Nitrogen mustard
 |
 |
 |
The nitrogen mustards are cytotoxic chemotherapy agents similar to mustard gas. Although their common use is medicinal, in principle these compounds can also be deployed as chemical warfare agents. Nitrogen mustards are nonspecific DNA alkylating agents. Nitrogen mustard gas was stockpiled by several nations during the Second World War, but it was never used in combat. As with all types of mustard gas, nitrogen mustards are powerful and persistent blister agents. The drug mustin was developed after a war accident in 1943 in Bari, Italy where civilians and soldiers were exposed to mustard gas. It was observed that survivors had a decreased number of lymphocytes suggesting a possible therapy to cure lymphomas.
Examples
The prototype nitrogen mustard drug is mustine, which is no longer commonly in use. It was the first anticancer chemotherapeutic. It is a schedule 1 substance in the Chemical Weapons Convention. Other nitrogen mustards include cyclophosphamide, chlorambucil, uramustine and melphalan.
Examples of nitrogen mustards that can be used for chemical warfare purposes and their weapon designations include:
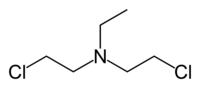
- HN1: Bis(2-chloroethyl) ethylamine
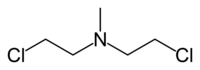
- HN2: Bis(2-chloroethyl) methylamine
- HN3: Tris(2-chloroethyl) amine
Mechanism of action
Nitrogen mustards form cyclic aminium ions (aziridinium rings) by intramolecular displacement of the chloride by the amine nitrogen. This azidirium group then alkylates basic centers on the DNA. Alkylated DNA malfunctions in replication. The therapeutically useful alkylating agents have more than one alkylating group per molecule, i.e. they are di- or polyalkylating agent. The effects are "radiomimetic," i.e. the DNA damage is similar to that which is seen when genetic material is exposed to radiation.
In the early 1960s, NMs were reported to form interstrand crosslinks (ICLs). At that time it was proposed that the ICLs were formed between N-7 atom of guanine residue in a 5’-d(GC) sequence.[1][2] Later was it clearly demonstrated that NM form a 1,3 ICL in the 5’-d(GNC) sequence.[3][4][5]
References
- ^ Geiduschek EP: "Reversible" DNA. Proc Natl Acad Sci U S A 1961, 47:950-955.
- ^ Brookes P, Lawley PD: The reaction of mono- and di-functional alkylating agents with nucleic acids. Biochem J 1961, 80(3):496-503.
- ^ Rink SM, Solomon MS, Taylor MJ, Rajur SB, McLaughlin LW, Hopkins PB: Covalent structure of a nitrogen mustard-induced DNA interstrand cross-link: An N7-to-N7 linkage of deoxyguanosine residues at the duplex sequence 5'-d(GNC). Journal of the American Chemical Society 1993, vol. 115, pp.2551-2557.
- ^ Dong Q, Barskt D, Colvin ME, Melius CF, Ludeman SM, Moravek JF, Colvin OM, Bigner DD, Modrich P, Friedman HS: A structural basis for a phosphoramide mustard-induced DNA interstrand cross-link at 5?-d(GAC). Proceedings of the National Academy of Sciences of the United States of America 1995, 92(26):12170-12174.
- ^ Bauer GB, Povirk LF: Specificity and kinetics of interstrand and intrastrand bifunctional alkylation by nitrogen mustards at a G-G-C sequence. Nucleic Acids Res 1997, volume 25, pages 1211-1218.
