Sigmodal
Appearance
From Wikipedia, the free encyclopedia
Chemical compound
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider |
|
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.013.575 |
| Chemical and physical data | |
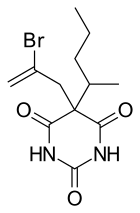
| Formula | C12H17BrN2O3 |
| Molar mass | 317.183 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Sigmodal (Rectidon) is a barbiturate derivative. It has sedative, hypnotic and anticonvulsant properties, and was used in surgical anaesthesia in the 1950s, and frequently appeared in drug mixtures in the 60s. [1] [2]
It was never widely used compared to better known barbiturates such as thiopental, and has now been replaced by newer drugs with a better safety profile.
References
[edit]| Inhalational | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injection |
| ||||||||||||||
| |||||||||||||||
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents | |
| Monoureides | |
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
Retrieved from "https://en.wikipedia.org/w/index.php?title=Sigmodal&oldid=1188722891"
Hidden categories:
- CS1 German-language sources (de)
- Articles with short description
- Short description matches Wikidata
- Articles with changed ChemSpider identifier
- ECHA InfoCard ID from Wikidata
- Articles with changed InChI identifier
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs missing an ATC code
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- All stub articles
