, Mythmina separata". Developmental and Comparative Immunology. 113: 103802. doi:10.1016/j.dci.2020.103802. ISSN 1879-0089. PMID 32712170. S2CID 220797641.
- ^ a b Atwood, H. L.; Klose, M. K. (1 January 2009), "Neuromuscular Transmission Modulation at Invertebrate Neuromuscular Junctions", in Squire, Larry R. (ed.), Encyclopedia of Neuroscience, Oxford: Academic Press, pp. 671–690, ISBN 978-0-08-045046-9, retrieved 10 July 2020
- ^ Roeder, T. (December 1999). "Octopamine in invertebrates". Progress in Neurobiology. 59 (5): 533–561. doi:10.1016/s0301-0082(99)00016-7. ISSN 0301-0082. PMID 10515667. S2CID 25654298.
- ^ Eckert, Manfred; Rapus, Jürgen; Nürnberger, Asja; Penzlin, Heinz (1992). "A new specific antibody reveals octopamine-like immunoreactivity in cockroach ventral nerve cord". Journal of Comparative Neurology (in French). 322 (1): 1–15. doi:10.1002/cne.903220102. ISSN 1096-9861. PMID 1430305. S2CID 41099770.
- ^ Sinakevitch, Irina G.; Geffard, Michel; Pelhate, Marcel; Lapied, Bruno (April 1994). "Octopamine-like immunoreactivity in the dorsal unpaired median (DUM) neurons innervating the accessory gland of the male cockroach Periplaneta americana". Cell and Tissue Research. 276 (1): 15–21. doi:10.1007/bf00354779. ISSN 0302-766X. S2CID 23485136.
- ^ Evans, P. D.; O'Shea, M. (April 1978). "The identification of an octopaminergic neurone and the modulation of a myogenic rhythm in the locust". The Journal of Experimental Biology. 73: 235–260. doi:10.1242/jeb.73.1.235. ISSN 0022-0949. PMID 25941.
- ^ Greenfield MD (November 2001). "Missing link in firefly bioluminescence revealed: NO regulation of photocyte respiration". BioEssays. 23 (11): 992–5. doi:10.1002/bies.1144. PMID 11746215.
- ^ Trimmer BA, Aprille JR, Dudzinski DM, Lagace CJ, Lewis SM, Michel T, et al. (June 2001). "Nitric oxide and the control of firefly flashing". Science. 292 (5526): 2486–8. doi:10.1126/science.1059833. PMID 11431567. S2CID 1095642.
- ^ Hopkin, Michael (2007). "How to make a zombie cockroach". Nature. doi:10.1038/news.2007.312.
- ^ Gal R, Rosenberg LA, Libersat F (December 2005). "Parasitoid wasp uses a venom cocktail injected into the brain to manipulate the behavior and metabolism of its cockroach prey". Archives of Insect Biochemistry and Physiology. 60 (4): 198–208. doi:10.1002/arch.20092. PMID 16304619.
- ^ Sabandal, John Martin; Sabandal, Paul Rafael; Kim, Young-Cho; Han, Kyung-An (20 May 2020). "Concerted Actions of Octopamine and Dopamine Receptors Drive Olfactory Learning". The Journal of Neuroscience. 40 (21): 4240–4250. doi:10.1523/JNEUROSCI.1756-19.2020. ISSN 0270-6474. PMC 7244198. PMID 32277043.
- ^ Burke, Christopher J.; Huetteroth, Wolf; Owald, David; Perisse, Emmanuel; Krashes, Michael J.; Das, Gaurav; Gohl, Daryl; Silies, Marion; Certel, Sarah; Waddell, Scott (December 2012). "Layered reward signalling through octopamine and dopamine in Drosophila". Nature. 492 (7429): 433–437. Bibcode:2012Natur.492..433B. doi:10.1038/nature11614. ISSN 0028-0836. PMC 3528794. PMID 23103875.
- ^ Schwaerzel, Martin; Monastirioti, Maria; Scholz, Henrike; Friggi-Grelin, Florence; Birman, Serge; Heisenberg, Martin (19 November 2003). "Dopamine and Octopamine Differentiate between Aversive and Appetitive Olfactory Memories in Drosophila". The Journal of Neuroscience. 23 (33): 10495–10502. doi:10.1523/JNEUROSCI.23-33-10495.2003. ISSN 0270-6474. PMC 6740930. PMID 14627633.
- ^ Schützler, Natalie; Girwert, Chantal; Hügli, Isabell; Mohana, Giriram; Roignant, Jean-Yves; Ryglewski, Stefanie; Duch, Carsten (26 February 2019). "Tyramine action on motoneuron excitability and adaptable tyramine/octopamine ratios adjust Drosophila locomotion to nutritional state". Proceedings of the National Academy of Sciences. 116 (9): 3805–3810. doi:10.1073/pnas.1813554116. ISSN 0027-8424. PMC 6397572. PMID 30808766.
- ^ Selcho, Mareike; Pauls, Dennis; el Jundi, Basil; Stocker, Reinhard F.; Thum, Andreas S. (1 November 2012). "The Role of octopamine and tyramine in Drosophila larval locomotion". The Journal of Comparative Neurology. 520 (16): 3764–3785. doi:10.1002/cne.23152. PMID 22627970. S2CID 17014658.
