Aprobarbital
This is an old revision of this page, as edited by DePiep (talk | contribs) at 12:33, 27 June 2015 (Infobox drug: rm/replace dedeprecated params. Fix unk parameters (via AWB script)). The present address (URL) is a permanent link to this revision, which may differ significantly from the current revision.
 | |
 | |
| Clinical data | |
|---|---|
| Other names | aprobarbital, Oramon, allylpropymal, Alurate, 5-isopropyl- 5-allylbarbituric acid |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| ATC code | |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.908 |
| Chemical and physical data | |
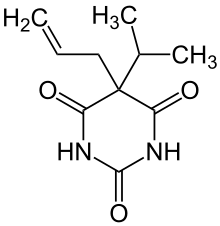
| Formula | C10H14N2O3 |
| Molar mass | 210.23 g/mol g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Aprobarbital (as known in the United States, or aprobarbitone (as known elsewhere), sold as Oramon, Somnifaine, and Allonal, is a barbiturate derivative invented in the 1920s by Ernst Preiswerk. It has sedative, hypnotic and anticonvulsant properties, and was used primarily for the treatment of insomnia.[1] Aprobarbital was never as widely used as more common barbiturate derivatives such as phenobarbital and is now rarely prescribed as it has been replaced by newer drugs with a better safety margin.
References
- ^ Reddemann H, Turk E. Oramon poisoning in infancy and childhood. Observations on 12 aprobarbital poisonings (German). Das Deutsche Gesundheitswesen. 1966 May 12;21(19):878-81.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids |
|
| Imidazoles | |
| Kava constituents | |
| Monoureides | |
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
- Articles with short description
- Short description is different from Wikidata
- ECHA InfoCard ID from Wikidata
- Chem-molar-mass both hardcoded and calculated
- Infobox-drug molecular-weight unexpected-character
- Pages using infobox drug with unknown parameters
- Drugs with no legal status
- Drugboxes which contain changes to watched fields
- All stub articles
