Estradiol/megestrol acetate
 | |
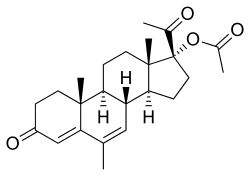 | |
| Combination of | |
|---|---|
| Estradiol | Estrogen |
| Megestrol acetate | Progestogen |
| Clinical data | |
| Trade names | Mego-E, Chinese injectable No. 2 |
| Other names | E2/MGA |
| Routes of administration | Intramuscular injection |
| Identifiers | |
| CAS Number | |
Estradiol/megestrol acetate (E2/MGA), sold under the brand names Mego-E and Chinese injectable No. 2, is a form of combined injectable birth control which is used in the People's Republic of China.[1][2][3][4][5][6][7] It contains 3.5 mg estradiol (E2), an estrogen, and 25 mg megestrol acetate (MGA), a progestin.[3][4][5] It is a microcrystalline aqueous suspension with a defined particle size range.[3][4][5] The medication is given once per month by injection into muscle.[3][4][5]
Studies of E2/MGA have been published.[8][9][10][11][12][13][14][15][16][17] The elimination half-life of MGA in this formulation is 14.35 ± 9.1 days.[10] The plasma protein binding of MGA to albumin is 82.4%, while none is bound to sex hormone-binding globulin.[10] Following an injection of E2/MGA, estradiol increased after 24 hours and persisted at high levels for 5 days, thereafter decreasing to low levels.[13]
In 1969, MGA was studied in China as an aqueous suspension for parenteral administration in animals.[18] The next year, it was studied in women as a progestogen-only injectable contraceptive, with a dosing interval of once every 3 months by intramuscular injection.[18] It was effective as a contraceptive but was associated with menstrual irregularities.[18] Starting in 1973, a combination of estradiol cypionate (EC) and MGA was studied in women as a combined injectable contraceptive over a period of 3 years.[18][1][6][2][19] E2/MGA, an "improvement" of EC/MGA, was studied in China in large clinical trials from 1977 to 1979 and was approved for use in this country in 1980.[1][6][12] By 1987, production of E2/MGA had reached 9 million units per year and had spread to over 22 Chinese provinces and cities.[12] E2/MGA appears to have been discontinued sometime between 2005 and 2008.[20]
A follow-up product consisting of 5 mg estradiol valerate (EV) and 15 mg MGA encapsulated in 50 to 80 μm-diameter microspheres as an aqueous suspension for use by intramuscular injection was developed and studied in China as well but was never marketed.[4][21][22][23][24][25][26] Following an injection, levels of MGA were higher than 2 ng/mL after a day, reached a peak of 3.2 ng/mL after 8 days, remained at levels of 2 ng/mL after 27 days, remained at 1 to 2 ng/mL after 27 to 45 days, and were below 1 ng/mL after 45 to 51 days (0.71 ng/mL on the 51st day).[26]
See also
[edit]- Combined injectable birth control § Available forms
- List of combined sex-hormonal preparations
- List of sex-hormonal aqueous suspensions
References
[edit]- ^ a b c De-Wei Z (1982). "Research activities in the field of oral contraceptives in the People's Republic of China". Acta Obstet Gynecol Scand. 105 (Suppl): 51–60. doi:10.3109/00016348209155319. PMID 6952745. S2CID 44858028.
- ^ a b Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 (Suppl 1): S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- ^ a b c d Garza-Flores J (April 1994). "Pharmacokinetics of once-a-month injectable contraceptives". Contraception. 49 (4): 347–59. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- ^ a b c d e Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ^ a b c d Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357.
- ^ a b c Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0. Archived from the original (PDF) on 28 August 2021. Retrieved 16 September 2018.; Hormonal Contraception and Post-menopausal Hormonal Therapy at Google Books
- ^ Yian JH, Min HD, Hu YT, Pan JX (1983). ""美尔伊"避孕针的临床观察及实验研究" [Mego-E injectable contraceptive: Analysis of clinical observations and laboratory findings]. Reproduction & Contraception. 3 (4): 16–20. ISSN 0253-357X.
- ^ Ding J, Guo Q, Pan J, Yan Y (1985). "避孕针剂"美尔伊"作用原理的探讨" [Study on the Mechanism of Injectable Contraceptive Mego-E.]. Universitatis Medicinalis Secondae Shanghai (5): 320–323+385. ISSN 0258-5898.[permanent dead link]
- ^ a b c Chu YH, Li Q, Zhao ZF (April 1986). "育龄妇女肌注复方甲地孕酮避孕针的药代动力学" [Pharmacokinetics of megestrol acetate in women receiving IM injection of estradiol-megestrol long-acting injectable contraceptive]. The Chinese Journal of Clinical Pharmacology (4): 224–229. doi:10.13699/j.cnki.1001-6821.1986.04.004. ISSN 1001-6821.