- ^ Saraswati, Sudipta; Fox, Lyle E.; Soll, David R.; Wu, Chun-Fang (March 2004). "Tyramine and octopamine have opposite effects on the locomotion ofDrosophila larvae". Journal of Neurobiology. 58 (4): 425–441. doi:10.1002/neu.10298. ISSN 0022-3034. PMID 14978721.
- ^ Ormerod, Kiel G.; Hadden, Julia K.; Deady, Lylah D.; Mercier, A. Joffre; Krans, Jacob L. (15 October 2013). "Action of octopamine and tyramine on muscles of Drosophila melanogaster larvae". Journal of Neurophysiology. 110 (8): 1984–1996. doi:10.1152/jn.00431.2013. hdl:10464/6361. ISSN 0022-3077. PMID 23904495.
- ^ Ormerod, Kiel G.; Jung, JaeHwan; Mercier, A. Joffre (3 July 2018). "Modulation of neuromuscular synapses and contraction in Drosophila 3rd instar larvae". Journal of Neurogenetics. 32 (3): 183–194. doi:10.1080/01677063.2018.1502761. ISSN 0167-7063. PMID 30303434. S2CID 52948972.
- ^ Andrews, Jonathan C.; Fernández, María Paz; Yu, Qin; Leary, Greg P.; Leung, Adelaine K. W.; Kavanaugh, Michael P.; Kravitz, Edward A.; Certel, Sarah J. (22 May 2014). Clandinin, Thomas (ed.). "Octopamine Neuromodulation Regulates Gr32a-Linked Aggression and Courtship Pathways in Drosophila Males". PLOS Genetics. 10 (5): e1004356. doi:10.1371/journal.pgen.1004356. ISSN 1553-7404. PMC 4031044. PMID 24852170.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Luo, Jiangnan; Lushchak, Oleh V.; Goergen, Philip; Williams, Michael J.; Nässel, Dick R. (12 June 2014). Broughton, Susan (ed.). "Drosophila Insulin-Producing Cells Are Differentially Modulated by Serotonin and Octopamine Receptors and Affect Social Behavior". PLOS ONE. 9 (6): e99732. Bibcode:2014PLoSO...999732L. doi:10.1371/journal.pone.0099732. ISSN 1932-6203. PMC 4055686. PMID 24923784.
- ^ Williams, Michael J; Goergen, Philip; Rajendran, Jayasimman; Klockars, Anica; Kasagiannis, Anna; Fredriksson, Robert; Schiöth, Helgi B (1 January 2014). "Regulation of Aggression by Obesity-Linked Genes TfAP-2 and Twz Through Octopamine Signaling in Drosophila". Genetics. 196 (1): 349–362. doi:10.1534/genetics.113.158402. ISSN 1943-2631. PMC 3872196. PMID 24142897.
- ^ Heberlein U, Wolf FW, Rothenfluh A, Guarnieri DJ (August 2004). "Molecular Genetic Analysis of Ethanol Intoxication in Drosophila melanogaster". Integrative and Comparative Biology. 44 (4): 269–74. CiteSeerX 10.1.1.536.262. doi:10.1093/icb/44.4.269. PMID 21676709. S2CID 14762870.
- ^ Tecott LH, Heberlein U (December 1998). "Y do we drink?". Cell. 95 (6): 733–5. doi:10.1016/S0092-8674(00)81695-5. PMID 9865690.
- ^ Williams, Ruth (22 June 2005). "Bar Flies: What our insect relatives can teach us about alcohol tolerance". Naked Scientist.
- ^ Vince, Gaia (22 August 2005). "'Hangover gene' is key to alcohol tolerance". New Scientist.
- ^ Selcho, Mareike; Pauls, Dennis (December 2019). "Linking physiological processes and feeding behaviors by octopamine". Current Opinion in Insect Science. 36: 125–130. doi:10.1016/j.cois.2019.09.002. PMID 31606580. S2CID 203470883.
- ^ Sayin, Sercan; De Backer, Jean-Francois; Siju, K.P.; Wosniack, Marina E.; Lewis, Laurence P.; Frisch, Lisa-Marie; Gansen, Benedikt; Schlegel, Philipp; Edmondson-Stait, Amelia; Sharifi, Nadiya; Fisher, Corey B. (November 2019). "A Neural Circuit Arbitrates between Persistence and Withdrawal in Hungry Drosophila". Neuron. 104 (3): 544–558.e6. doi:10.1016/j.neuron.2019.07.028. PMC 6839618. PMID 31471123.
- ^ Jia, Yicong; Jin, Shan; Hu, Kunkun; Geng, Lei; Han, Caihong; Kang, Ruxue; Pang, Yuxin; Ling, Erjun; Tan, Eng King; Pan, Yufeng; Liu, Wei (December 2021). "Gut microbiome modulates Drosophila aggression through octopamine signaling". Nature Communications. 12 (1): 2698. Bibcode:2021NatCo..12.2698J. doi:10.1038/s41467-021-23041-y. ISSN 2041-1723. PMC 8113466. PMID 33976215.
- ^ Schretter, Catherine E.; Vielmetter, Jost; Bartos, Imre; Marka, Zsuzsa; Marka, Szabolcs; Argade, Sulabha; Mazmanian, Sarkis K. (November 2018). "A gut microbial factor modulates locomotor behaviour in Drosophila". Nature. 563 (7731): 402–406. Bibcode:2018Natur.563..402S. doi:10.1038/s41586-018-0634-9. ISSN 0028-0836. PMC 6237646. PMID 30356215.
- ^ Nall, Aleksandra; Sehgal, Amita (2014). "Monoamines and sleep in Drosophila". Behavioral Neuroscience. 128 (3): 264–272. doi:10.1037/a0036209. ISSN 1939-0084. PMID 24886188.
- ^ Erion, Renske; DiAngelo, Justin R.; Crocker, Amanda; Sehgal, Amita (September 2012). "Interaction between Sleep and Metabolism in Drosophila with Altered Octopamine Signaling". Journal of Biological Chemistry. 287 (39): 32406–32414. doi:10.1074/jbc.M112.360875. PMC 3463357. PMID 22829591.
- ^ Sujkowski, Alyson; Gretzinger, Anna; Soave, Nicolette; Todi, Sokol V.; Wessells, Robert (24 June 2020). Bai, Hua (ed.). "Alpha- and beta-adrenergic octopamine receptors in muscle and heart are required for Drosophila exercise adaptations". PLOS Genetics. 16 (6): e1008778. doi:10.1371/journal.pgen.1008778. ISSN 1553-7404. PMC 7351206. PMID 32579604.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Cobb, Tyler; Sujkowski, Alyson; Morton, Courtney; Ramesh, Divya; Wessells, Robert (July 2020). "Variation in mobility and exercise adaptations between Drosophila species". Journal of Comparative Physiology A. 206 (4): 611–621. doi:10.1007/s00359-020-01421-x. ISSN 0340-7594. PMC 7314734. PMID 32335730.
- ^ Ahmed, Mohamed Ahmed Ibrahim; Vogel, Christoph Franz Adam (August 2020). "Hazardous effects of octopamine receptor agonists on altering metabolism-related genes and behavior of Drosophila melanogaster". Chemosphere. 253: 126629. Bibcode:2020Chmsp.253l6629A. doi:10.1016/j.chemosphere.2020.126629. PMID 32283422. S2CID 215757990.
- ^ Li, Yong; Hoffmann, Julia; Li, Yang; Stephano, Flora; Bruchhaus, Iris; Fink, Christine; Roeder, Thomas (December 2016). "Octopamine controls starvation resistance, life span and metabolic traits in Drosophila". Scientific Reports. 6 (1): 35359. Bibcode:2016NatSR...635359L. doi:10.1038/srep35359. ISSN 2045-2322. PMC 5069482. PMID 27759117.
- ^ Stohs SJ (January 2015). "Physiological functions and pharmacological and toxicological effects of p-octopamine". Drug and Chemical Toxicology. 38 (1): 106–12. doi:10.3109/01480545.2014.900069. PMID 24654910. S2CID 21901553.
- ^ Haller CA, Benowitz NL, Jacob P (September 2005). "Hemodynamic effects of ephedra-free weight-loss supplements in humans". The American Journal of Medicine. 118 (9): 998–1003. doi:10.1016/j.amjmed.2005.02.034. PMID 16164886.
- ^ "Prohibited In Competition – Stimulants". WADA. Retrieved 6 May 2019.
- ^ Enan, Essam (1 November 2001). "Insecticidal activity of essential oils: octopaminergic sites of action". Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology. 130 (3): 325–337. doi:10.1016/S1532-0456(01)00255-1. ISSN 1532-0456. PMID 11701389.
- ^ Rattan, Rameshwar Singh (1 September 2010). "Mechanism of action of insecticidal secondary metabolites of plant origin". Crop Protection. 29 (9): 913–920. doi:10.1016/j.cropro.2010.05.008. ISSN 0261-2194.
- ^ Maguire JJ, Davenport AP (20 February 2018). "Trace amine receptor: TA1 receptor". IUPHAR/BPS Guide to PHARMACOLOGY. International Union of Basic and Clinical Pharmacology. Retrieved 16 July 2018.
- ^ Michele L. R. Heffernan; Lee W. Herman; Scott Brown; Philip G. Jones; Liming Shao; Michael C. Hewitt; et al. (2022). "Ulotaront: A TAAR1 Agonist for the Treatment of Schizophrenia". ACS Med. Chem. Lett. 13 (1) (published 6 December 2021): 92–98. doi:10.1021/acsmedchemlett.1c00527. PMC 8762745. PMID 35047111.
- ^ "Gene Group : OCTOPAMINE RECEPTORS", FlyBase, October 16, 2018.
- ^ Zhou C, Rao Y, Rao Y (September 2008). "A subset of octopaminergic neurons are important for Drosophila aggression". Nature Neuroscience. 11 (9): 1059–67. doi:10.1038/nn.2164. PMID 19160504. S2CID 1134848.