- ^ Zheng H, Tan Y, Bao Y, Ding Y, Wang L, Jiang X, Zhu L, Zhang Z (1986). "复方甲地孕酮注射液的遗传学效应研究" [A study on the genetic effects of "compound megestrol injection"]. Genetics and Diseases (3): 149–153. ISSN 1003-9406.[permanent dead link]
- ^ a b c Pan J (1987). "复方甲地孕酮避孕针临床研究进展" [Progress in clinical research of compound megestrol acetate injectable needle.]. Journal of Shanghai Second Medical University (S1): 71–73. ISSN 0258-5898.[permanent dead link]
- ^ a b Yan Y, Pan J, Zhang Y, Kang J (1987). "The effect of monthly injectable contraceptive megestrol acetate compound on pituitary-ovarian function". Journal of Shanghai Second Medical University (2): 7–12. ISSN 1001-6686.[permanent dead link]
- ^ Pan J, Yan Y, Li X, Zhang Y, Li M (1987). "潘家骧,严隽鸿,黎小燕,张亚琴,李明光" [Effects of injection Mego-E on blood glucose and serum insulin levels.]. Journal of Shanghai Second Medical University (2): 48–55. ISSN 1001-6686.[permanent dead link]
- ^ Zhang L, Qi Y, Zhang H (1987). "血浆甲地孕酮的高效液相色谱测定法" [HLPC determination of megestrol in blood plasma. / Determination of plasma megestrol by high performance liquid chromatography.]. Pharmaceutical Industry (7): 311–313. doi:10.16522/j.cnki.cjph.1987.07.006.[permanent dead link]
- ^ Zhou M, Zhang L, Qi Y (1988). "醋酸甲地孕酮与血浆蛋白的结合" [Studies on plasma protein binding of megestrol acetate.]. Journal of Shanghai Medical University (1): 13–17. ISSN 0257-8131.[permanent dead link]
- ^ Cheng XF, Shao HZ, Chen ZP (1988). "复方甲地孕酮避孕针对人凝血、抗凝血、纤溶及血小板聚集性的影响" [Effect of megestrol acetate compound (injectable contraceptive) on human blood coagulation, anticoagulation activity, fibrinolysis and platelet aggregation]. Shengzhi Yu Biyun (in Chinese). 8 (1): 22–6. ISSN 0253-357X. PMID 12315407.
- ^ a b c d Cheng X, Shao H, Yang J, Zhi L, Wang H, Wang Z, et al. (September 1977). "复方甲地孕酮避孕针的药理研究" [Pharmacological studies on the contraceptive injection of megestrol acetate.]. Acta Zoologica Sinica. 23 (3): 231–237. ISSN 0001-7302.[permanent dead link]
- ^ Mokhtar K. Toppozada (1983). "Monthly Injectable Contraceptives". In Goldsmith A, Toppozada M (eds.). Long-Acting Contraception. pp. 93–103. OCLC 35018604.
- ^ Gallo MF, Grimes DA, Lopez LM, Schulz KF, d'Arcangues C (2013). "Combination injectable contraceptives for contraception". The Cochrane Database of Systematic Reviews. 3: CD004568. PMID 23641480.
- ^ Han Z, Xiao R (1982). "复方甲地孕酮微囊注射液四种配伍量的临床选择" [Clinical evaluation of intramuscular injection of microencapsulated compound megestrol acetate.]. Journal of Sichuan Medical College (1): 93–99. ISSN 1672-173X.[permanent dead link]
- ^ Han ZY, Xiao RQ (August 1984). "Clinical evaluation of intramuscular injection of microencapsulated compound megestrol acetate". Int J Gynaecol Obstet. 22 (4): 319–24. doi:10.1016/0020-7292(84)90091-2. PMID 6152804. S2CID 72806071.
- ^ Han ZY, Xiao RQ (June 1985). "A follow-up study of the efficacy and safety of injectable microencapsulated megestrol acetate and a discussion on its contraceptive mechanism". Int J Gynaecol Obstet. 23 (3): 207–11. doi:10.1016/0020-7292(85)90106-7. PMID 2865183. S2CID 74276439.
- ^ Lu B, Li TL, Guan Q, Yang HL (March 1985). "微囊化与未微囊化复方甲地孕酮注射液动物肌内残留率的对比研究" [Comparative study of the residual rates of microencapsulated and unmicroencapsulated compound megestrol acetate injection in animal muscle]. Sichuan Yi Xue Yuan Xue Bao (in Chinese). 16 (1): 44–8. ISSN 1672-173X. PMID 4012589.
- ^ Lu B, Meng JX, Xie ZY, Pu JX (March 1989). "复方甲地孕酮微囊注射液溶出度的研究" [Study on dissolution rate of injection of microencapsulated compound megestrol acetate]. Hua Xi Yi Ke da Xue Xue Bao (in Chinese). 20 (1): 81–84. PMID 2793150.
- ^ a b Xu LZ, Han ZY, Yue YC, Yang SZ (March 1988). "复方甲地孕酮微囊注射液肌注后血药浓度及对垂体-卵巢功能影响的探讨" [Blood levels of megestrol acetate following injection of the microencapsulated compound megestrol acetate and its effect on pituitary-ovarian function]. Hua Xi Yi Ke da Xue Xue Bao (in Chinese). 19 (1): 97–101. ISSN 1672-173X. PMID 3391610.
